| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 28, December 2024, pages 76-87
Antioxidant and anti-inflammatory activities, bioaccessibility, transmembrane transport of major phenolics from selected floral honeys using Caco-2 BBe1 cell model
Yan Zhua, Ronghua Liua, Lili Matsa, Honghui Zhua, Tauseef Khanb, c, d, John Sievenpiperb, c, d, e, f, Dan Ramdatha, Rong Tsaoa, *
aGuelph Research and Development Centre, Agriculture and Agri-Food Canada, 93 Stone Road West, Guelph, Ontario N1G 5C9, Canada
bDepartment of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Toronto, Canada
cToronto 3D Knowledge Synthesis and Clinical Trials Unit, Clinical Nutrition, St Michael’s Hospital, Toronto, Ontario, Canada.
dClinical Nutrition and Risk Factor Modification Centre, St Michael’s Hospital, Toronto, Ontario, Canada
eDivision of Endocrinology and Metabolism, St. Michael’s Hospital, Toronto, Ontario, Canada
fLi Ka Shing Knowledge Institute, St Michael’s Hospital, Toronto, Ontario, Canada.
*Corresponding author: Rong Tsao, Guelph Research and Development Centre, Agriculture and Agri-Food Canada, 93 Stone Road West, Guelph, Ontario N1G 5C9, Canada. E-mail: rong.cao@agr.gc.ca
DOI: 10.26599/JFB.2024.95028398
Received: July 30, 2024
Revised received & accepted: December 11, 2024
| Abstract | ▴Top |
In the present study, we assessed the antioxidant activity of the phenolic extracts and major phenolic compounds of alfalfa, buckwheat, clover and orange honeys collected in North America using chemical-based and cell-based antioxidant assays (CAA). Cell culture models using Caco-2 BBe1 were established to evaluate the effect of honey phenolics on antioxidant enzyme activities and secretion of interleukin 8 (IL-8). Moreover, bioaccessibility, transmembrane transport and cellular uptake of honey phenolics were also studied. Based on the same quantity of the honey studied, phenolic extract of buckwheat honey showed the highest FRAP, DPPH, ORAC and CAA values, and strongest activity in restoring activities of antioxidant enzymes (GR, SOD and CAT) and in reducing TNF-α-induced IL-8 secretion. Our results showed that compared to the major phenolic component of honey of plant origin, minor phenolics or combination of different phenolic compounds, particularly those derived from propolis, and their phase II metabolites may play contribute more to the overall antioxidant and anti-inflammatory effects. Future research will focus on bioavailability of honey phenolics and their metabolites, and the molecular mechanism of the antioxidant, anti-inflammatory activities.
Keywords: Honey; Phenolics; antioxidant; Anti-inflammatory; Bioaccessibility; Cellular uptake; Transmembrane transport; Metabolism
| 1. Introduction | ▴Top |
Honey has been appreciated by humans as a natural sweetener since antiquity, but in recent years, it has been found to have additional health benefits beyond being merely a sweetener and energy source (Battino et al., 2021; Zammit Young and Blundell, 2023). In the meantime, in addition to sugars, its main component, other nutrients and bioactive components have been identified (Machado De-Melo et al., 2018; Zhu et al., 2024). The potential health benefits of honey have been mainly associated with the antioxidant and anti-inflammatory activities, which has been attributed to mainly the phenolic content of honey (Sultana et al., 2022; Tanleque-Alberto et al., 2020; Tel-Çayan et al., 2023; Yu et al., 2023; Zammit Young and Blundell, 2023). Majority of the studies focus on the phenolic extracts and examine the antioxidant and anti-inflammatory activities in vitro (Giordano et al., 2018; Ruiz-Ruiz et al., 2017; Tanleque-Alberto et al., 2020). However, the phenolic composition varies significantly among different floral honeys, and in fact, phenolic profile, along with other unique honey components have been used as chemical markers to identify botanic origin, geographic origin, and environmental origins (Wang et al., 2022; Zhu et al., 2024). (Tomás-Barberán et al., 2001). Other factors, such as extraction and analytical methods may also add complexity to the composition of honey phenolics, consequently their bioactivities. There is no consented extraction method on honey phenolics. In some studies, honey phenolics were simply extracted by methanol or acetonitrile/10%NaCl partitioning and used for compositional analysis (Ruiz-Ruiz et al., 2017; Yu et al., 2023), and in others adsorbent such as XAD-2 resin was used, following liquid-liquid partitioning between ethyl acetate and water (Sun et al., 2020). Solid state extraction (SPE) was found to be a simple and valid extraction and semi-purification method for honey phenolics (Bertoncelj et al., 2011; Tanleque-Alberto et al., 2020), therefore, it was adopted in our recent study (Zhu et al., 2024). We recently reported that up to 29 phenolic compounds, mostly phenolic acids and flavonoids, were found in selected North American floral honeys (Zhu et al., 2024). Among them, pinobanksin-5-methyl ether, pinobanksin and pinocembrin are propolis-derived flavonoids which are rarely reported in honey (Tomás-Barberán et al., 2001).
