| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 22, June 2023, pages 53-65
Effects of solid-state fermentation on the phytochemical composition and antioxidant activities of oriental mustard (Brassica juncea) and yellow mustard (Sinapis alba) bran
Joy Roasaa, b, Ray De Villaa, b, Lili Matsb, Honghui Zhub, Yan Zhub, Ronghua Liub, Yoshinori Minea, Rong Tsaob, *
aDepartment of Food Science, University of Guelph, Guelph, Ontario N1G 2W1, Canada
bGuelph Research and Development Centre, Agriculture and Agri-Food Canada, 93 Stone Road West, Guelph, Ontario N1G 5C9, Canada
*Corresponding author: Rong Tsao, Guelph Research and Development Centre, Agriculture and Agri-Food Canada, 93 Stone Road West, Guelph, Ontario N1G 5C9, Canada. E-mail: rong.cao@agr.gc.ca
DOI: 10.31665/JFB.2023.18348
Received: June 11, 2023
Revised received & accepted: June 27, 2023
| Abstract | ▴Top |
Mustard bran is enriched with bioactive phenolic compounds and glucosinolates, yet it is underutilized as a low-value processing by-product. Here, we investigate the effects of solid-state fermentation (SSF) using various food-grade microorganisms (Aspergillus spp., Rhizopus spp., Bacillus subtilis, Saccharomyces cerevisiae) on the phytochemical composition and antioxidant activities of oriental mustard and yellow mustard brans. The total phenolic contents (TPC) and antioxidant activities (FRAP, DPPH assays) of oriental and yellow mustard brans were significantly improved (p < 0.05) after fermentation, especially by R. oligosporus and R. oryzae. Moreover, SSF by R. oligosporus and R. oryzae significantly increased (p < 0.05) the levels of p-hydroxybenzoic acid, syringic acid, protocatechuic acid, sinapic acid and kaempferol-3-O-glucoside in both mustard brans. Conversely, a significant reduction (p < 0.05) of major glucosinolates in oriental and yellow mustard brans were observed after SSF by R. oligosporus. Findings from this study show that SSF by filamentous fungi is a promising strategy to enhance the phenolic contents, antioxidant properties and overall value of oriental and yellow mustard brans.
Keywords: Antioxidants; Glucosinolates; Mustard bran; Polyphenols; Solid-state fermentation
| 1. Introduction | ▴Top |
Oriental mustard (Brassica juncea) and yellow mustard (Sinapis alba) belong to the Brassicaceae family and are widely known around the world for their various food applications. Mustard seeds, in particular, are commonly used to produce condiments, sauces, spices, and edible oils. Aside from having desirable flavour characteristics, mustard seeds are also enriched with a variety of bioactive components, especially glucosinolates and phenolic compounds (Mayengbam et al., 2014; Torrijos et al., 2023). Glucosinolates (GSL) are plant secondary metabolites that are ubiquitously distributed in cruciferous plants, such as broccoli, cauliflower, and mustard. GSLs are composed of a thiohydroximate-O-sulfonate group linked to a glucose molecule and an alkyl, aryl or indolyl side chain. These compounds remain relatively inert until hydrolyzed into biologically active compounds by myrosinase, a β-thioglucoside glucohydrolase enzyme (Sikorska-Zimny and Beneduce, 2021). Upon plant tissue damage and in the presence of water, myrosinases released from specialized myrosin cellular compartments can hydrolyze GSLs to produce isothiocyanates (ITCs), nitriles and thiocyanates (Ishida et al., 2014). These hydrolysis products are responsible for the sharp and pungent flavours associated with cruciferous plants. GSL hydrolysis products, especially ITCs, have been found to exert antimicrobial, antioxidant, anti-inflammatory and anticarcinogenic effects (García-Ibañez et al., 2022; Sikorska-Zimny and Beneduce, 2021). Studies have identified sinigrin and sinalbin as the main GSLs in oriental and yellow mustard seeds, respectively (Bouranis et al., 2021; Pan et al., 2022).
In addition to glucosinolates, mustard seeds are also a source of phenolic compounds, which can occur in either soluble or insoluble forms (Torrijos et al., 2023). Soluble phenolic compounds can occur as free forms or conjugated forms, esterified or covalently bound to sugars or small peptides (Shahidi and Yeo, 2016). Conversely, insoluble or bound phenolics are associated with plant cell wall structures, such as pectin, cellulose, hemicellulose and structural proteins, through covalent bonding, hydrogen bonding and hydrophobic interactions (Shahidi and Yeo, 2016; Zhang et al., 2020). Phenolic compounds are known for their antioxidant properties, which have been linked to the reduced incidence of various inflammation-related chronic diseases, including type-2 diabetes, cardiovascular disease, obesity and certain cancers (Anhê et al., 2015; Hashemzaei et al., 2017; Kleemann et al., 2011; Li et al., 2015). The phenolic composition of mustard seeds have generally been found to consist of sinapic acid and its ester derivatives, sinapoyl choline (sinapine), sinapoyl malate and sinapoyl glucose; although, other phenolic compounds have been detected as well (Nguyen et al., 2021; Torrijos et al., 2023).
A recent study has shown that the bran fractions of oriental and yellow mustard seeds are highly enriched with various phenolic acids, including p-hydroxybenzoic acid, salicylic acid, ferulic acid and especially, sinapic acid (Torrijos et al., 2023). Despite being rich sources of these bioactive compounds, the bran layer of mustard seeds are commonly removed during the milling process and discarded as a by-product (Mustard 21 Canada Inc., 2019). A major limitation that may hinder the utilization and recovery of phenolic compounds from bran substrates is the low bioaccessibility of these compounds. Studies have shown that the phenolic compounds present in the mustard bran fraction mainly occur in bound forms, which have poor bioaccessibility and typically require the use of acid or base hydrolysis to facilitate their release from the cell wall matrix (Torrijos et al., 2023). Therefore, to improve the economic and functional values of mustard bran, strategies are needed to enhance the soluble phenolic contents in this substrate.
Solid-state fermentation (SSF) is a bioprocessing treatment that has been traditionally used in the production of fermented foods and is a potential strategy to enhance the bioaccessibility of phenolic compounds from bran by-products. It involves the growth of microorganisms on solid substrates in a low moisture environment, without a free-flowing aqueous phase. Advantages of SSF over conventional submerged fermentation systems include: having lower water and energy requirements, lower demand for sterility, and the capability of utilizing low-value agro-industrial residues as substrates (Bhanja Dey et al., 2016). Filamentous fungi, yeasts, and bacteria have shown SSF to be an effective treatment to enhance total phenolic contents (TPC) and antioxidant activities of substrates, such as wheat bran (Călinoiu et al., 2019; Moore et al., 2007; Roasa et al., 2021; Zhang et al., 2014). The TPC and individual phenolic components of wheat bran, including ferulic acid, vanillic acid, dihydroxybenzoic acid, and apigenin glucoside, significantly increased after 3 days of fermentation by Saccharomyces cerevisiae, which was also accompanied by higher DPPH radical scavenging activities (Călinoiu et al., 2019).
Mustard bran is an excellent source of bioactive compounds with health-promoting properties, making it a promising candidate for human health applications. Currently, the impacts of SSF on the phenolic and GSL composition, and potential health attributes of mustard bran are largely unknown. Therefore, as an unexplored area of research, our objective was to evaluate the effects of SSF on the phytochemical composition and antioxidant activities of oriental mustard and yellow mustard brans. Furthermore, an additional goal was to determine the most suitable microorganism to yield the desired results from each bran substrate; hence, SSF experiments were carried out using seven different microorganisms (Aspergillus awamori, A. niger, A. oryzae, Rhizopus oligosporus, R. oryzae, Bacillus subtilis, Saccharomyces cerevisiae) over a 7-day fermentation period. Results from this study may eventually lead to the development of mustard bran products with added value and greater health attributes.
| 2. Materials and methods | ▴Top |
2.1. Samples
Oriental (Lot No.: Jan 08/20) and yellow (Lot No.: Dec 14/19) mustard bran samples were supplied by G.S. Dunn Ltd. (Hamilton, ON). All bran samples were stored in sealed plastic bags in 4 oC prior to analysis. Aspergillus oryzae (ATCC 42149), A. niger (ATCC 9029), A. awamori Nakazawa (ATCC 38854), Rhizopus oryzae (ATCC 9363) and Saccharomyces cerevisiae L17 were provided in-house by the Guelph Research and Development Centre (Guelph, ON). Commercially available Tempeh starter culture (R. oligosporus) was purchased from Cultures For Health (Akron, OH, USA) and Bacillus subtilis natto was purchased from T&T Supermarket (Mississauga, ON).