Majority of the studies use in vitro chemical-based assays e.g., ferric reducing antioxidant power (FRAP), 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and oxygen radical absorption capacity (ORAC), to assess the antioxidant activity of honey phenolics (Poulsen-Silva et al., 2023; Sun et al., 2020; Tanleque-Alberto et al., 2020; Tel-Çayan et al., 2023), and cell culture models for anti-inflammatory activities (Ruiz-Ruiz et al., 2017; Silva et al., 2021; Sun et al., 2020); however, in most of these studies, contributions of honey phenolics to the antioxidant and anti-inflammatory activities were made by correlating those activities with total phenolic content (TPC) or concentrations of individual phenolics by statistic analyses, (Shen et al., 2019; Tanleque-Alberto et al., 2020). These activities have not been verified by measuring the activities of individual phenolic compounds of honey using the same in vitro assays.
Bioaccessibility and bioavailability are important properties for phenolics to exert their in vivo bioactivities. Bioaccessibility of honey phenolics is rarely studied. One report showed that in vitro gastric digestion did not significantly affect the TPC, thus it concluded that phenolics of honey had high bioaccessibility (O’Sullivan et al., 2013); however, its effect on individual honey phenolics was not evaluated. The same study also showed that that in vitro gastric digestion had mixed effects on FRAP, DPPH activities. While bioavailability of phenolics need to be assessed in vivo, intestinal epithelial cells such as Caco-2 cells have been used as a model to evaluate potential bioavailability of these bioactives, by assessing cellular uptake and transmembrane transport of phenolics across the Caco-2 monolayer (Chen et al., 2021; Zhang et al., 2020).
In the present study, we assessed the antioxidant activity of the phenolic extracts and major phenolic compounds of these honeys using cell-based antioxidant assay (CAA) in addition to the chemical-based assays. Caco-2 BBe1 cells was used to measure the effects of honey phenolics on antioxidant enzyme activities and secretion of interleukin 8 (IL-8). Moreover, bioaccessibility, transmembrane transport and cellular uptake of p-hydroxybenzoic acid, caffeic acid, p-coumaric acid, isoferulic acid, pinobanksin-5-methyl ether, pinobanksin, kaempferol and pinocembrin, the major phenolic compounds identified in four North American alfalfa, buckwheat, clover and orange honeys (Zhu et al., 2024). Bioaccessibility of honey phenolics was also assessed. The objective of this study is to better understand the roles of individual honey phenolics in the antioxidant and anti-inflammatory activities, and provide initial insight into their bioaccessibility and bioavailability.
| 2. Material and methods | ▴Top |
2.1. Samples, chemicals
Honey samples were provided by the US National Honey Board. Four floral honey varieties including alfalfa, buckwheat, clover, and orange were collected and produced between 2020 and 2022. All samples were stored at −20 °C before analysis.
Phenolic standards, including p-hydroxybenzoic acid (PHBA), caffeic acid (CA), p-coumaric acid (PCA), isoferulic acid (IFA), pinobanksin-5-methyl ether (P5ME), pinobanksin (PBK), kaempferol (KAE), pinocembrin (PCB), and other solvents and reagents including acetonitrile, formic acid, dimethyl sulfoxide (DMSO), L-ascorbic acid, ferric chloride, 2,2′-Azobis(2-methylpropionamidine) dihydrochloride (AAPH), (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were obtained from Sigma–Aldrich (Oakville, ON, Canada). 2,4,6-Tri(2-pyridyl)-1,3,5-triazine (TPTZ) and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) were obtained from Fisher Scientific (Nepean, ON, Canada). LC-MS grade methanol was obtained from Fisher Scientific (Ottawa, ON, Canada). Distilled and deionized water was purified in-house using a Milli-Q system (Bedford, MA, USA).
2.2. Extraction of phenolic compounds in honey
A total of 300 g of each honey sample was weighed and diluted with 1% aqueous formic acid (v/v) to 30% (w/v). One liter of this acidified honey solution was then purified using 3 Strata-X 33 polymeric SPE cartridges (2 g, Phenomenex, Torrance, CA, USA), the absorbed phenolic compounds were eluted with 20 mL 1% (v/v) formic acid in methanol per cartridge. The eluates were combined and dried by a vacuum concentrator (Savant Speedvac SPD1030, ThermoFisher Scientific, Mississauga, ON, Canada). This yielded 259.4, 190.9, 312.8 and 429.9 mg of dried extract from clover, alfalfa, orange and buckwheat honey, or 0.09%, 0.06%, 0.10% and 0.14% by weight, respectively. Stock solutions (2 mL) of honey phenolic extracts were prepared by dissolving the dried extract in DMSO at a concentration of 150 g honey equivalent extract per mL (HE/mL), and stored in −20 °C freezer before use. HE concentrations were used in this study to compare the strength different bioactivities of different honeys.
2.3. Antioxidant activities assays
2.3.1. Ferric-reducing antioxidant power (FRAP) assay
FRAP was measured following a previously reported procedure (Li et al., 2012) with slight modifications. Briefly, 10 µL of diluted extract or phenolic standards or L-ascorbic acid was allowed to react with 300 µL of ferric-TPTZ reagent in wells of a 96-well microplate for 2 h at room temperature. The absorbance was read at 593 nm using the visible–UV microplate reader (PowerWave XS2, Bio-Tek Instruments, Inc., Winooski, VT, USA). FRAP value was expressed as µmol L-ascorbic acid equivalent per gram sample (µmol AAE/g).