2.2. Chemicals and reagents
HPLC-grade chemicals and solvents were obtained from EMD Chemicals (Gibbstown, NJ, USA), VWR (Mississauga, ON) and Caledon Labs (Georgetown, ON) unless otherwise specified. Analytical standards (e.g., gallic acid, Trolox) were obtained from Sigma-Aldrich (Oakville, ON). Culture media were purchased from VWR (Mississauga, ON) or Fisher Scientific (Nepean, ON) and Tween 20 was purchased from Sigma-Aldrich. Reagents for antioxidant and total phenolic content assays, including 1,3,5-tri(2-pyridyl)-2,4,6-triazine (TPTZ), L-ascorbic acid, Folin-Ciocalteu’s phenol reagent, sodium bicarbonate, fluorescein, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azobis-(2-methylpropionamidine) dihydrochloride (AAPH), were also purchased from Sigma-Aldrich. Sodium acetate, ferric chloride hexahydrate, sodium phosphate monobasic and sodium phosphate dibasic were purchased from Caledon Labs. Distilled and deionized water were obtained from a Thermo Scientific Barnstead Nanopure ultrapure water purification system (Ottawa, ON).
2.3. Solid-state fermentation (SSF)
Preparation of microbial cultures and SSF experiments were performed using modified versions of previously reported methods (Bhanja Dey and Kuhad, 2014; Chen et al., 2020; Moore et al., 2007; Sandhu et al., 2016; Shin et al., 2019; Torino et al., 2013). Aspergillus spp. and Rhizopus spp. were cultured on potato dextrose agar (PDA, 15 g/L agar, 20 g/L dextrose, 4 g/L potato extract) plates and incubated at 30 °C for 3–5 days. Spore suspensions were prepared by suspending the top mycelia on each PDA plate using 10 mL 0.1% Tween 20, followed by filtration of spores through a sterilized cheese cloth. Filtration was repeated twice more using 10 mL of 0.1% Tween 20 and the volume was adjusted to obtain a final suspension volume of 50 mL (approximately 105–106 spores/mL). An isolated pure colony obtained from streak plating Bacillus subtilis natto on PDA was aseptically transferred into 5 mL of lysogeny broth (LB, 10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl) and incubated at 37 °C for 24 h, with constant shaking at 150 rpm. The resulting broth culture (5 mL) was transferred into 100 mL of LB broth and incubated at 37 °C for 24 h, with constant shaking at 150 rpm. An isolated pure colony obtained from streak plating S. cerevisiae L17 on PDA was aseptically transferred into 5 mL of yeast malt (YM, 10 g/L glucose, 3 g/L malt extract, 5 g/L peptone, 3 g/L yeast extract) broth and was incubated at 33 °C for 24 h under anaerobic conditions, with constant shaking at 150 rpm. The broth culture (5 mL) was then transferred into 100 mL of YM broth and incubated once more at 33 °C for 24 h under anaerobic conditions, with constant shaking at 150 rpm. The bacterial suspension (105 colony forming units (CFU)/mL) was kept at room temperature for immediate use in SSF experiments. Fungal spore and yeast suspensions (106 CFU/mL) were stored in 4 °C prior to experiment use.
Mustard bran samples were weighed (∼30 g) into aluminum trays, which were then loosely covered with aluminum foil and were autoclaved (121 °C, 20 min). Once cooled, 1.0 g of each bran was accurately weighed into 15 mL tubes in triplicate. Each tube was inoculated aseptically at a 10% inoculum ratio using the prepared fungal spore, bacterial and yeast suspensions. The moisture contents of Aspergillus spp. and Rhizopus spp. were adjusted to 50% using sterilized modified Czapek-Dox broth (3.0 g/L NaNO3, 0.5 g/L MgSO4, 0.5 g/L KCl, 1.0 g/L K2HPO4, 0.01 g/L FeSO4), whereas B. subtilis and S. cerevisiae groups were adjusted to 70%. Non-fermented samples (0 h) were immediately stored at −20 °C after inoculation. Tubes with Aspergillus spp. and Rhizopus spp. groups were partially covered and incubated for 24, 48, 72, 96, 120 and 168 h at 30 °C in static mode. B. subtilis tubes were also partially covered and incubated for the specified fermentation duration at 37 °C, with constant shaking at 150 rpm. S. cerevisiae tubes were sealed (anaerobic) and incubated at 33 °C, with constant shaking at 150 rpm. Fermented bran samples were stored in −20 °C prior to further analysis.
2.4. Extraction
Extractable phenolics and glucosinolates were extracted from raw (non-autoclaved, untreated), non-fermented (0 h) and fermented (24, 48, 72, 96, 120 and 168 h) mustard bran samples using aqueous methanol according to a slightly modified version of previously reported methods (Alrifai et al., 2021; Chen et al., 2015). Briefly, 5 mL of 70% methanol with 0.1% formic acid (v/v) was added into each 15 mL tube containing 1.0 g bran. Each tube was then vortexed for 30 s by an IKA MS1 Minishaker (IKA Works, Wilmington, NC) and sonicated for 15 min at room temperature (VWR, Mississauga, ON), which was followed by constant rolling on a rotary shaker (Scientific Industries Inc., Bohemia, NY) at 150 rpm for 18 h. The mixture was centrifuged at 4,000 rpm for 10 min by Eppendorf centrifuge (5810R, Brinkman Instruments Inc., Westbury, NY, USA) and the extraction was repeated twice more with 5 mL extraction solvent for 2 h each. The volumes of the pooled crude extracts were adjusted 15 mL. Crude extracts were filtered through Phenex-NY syringe filters (4mm, i.d.; 0.2μm particle size; Sigma-Aldrich) prior to LC-MS/MS analyses.
2.5. Total phenolic contents
The TPC of bran extracts was determined by the Folin-Ciocalteu method (Li et al., 2012). Authentic gallic acid standards were prepared at concentrations: 0, 0.0313, 0.0625, 0.125, 0.250, and 0.500 mg/mL. Briefly, 25 μL of extracts or standards were mixed with 125 μL of 0.2 M Folin-Ciocalteu reagent in a 96-well microplate, which were allowed to react for 6 min at room temperature. Subsequently, 125 μL of 15% Na2CO3 was added into each of the wells and was allowed to react for 30–120 min at room temperature. The absorbance was measured at 765 nm using a UV-Vis microplate kinetic reader (EL 340, Bio-Tek Instruments, Inc., Winooski, VT, USA). TPC was determined using the gallic acid calibration curve and was expressed as mg gallic acid equivalents per g dry weight bran (mg GAE/g DW).
2.6. Antioxidant assays
2.6.1. Ferric-reducing antioxidant power (FRAP) assay
FRAP activities of mustard bran extracts were determined using a previously reported procedure (Li et al., 2012). Briefly, ferric-TPTZ reagent was first prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM TPTZ in 40 mM HCl, and 20 mM FeCl3•6H2O at a ratio of 10:1:1 (v/v/v). Diluted extracts (10 μL) or ascorbic acid standards (62.5, 125, 250, 500, 750 and 1,000 μM) were allowed to react with 300 μL of ferric-TPTZ reagent in a 96-well microplate for 2 h at room temperature. The absorbance was read at 593 nm using a UV-Vis microplate kinetic reader (EL 340, Bio-Tek). FRAP activities were calculated using the ascorbic acid calibration curve and were expressed as μmol ascorbic acid equivalents (AAE) per g bran on a dry weight basis (μmol AAE/g DW).
2.6.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay
The radical scavenging activities of mustard bran extracts were determined according to a previously reported method with minor modifications (Li et al., 2012). Briefly, 25 μL of appropriately diluted extracts or Trolox standards (125, 250, 500, 750, 1,000 and 1,250 μM) were mixed with 225 μL of 350 μM DPPH• in methanol in a 96-well microplate. The mixtures were allowed to react for 6 h under subdued light at room temperature. The absorbance was measured at 517 nm using a UV-Vis microplate kinetic reader (EL 340, Bio-Tek). The DPPH radical scavenging activity was calculated using the Trolox calibration curve and was expressed as μmol Trolox equivalents per g bran on a dry weight basis (μmol TE/g DW).
2.6.3. Oxygen radical absorption capacity (ORAC) assay
ORAC activities of mustard bran extracts were determined using a previous method with minor modifications (Li et al., 2012). Briefly, 25 μL of blank, Trolox (6.25, 12.5, 25, 50, and 100 μM) or diluted bran extracts were mixed with 150 μL of 8.68 × 10−5 mM fluorescein solution in phosphate buffer (pH 7.4) in a 96-well microplate, which was then incubated for 30 min at 37 °C. The reaction was initiated by addition of 25 μL 153 mM AAPH solution (phosphate buffer, pH 7.4), followed by shaking for 10 s. The fluorescence (excitation at 485 nm; emission at 520 nm) was monitored kinetically, with data read every minute for about 120 min, in a Bio-Tek Fluorescence Spectrophotometer equipped with an automatic thermostatic holder (PLX 800, Bio-Tek). The calibration curve was constructed by plotting the difference between the area under the fluorescein decay curve of known standards and the blank against their respective concentrations. ORAC results were expressed as μmol TE per g bran on a dry weight basis (μmol TE/g DW).
2.7. LC-MS/MS analysis
A targeted, quantitative profiling approach using LC-MS/MS was performed on extracts of selected mustard bran samples using a slightly modified protocol (Alrifai et al., 2021). LC-MS/MS analysis was conducted using a Thermo® Scientific Q-Exactive™ Orbitrap mass spectrometer equipped with a Vanquish™ Flex Binary UPLC System (Waltham, MA, USA). The chromatographic separation was performed on a Kinetex XB-C18 100A HPLC column (100 x 4.6 mm, 2.6 µm, Phenomenex Inc., Torrance, CA, USA). The binary mobile phase consisted of solvent A (99.9% H2O/0.1% formic acid) and solvent B (94.9% MeOH/5%/0.1% formic acid). High purity LC-MS grade methanol, acetonitrile and formic acid were used for LC-MS analysis (Optima grade, ThermoFisher Scientific, Mississauga, ON). Most phenolic and GSL compounds were detected by using negative ionization mode and two compounds (sinapine and syringaldehyde) were determined by using positive ionization mode. The negative mode solvent gradient was: 0–5 min, 0% to 12% B; 5–15 min, 12% to 23% B; 15–30 min, 23% to 50% B; 30 - 40 min, 50% to 80% B; 40–42 min, 80% to 100% B; 42–45 min, 100% B; 45–46 min, 100% to 0% B; 46–52 min, 0% B. The positive mode solvent gradient was: 0–5 min 20% B; 5–6 min, 20% to 100% B; 6–9 min, 100% B; 9–10 min, 100% to 20% B; 10–16 min, 20% B. The column temperature was set at 40 °C, the flow rate was set at 0.700 mL/min, and the injection volume was 2 µL; UV peaks were monitored at 280 nm, 320 nm, 360 nm and 520 nm. The spray voltages for negative and positive modes were set at 4.5 kV an 3.3 kV, respectively. Mass spectrometry data were collected using either Full-MS mode for quantification, or DDMS2 (TopN = 10, NCE = 30) mode for identification. Data were visualized and analysed using Thermo FreeStyle™ 1.7PS2 software. Quantification was achieved using calibration curves generated from selected analytical standards in serial dilutions (0.78–200 mg/L; r2 = 0.99).
2.8. Statistical analysis
All mustard bran samples were analysed in triplicates and results were expressed as mean ± standard deviation (n = 3). A two-way analysis of variance (ANOVA) followed by Tukey’s Honest Significant Difference (HSD) test was performed to analyze the effect of fermentation duration and microbial group on the TPC and antioxidant activities of mustard bran samples. Moreover, one-way ANOVA followed by Tukey’s HSD test was used to evaluate the effect of fermentation per microbial group on the glucosinolate and phenolic contents of each mustard bran. Significant differences were considered at p < 0.05. Pearson’s correlation coefficient (r) was used to evaluate the correlations among variables. Principal component analyses (PCA) were performed to gain an overview of the relationships between individual phenolic and glucosinolate compounds throughout fermentation. Statistical analysis was performed using RStudio Software (Boston, MA, USA).
| 3. Results and discussion | ▴Top |
3.1. Total phenolic contents
To the best of our knowledge, the impacts of SSF on mustard processing by-products have not been extensively investigated in literature. Some studies have evaluated the use of mustard oilseed cakes as fermentation substrates for the production of industrially relevant enzymes, such as amylase and lipase (Saxena and Singh, 2011; Sethi et al., 2016); however, the effects of SSF on the phenolic and GSL composition of mustard bran have not been reported. It is important to note that the TPC of mustard bran samples in the present study mainly refer to the total soluble phenolic contents. Mixtures of aqueous-organic solvents are widely used to extract phenolic compounds from plant materials. This method, however, preferentially isolates soluble phenolics while neglecting insoluble or bound phenolic compounds, which remain associated with structural components in the plant cell wall matrix (Saura-Calixto and Pérez-Jiménez, 2018). Therefore, results in this study mainly reflect the effects of SSF on the composition of extractable phenolics in oriental and yellow mustard brans.
Prior to SSF experiments, a sterilization step was employed for the removal of endogenous microbes and the thermal inactivation of myrosinases to prevent the premature hydrolysis of glucosinolates in the mustard bran samples. Interestingly, the TPC of oriental mustard bran was found to decrease after autoclaving while the TPC of yellow mustard bran generally increased (Figure S1–2). These results could be explained by the stability and initial composition of phenolic compounds in each bran. The soluble phenolics present in the native state of oriental mustard bran may be sensitive to high temperatures and susceptible to degradation, resulting in a decrease in TPC after autoclaving. Conversely, the increase in soluble phenolic contents in yellow mustard bran after thermal treatment may be due to the release of bound phenolics from the bran matrix. Similar results have been observed after the thermal treatment of other bran substrates, such as wheat bran (Spaggiari et al., 2020). The fermentation process was also found to significantly enhance (p < 0.05) the soluble TPC of both oriental and yellow mustard bran, which greatly depended on fermentation duration and the microbe used (Figures 1a and 2a). A minimum fermentation duration of 24 h for R. oligosporus and R. oryzae, and 48 h for A. awamori and A. niger were sufficient at significantly improving the TPC of oriental mustard bran. The TPC of bran samples continued to increase after 48 h, reaching maximum TPC results at different fermentation times based on the treatment group. The TPC of oriental mustard bran fermented by A. awamori had a maximum increase of +63.5% after 72 h of fermentation, whereas R. oligosporus fermented samples had a maximum increase of +54.3% after 96 h of treatment relative to non-fermented bran. SSF by A. niger (+109.6%) and R. oryzae (+79.5%) led to the highest increases in TPC after 168 h of treatment relative to non-fermented bran. No significant changes in TPC were observed in oriental mustard bran fermented by A. oryzae and B. subtilis. Conversely, significant changes in the TPC of yellow mustard bran was only observed in samples fermented by R. oligosporus and R. oryzae. The TPC of yellow mustard bran was significantly improved as early as 24 h for both R. oligosporus (+8.9%) and R. oryzae (+15.4%) fermented samples. Notably, the TPC of yellow mustard bran fermented by these Rhizopus spp. did not significantly change past 24 h of treatment.
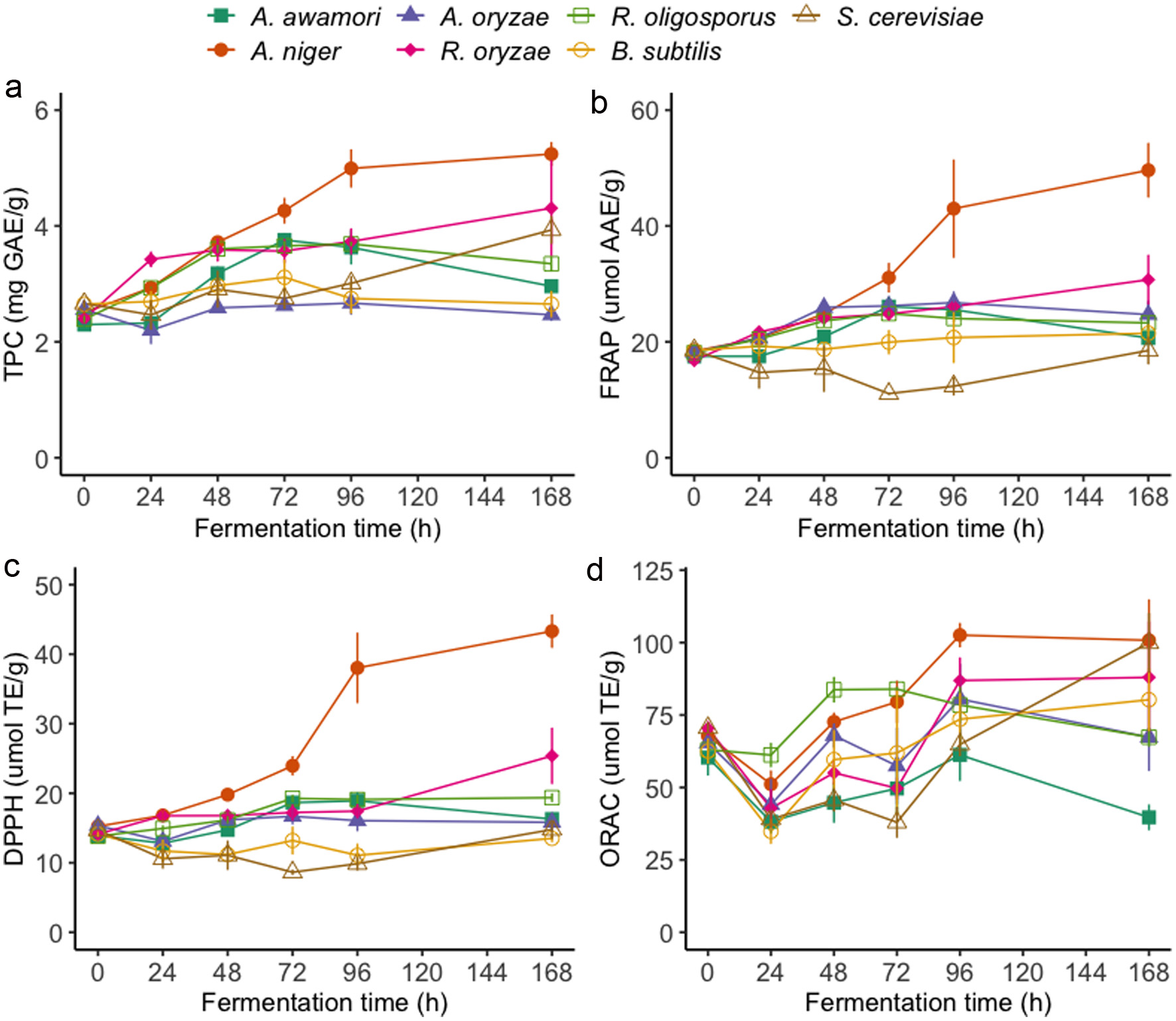 Click for large image | Figure 1. Total phenolic contents (a) and antioxidant activities FRAP (b), DPPH (c), and ORAC (d) of oriental mustard bran extracts during solid-state fermentation by various microbes. |
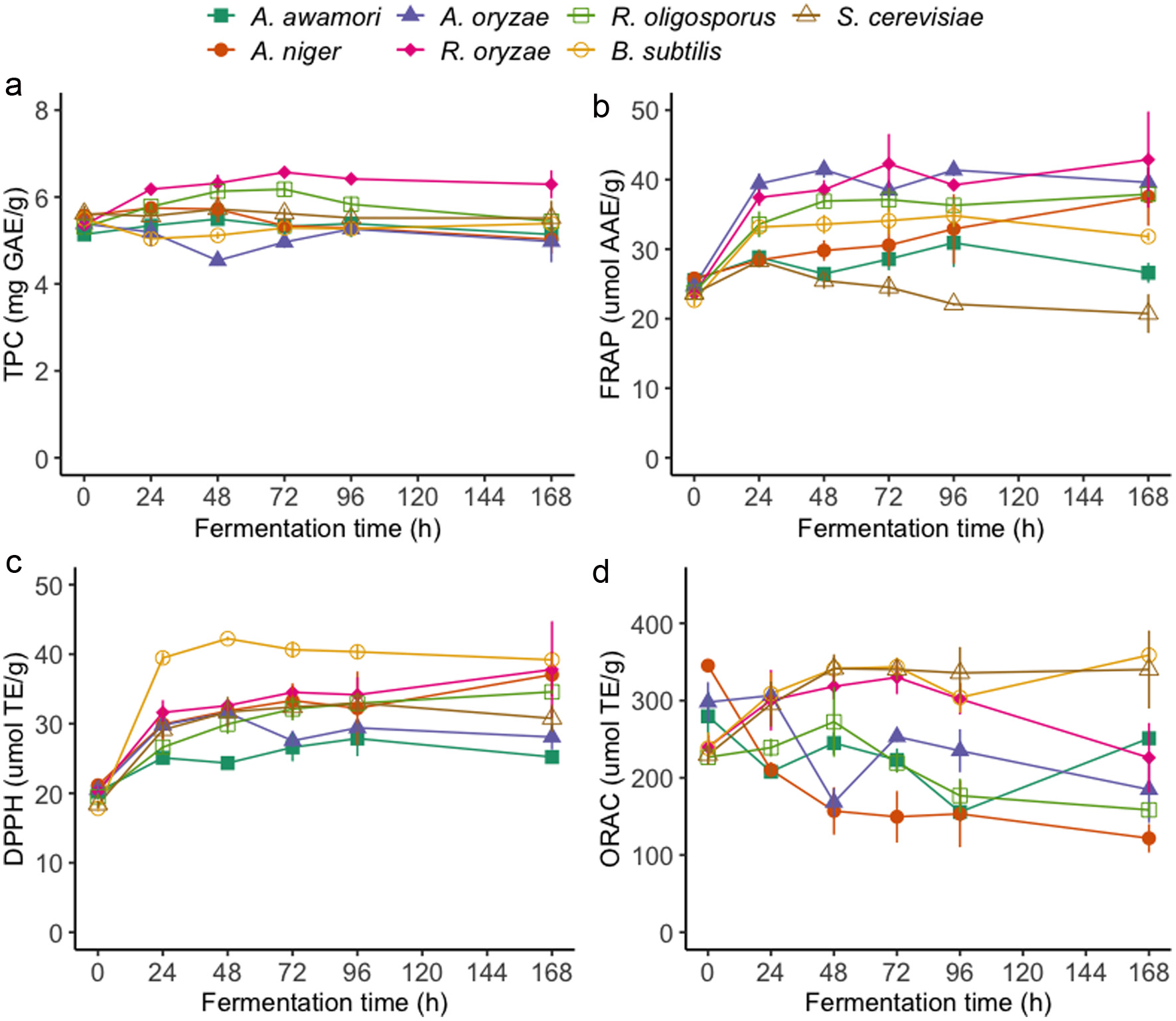 Click for large image | Figure 2. Total phenolic contents (a) and antioxidant activities FRAP (b), DPPH (c), and ORAC (d) of yellow mustard bran extracts during solid-state fermentation by various microbes. |
Currently, there is a lack of research on the impacts of SSF on the phenolic contents of mustard bran; hence, this research provides new information on this area. Results from this study are in line with research on other substrates, such as wheat bran, which show that SSF is an effective method to improve the extractable phenolic contents of bran (Călinoiu et al., 2019; Moore et al., 2007). The significant improvement of TPC may be attributed to the activity of microbial enzymes, which can catalyze the degradation of cell wall structures and liberate bound phenolics from the bran matrix. Consequently, differences in TPC results may be due to the release of different enzymes, such as amylase, protease, esterase and cellulase, by each microorganism used in this study (Sharma et al., 2020). Filamentous fungi are generally considered to be more adapted for SSF than yeast or bacteria due to the ability of fungal hyphae to penetrate the surface of solid substrates. R. oryzae and R. oligosporus have been traditionally used to produce solid fermented food, such as tempeh (Cantabrana et al., 2015). Therefore, due to their pre-existing food applications and effectiveness at enhancing the soluble phenolic contents of mustard bran, R. oryzae and R. oligosporus may be suitable in transforming mustard bran into a fermented food product or ingredient.
3.2. Antioxidant activities
The antioxidant activities of oriental mustard bran samples, measured through the FRAP, DPPH and ORAC assays, throughout the fermentation period are summarized in Figure 1b–d and Table S1. Significant increases in FRAP activities were observed in oriental mustard bran fermented by all Aspergillus spp. and Rhizopus spp. while bran fermented by B. subtilis had no significant change in FRAP activities relative to non-fermented bran. SSF by A. niger (+35.0%), A. oryzae (+42.6%), R. oligosporus (+30.2%) and R. oryzae (+43.5%) significantly enhanced the reducing power of mustard bran after 48 h of treatment. In contrast, a minimum of 72 h of fermentation by A. awamori was required to attain a significant improvement in FRAP activities. Trends in FRAP results of oriental mustard bran throughout SSF treatment generally corresponded well with DPPH radical scavenging activities. B. subtilis, S. cerevisiae and A. oryzae treatment groups had no significant effects on DPPH radical scavenging activities of oriental mustard bran. For all treatment groups, ORAC activities of oriental mustard bran samples were found to decrease after 24 h of fermentation, which was subsequently followed by a general upward trend in ORAC results as fermentation time increased. SSF by A. niger and R. oligosporus led to the highest percent increases in ORAC activities in oriental mustard bran, reaching +51.6% after 96 h and +32.9% after 48 h of fermentation, respectively.
Regarding the antioxidant activities of yellow mustard bran samples, significant differences were observed between fermented and non-fermented bran, which varied based on the treatment group (Figure 2b–d; Table S2). Similar to the effects of SSF on the TPC of yellow mustard bran, R. oligosporus and R. oryzae were the most efficient at enhancing the FRAP activities of bran samples, increasing by +41.0% and +58.8% after 24 h of fermentation, respectively. Interestingly, DPPH scavenging activities of yellow mustard bran were significantly improved by all microorganisms as early as 24 h of fermentation. Percent increases in the DPPH scavenging activities of yellow mustard bran samples after 24 h of treatment ranged from +24.1–121.4%, which were reached by each microorganism in the following order: A. awamori < R. oligosporus < A. niger < A. oryzae < R. oryzae < S. cerevisiae < B. subtilis. In contrast to FRAP and DPPH results, changes in the ORAC activities of yellow mustard bran samples throughout SSF generally followed different trends. SSF by R. oryzae, B. subtilis and S. cerevisiae led to significant enhancement of ORAC results compared with non-fermented bran, increasing by +25.3, 29.5 and 28.7%, respectively. On the other hand, the ORAC values of yellow mustard bran fermented by the remaining microorganisms, especially by A. awamori, A. niger and A. oryzae, were significantly reduced throughout fermentation.
The favourable effects of SSF on the antioxidant activities of mustard bran are in line with results reported by studies on other substrates (Zhang et al., 2014). In one study, Zhang et al (2014) evaluated the effects of fermentation using various microbes on the TPC and antioxidant activites of wheat bran and found that R. oryzae could significantly increase the antioxidant capacity of wheat bran. The discrepancy between trends in antioxidant activities measured through FRAP, DPPH and ORAC assays could be attributed to the individual activities of various phenolic compounds or other antioxidant metabolites released or altered by SSF treatment. FRAP, DPPH and ORAC assays are widely used chemical-based methods to measure antioxidant activities; however, each method is characterised by different mechanisms of action. For instance, electron transfer reactions are the basis for FRAP and DPPH assays while the ORAC method is based on hydrogen atom transfer (HAT) (Amorati and Valgimigli, 2015). The antioxidant activities of phenolic and related compounds are greatly influenced by their chemical structures. As such, differences in the phytochemical profiles of fermented bran samples can lead to discrepancies in results between the antioxidant assays used.
3.3. Characterization of phenolic and glucosinolate profiles
The phenolic and glucosinolate (GSL) contents of mustard bran samples were analyzed and quantified using LC-MS/MS. After an initial, untargeted pre-screening to determine major compounds that were significantly altered by SSF treatment of oriental (Table 1) and yellow (Table 2) mustard brans, a total of 4 GSL and 9 phenolic compounds were selected for quantification. The extracted ion chromatograms (XIC) of relevant phenolic compounds that were most affected during fermentation are also shown in Figure 3 and 4. Due to the favourable performance of filamentous fungi, especially by R. oligosporus and R. oryzae, at improving the TPC of both oriental and yellow mustard bran, these treatment groups were considered for individual quantification of phenolic and GSL compounds. Bran samples fermented by A. awamori were also included in the LC-MS/MS analysis for comparison.
 Click to view | Table 1. Quantification of main glucosinolates and phenolic compounds in oriental mustard bran fermented by A. awamori, R. oligosporus and R. oryzae |
 Click to view | Table 2. Quantification of main glucosinolates and phenolic compounds in yellow mustard bran fermented by A. awamori, R. oligosporus and R. oryzae |
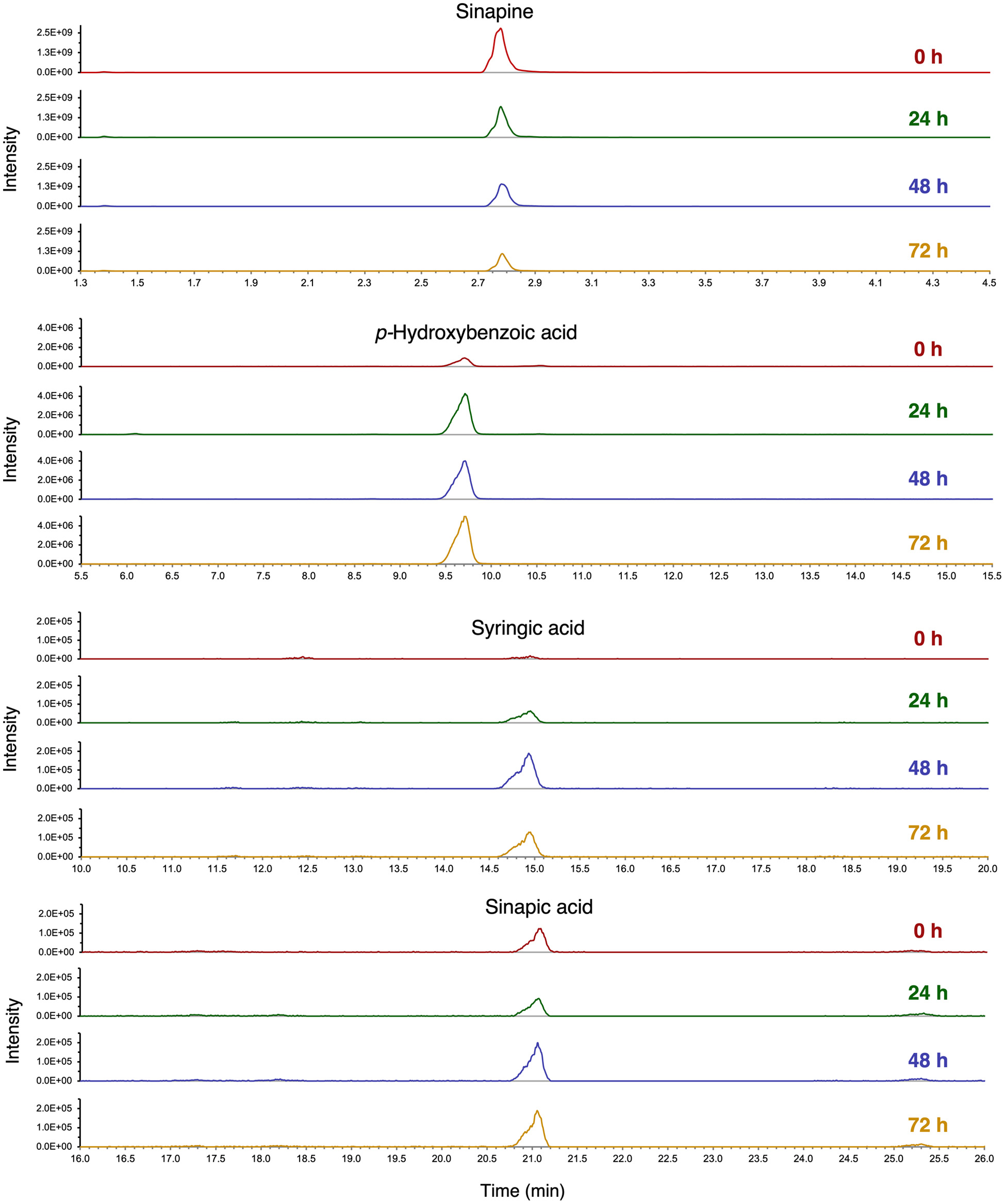 Click for large image | Figure 3. Extracted ion chromatograms (XIC) of sinapine, p-hydroxybenzoic acid, syringic acid and sinapic acid in oriental mustard bran after 0, 24, 48 and 72 h of fermentation by R. oryzae. Individual compounds were identified and quantified using analytical standards. |
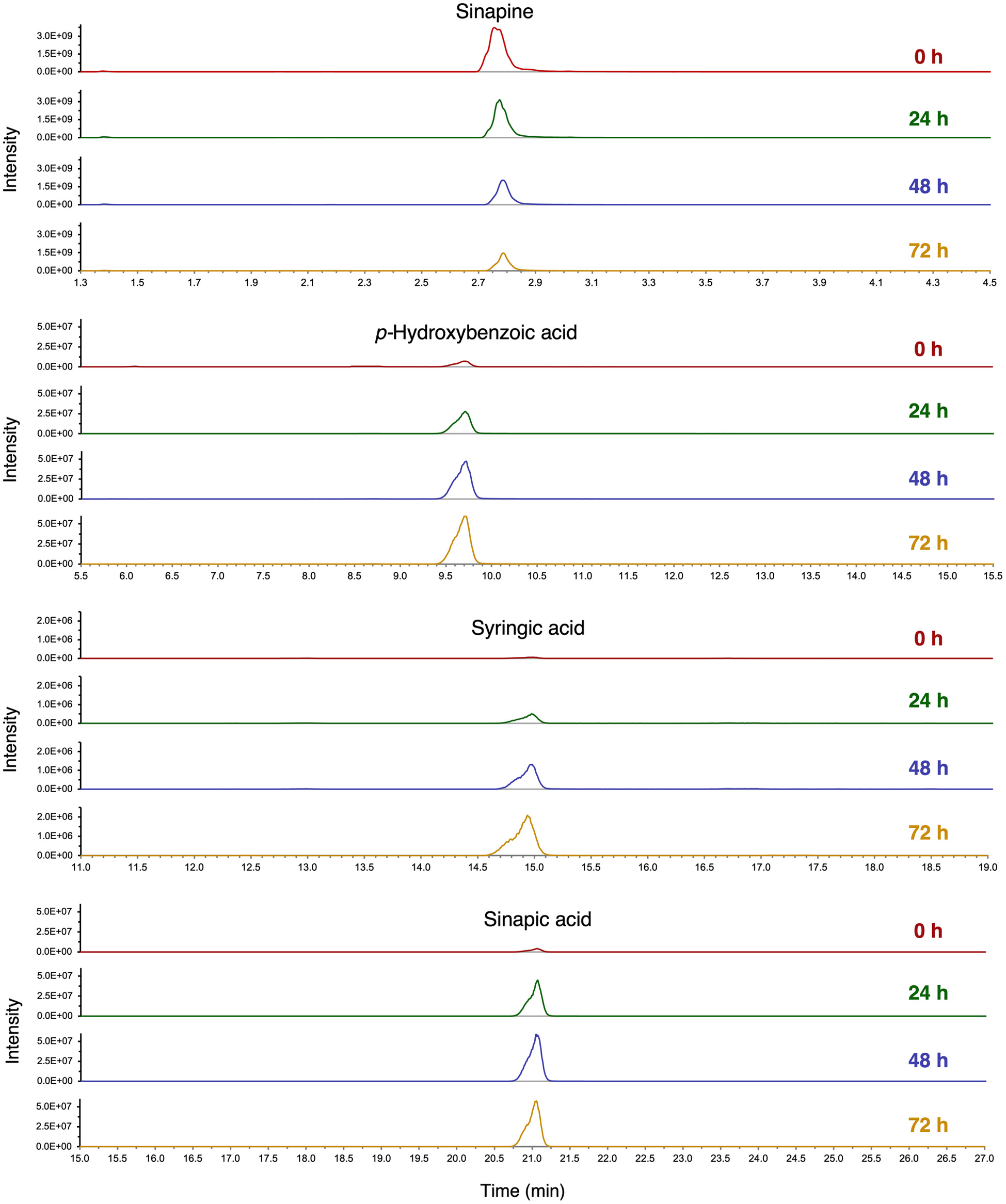 Click for large image | Figure 4. Extracted ion chromatograms (XIC) of sinapine, p-hydroxybenzoic acid, syringic acid and sinapic acid in yellow mustard bran after 0, 24, 48 and 72 h of fermentation by R. oryzae. Individual compounds were identified and quantified using analytical standards. |
Notable phenolic compounds in oriental and yellow mustard brans that increased significantly during the first 72 h of SSF by R. oligosporus and R. oryzae include: syringic acid, p-hydroxybenzoic acid, sinapic acid, protocatechuic acid and kaempferol-3-O-glucoside. In contrast, phenolic compounds that significantly decreased in both mustard brans after fermentation, include salicylic acid, sinapine and syringaldehyde. GSL contents, including sinigrin, sinalbin, gluconasturtiin and glucobrassicin, were found to either decrease or remain constant throughout SSF treatment. In oriental mustard bran samples fermented by R. oligosporus, the sinigrin contents decreased significantly by −67.9% after 72 h of treatment, whereas no significant change was observed in the sinigrin contents of the R. oryzae group. Likewise, the sinalbin contents of yellow mustard bran after 72 h of SSF by R. oligosporus significantly decreased by −68.4% while the sinalbin contents of R. oryzae fermented bran remained relatively constant. These results may be attributed to the microbial release of myrosinase-like enzymes by R. oligosporus capable of hydrolyzing GSLs in the mustard bran (Zhang et al., 2022).
In this study, the levels of phenolic compounds in oriental and yellow mustard brans prior to fermentation treatments were generally comparable with the contents reported by Torrijos et al (2023). Any differences in initial contents of individual phenolics may be due to the impact of sterilization, which may have led to degradation. Comparatively, yellow mustard bran contained higher phenolic contents than oriental mustard bran, both before and after SSF. In fact, a +760.5%, +3,181% and 1,380% increase in p-hydroxybenzoic acid, syringic acid and sinapic acid, respectively, was observed in yellow mustard bran after 72 h of SSF. The significant improvement in sinapic acid contents may be due to the conversion of sinapine into sinapic acid and sinapate esters, such as sinapoyl malate, by microbial enzymes (Nguyen et al., 2021). Moreover, due to having higher contents of phenolic compounds, these findings suggest that yellow mustard bran may exhibit more potent antioxidant activities than oriental mustard bran.
3.4. Correlation and principal component analyses
Pearson’s correlation analysis was performed to determine the correlation between the total phenolic contents, antioxidant activities and individual compounds quantified in oriental and yellow mustard bran (Figure 5). Based on the LC-MS/MS analysis, R. oligosporus, R. oryzae and A. awamori treatment groups were used to evaluate correlation between variables. The FRAP and DPPH activities of oriental mustard bran samples in all three treatment groups were found to highly correlate with TPC results. On the other hand, the R. oligosporus treatment group was the only one observed to exhibit a strong correlation between TPC and ORAC results. Regarding individual compounds, the TPC of R. oryzae fermented samples was highly correlated with p-hydroxybenzoic acid, syringic acid and kaempferol-3-O-glucoside, suggesting that these compounds may contribute greatly to the TPC, FRAP and DPPH activities of oriental mustard bran throughout fermentation. Likewise, oriental mustard bran fermented by R. oligosporus exhibited strong positive correlations between TPC and the contents of syringic acid, sinapic acid and kaempferol-3-O-glucoside. In contrast, the TPC of bran samples fermented by A. awamori did not exhibit high correlation with any of the individual phenolic compounds.
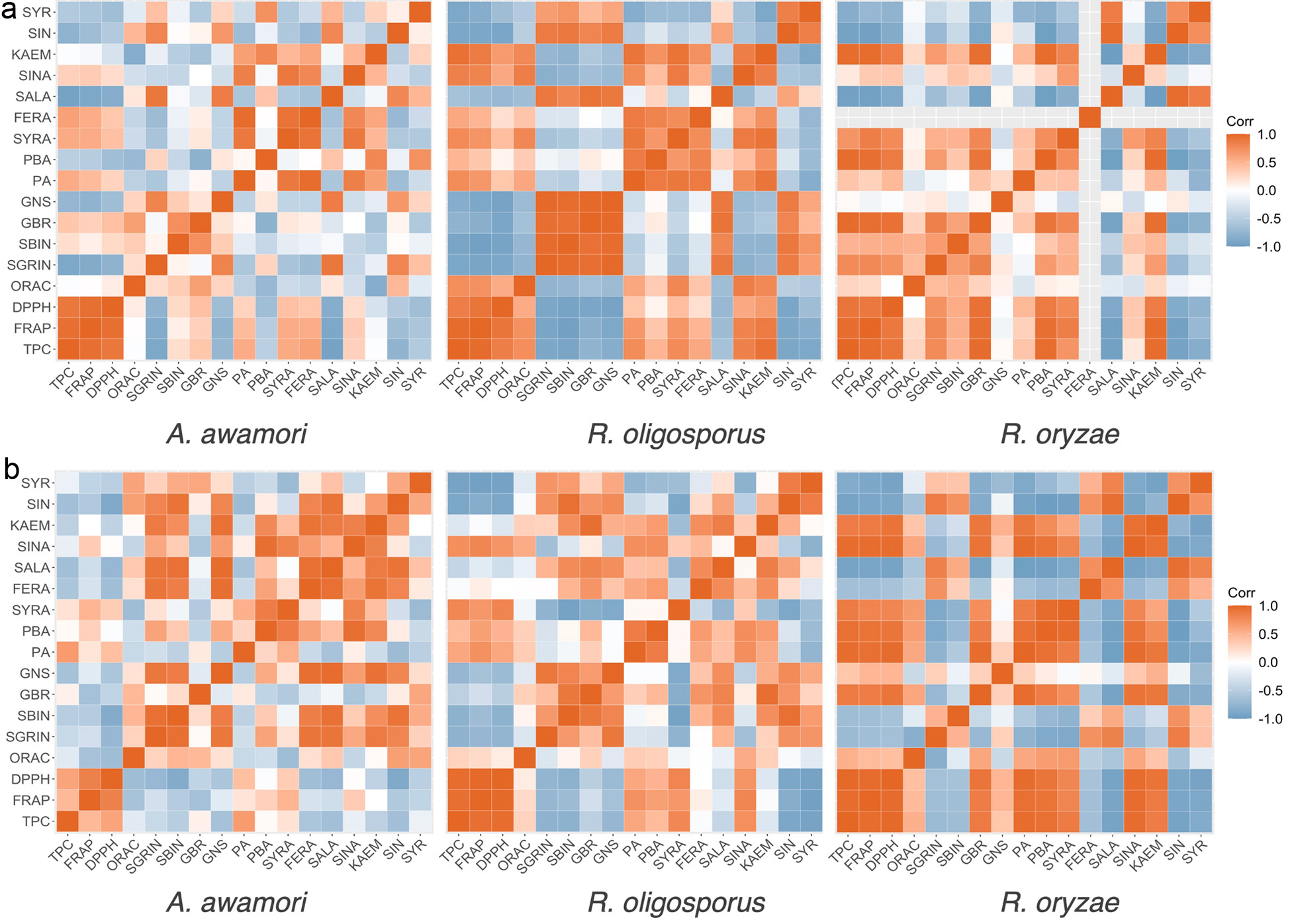 Click for large image | Figure 5. Correlation heatmaps between the total phenolic contents (TPC), antioxidant activities (FRAP, DPPH, ORAC), and glucosinolate and phenolic contents of (a) oriental mustard bran and (b) yellow mustard bran fermented by A. awamori, R. oligosporus and R. oryzae. Strong positive correlations are denoted by a deep red color. Strong negative correlations are denoted by a deep blue color. FERA: trans-ferulic acid, GBR: glucobrassicin, GNS: gluconasturtiin, KAEM: kaempferol-3-O-glucoside, PA: protocatechuic acid, PBA: p-hydroxybenzoic acid, SALA: salicylic acid, SBIN: sinalbin, SGRIN: sinigrin, SIN: sinapine, SINA: sinapic acid, SYR: syringaldehyde, SYRA: syringic acid. |
The TPC of yellow mustard bran was found to highly correlate with FRAP and DPPH activities for both R. oligosporus and R. oryzae treatment groups. Compared with individual phenolic compounds, the TPC of R. oryzae fermented bran showed strong positive correlations with several phenolic compounds, including p-hydroxybenzoic acid, protocatechuic acid, syringic aid, sinapic acid and kaempferol-3-O-glucoside. Conversely, the TPC of bran samples fermented by R. oligosporus showed weak to moderate positive correlations against p-hydroxybenzoic acid, protocatechuic acid, syringic aid and sinapic acid. Similar to oriental mustard bran, the TPC of yellow mustard bran fermented by A. awamori did not yield strong positive correlations with any individual phenolic compounds. Aside from potentially catalyzing the liberation of bound phenolics from the bran matrix, which may in turn lead to an increase in total soluble phenolic contents, the microbial enzymes released by the microbes may also produce fermentation metabolites that can influence the TPC results. This may explain the disagreement between the increase in TPC yet lack of significant changes in individual phenolic contents of oriental mustard bran fermented by A. awamori (Spaggiari et al., 2020).
To gain further insights on the impacts of SSF using different microorganisms on the GSL and phenolic contents of oriental and yellow mustard bran, principal component analysis (PCA) was conducted (Figure 6). PCA is a useful method to reduce the dimensionality of large datasets by projecting data points onto a few principal components, while preserving the total variation. As shown in Figure 6a, the first three principal components explain about 83.9% of the total variance in the oriental mustard bran dataset; hence, principal components 1, 2 and 3 were used to create the PCA biplot. For yellow mustard bran, the first three principal components were also considered in the analysis since they accounted for about 84.1% of the total variance in the data set (Figure 6b). Loading variables, which represent individual GSL and phenolic compounds, are denoted as vectors while individual results for each day of fermentation per microorganism (A. awamori, R. oligosporus, R. oryzae) are denoted as points.
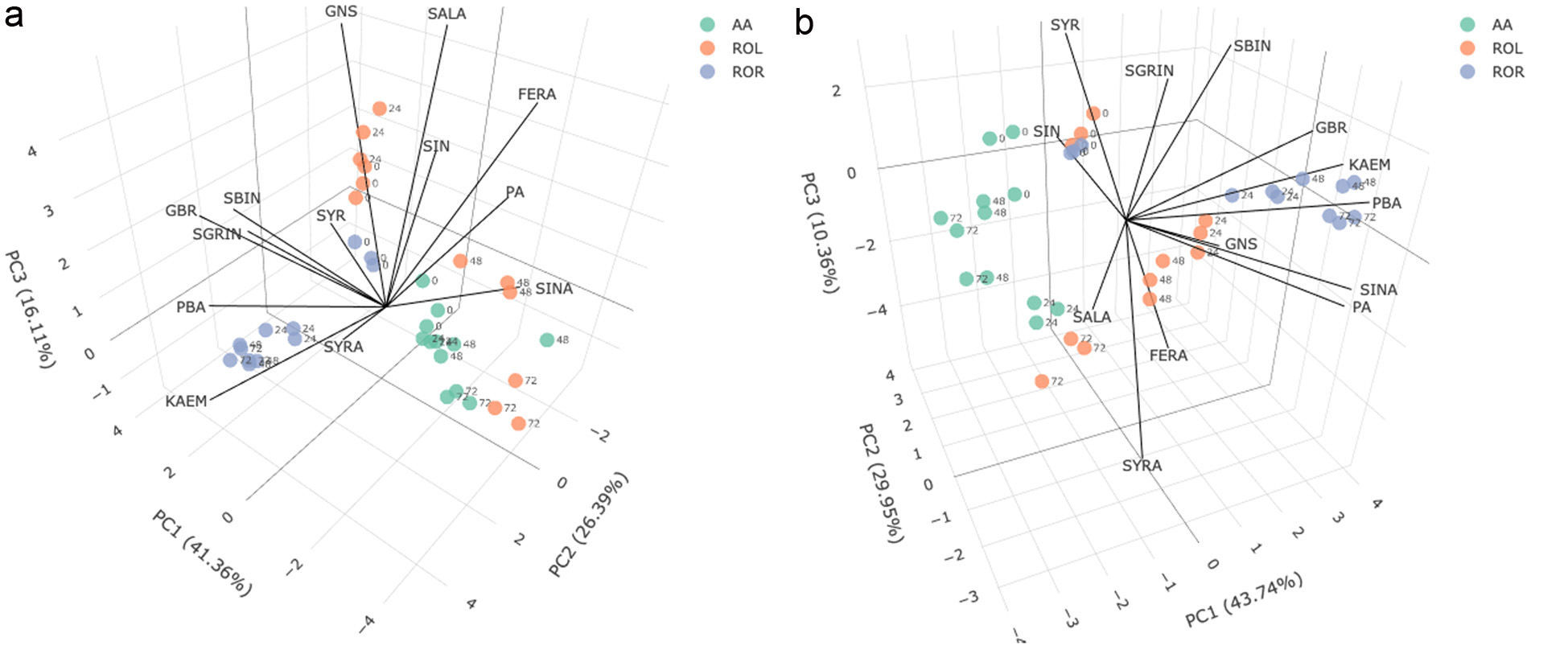 Click for large image | Figure 6. Principal component analysis biplots of glucosinolate and phenolic contents in (a) oriental mustard bran and (b) yellow mustard bran during SSF by A. awamori (AA), R. oligosporus (ROL) and R. oryzae (ROR) over a 3-day period. Data points are labelled based on fermentation duration: 0, 24, 48 or 72 h. FERA: trans-ferulic acid, GBR: glucobrassicin, GNS: gluconasturtiin, KAEM: kaempferol-3-O-glucoside, PA: protocatechuic acid, PBA: p-hydroxybenzoic acid, SALA: salicylic acid, SBIN: sinalbin, SGRIN: sinigrin, SIN: sinapine, SINA: sinapic acid, SYR: syringaldehyde, SYRA: syringic acid. |
PCA results show that GSL and phenolic contents significantly differ between non-fermented and fermented samples, for both types of mustard bran. SSF of oriental mustard bran by R. oryzae was generally found to favour an increase in p-hydroxybenzoic acid, syringic acid and kaempferol-3-O-glucoside contents, whereas SSF by R. oligosporus led to increases in the contents of ferulic acid, protocatechuic acid and sinapic acid. On the other hand, R. oryzae was found to be effective at increasing the p-hydroxybenzoic acid, sinapic acid, protocatechuic acid and kaempferol-3-O-glucoside contents in yellow mustard bran after fermentation. These results further demonstrate the suitability of Rhizopus spp. at enhancing the soluble phenolic contents of mustard bran.
| 4. Conclusions | ▴Top |
Currently, mustard bran remains an underutilized by-product of mustard seed processing and their applications for human consumption have been largely unexplored. Results from this study reveal that SSF is an effective strategy to enhance the TPC of both oriental and yellow mustard bran, which was dependent on both fermentation duration and microbial treatment group. The antioxidant activities of fermented mustard brans were found to positively correlate with TPC results, especially with FRAP and DPPH activities. Results from the LC-MS/MS analysis revealed that fermentation by R. oligosporus and R. oryzae led to significant improvement in the contents of p-hydroxybenzoic acid, syringic acid, protocatechuic acid, sinapic acid and kaempferol-3-O-glucoside in both oriental and yellow mustard brans. Yellow mustard bran was also generally found to possess higher levels of individual phenolic compounds than oriental mustard bran, both before and after fermentation. Conversely, SSF led to a significant decrease in the contents of salicylic acid, sinapine and syringaldehyde in both mustard brans. Regarding the levels of major glucosinolates, SSF by R. oligosporus was found to significantly reduce the sinigrin and sinalbin contents of oriental and yellow mustard brans. These results collectively support SSF as an efficient strategy to improve the soluble phenolic contents and antioxidant activities of oriental and yellow mustard brans. Overall, findings from this research provide valuable groundwork that may lead to the development of value-added mustard bran products or ingredients with health-promoting properties.
| Supplementary Material | ▴Top |
Figure S1. Total phenolic contents (TPC) of oriental mustard bran before (raw) and after autoclaving.
Figure S2. Total phenolic contents (TPC) of yellow mustard bran before (raw) and after autoclaving.
Table S1. Total phenolic contents and antioxidant activities of oriental mustard bran extracts during solid-state fermentation by various microbes.
Table S2. Total phenolic contents and antioxidant activities of yellow mustard bran extracts during solid-state fermentation by various microbes.
Acknowledgments
This study was supported by the A-Base funds of Agriculture & Agri-Food Canada (AAFC) (Project #J-002252).
Conflict of interest
The authors declare no conflict of interest.
| References | ▴Top |