2.3.2. DPPH assay
Measurement of DPPH antioxidant activity also followed Li et al. (Li et al., 2012) with slight modifications. Briefly, 25 µL of diluted extract or phenolic standards of Trolox was mixed with 200 µL of 350 µM DPPH in methanol in a 96-well plate. The mixtures were reacted for 6 h in darkness at room temperature. The absorbance was measured at 517 nm using the microplate reader as stated above. The DPPH antioxidant activity was expressed as µmol of Trolox equivalents per gram sample (µmol TE/g).
2.3.3. Oxygen radical absorption capacity (ORAC) assay
The ORAC assay also followed the procedure by Li et al. (Li et al., 2012). Briefly, 25 µL of blank, Trolox or diluted extracts or phenolic standards were mixed with 200 µL fluorescein solution (0.0868 µM) and incubated for 30 min at 37 °C before injection of 25 µL AAPH (153 mM) in a black 96-well microplate. The fluorescence (excitation: 485 nm, emission: 528 nm) was measured every minute for 120 min until the signal reached zero in a Bio-Tek Fluorescence Spectrophotometer equipped with an automatic thermostatic holder (FLX 800, Bio-Tek Instruments, Inc., Winooski, VT, USA). A calibration curve was constructed by plotting the calculated differences of area under the fluorescein decay curve between the blank and a series of Trolox solutions. The results were expressed as µmol Trolox equivalent per gram sample (µmol TE/g).
2.4. Cell culture of Caco-2 BBe1
The Caco-2 BBe1 human intestinal cell line (American Type Culture Collection, Rockville, MD, USA) was grown in Dulbecco's Modified Eagle Medium (DMEM; Gibco, Burlington, ON, Canada) with 10% foetal bovine serum (FBS; Hyclone Co., Logan, UT, USA) and 1X Antibiotic-Antimycotic (100 units/mL of penicillin, 100 μg/mL of streptomycin, and 250 ng/mL of Gibco Amphotericin B; Gibco). The cells were incubated at 37 °C in 5% CO2 and the medium was replaced every 3 days. Cell passages of 60–80 were used in all monolayers for this experiment. Caco-2 BBe1 cells were cultured in T-75 culture flasks at 2 × 105 cells/mL and grown for 5–7 days to reach 80–90% confluency. Cells were sub-cultured to appropriate culture plates depending on the experiment to be performed.
2.5. Cell viability assay (WST-1 assay)
Caco-2 BBe1 cells were incubated with honey extracts at concentrations of 0.25, 0.5, 1.0, and 2.0 g HE/mL, as well as 1 mM of phenolic standards (PHBC, CA, PCA, IFA, P5ME, PBK, KAE, and PCB) for 6 h at 37 °C in 5% CO2 , followed by adding water-soluble tetrazolium (WST-1) at a final concentration of 250 µM and incubating for 2 h. The absorbance was measured at 450 nm using a UV/vis microplate reader mentioned above. Untreated cells were used as control. The cell viability was reported as the ratio of optical density of samples to control.
2.6. Cellular antioxidant activity (CAA) assay
CAA was evaluated using a published method with slight modifications (Li et al., 2014). Briefly, Caco-2 BBe1 cells were grown in a 96-well black/clear flat bottom plate until formation of monolayer. Cells were simultaneously treated with 50 µL of DCFH-DA (100 µM) and 50 µL of FBS-free DMEM (as control), honey extracts (0.1, 0.2, 0.5, and 1.0 g HE/mL), or phenolic standards (1 mM) for 1 h at 37 °C in 5% CO2. After washing twice with 200 µL of HBSS, media were removed and replaced with 100 µL of 0.6 mM AAPH. Background samples were prepared by replacing media with 100 µL of HBSS. The intensity of fluorescence was measured using a fluorescence spectrophotometer mentioned above at an excitation wavelength of 485 nm and an emission wavelength of 528 nm for 2 h. The results were calculated according to the Equation (1):
2.7. Interleukin-8 (IL-8) immunoassay
Caco-2 BBe1 cells were grown in a 48-well plate until reaching a 80–90% confluence. Cells were treated with 200 µL of DMEM with 5% FBS (as control) or honey extracts (0.25, 0.5, and 1.0 g HE/mL) for 2 h at 37 °C in 5% CO2 . Oxidative stress was induced by adding 2 ng/mL of TNF-α to both control and honey extracts samples (TNF-α +). A parallel groups of samples were treated with HBSS (TNF-α -) to identify the baseline of IL-8 release. All samples were incubated for 4 h and the supernatant was collected and stored in −80 °C before analysis.
Released IL-8 was determined by using a human IL-8 enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s instructions (eBioscience, Inc., San Diego, CA, USA). Mouse anti-human IL-8 antibodies were coated in 100 µL of coating buffer in a 96-well plate incubated overnight at 4 °C. The wells were washed 3 times with 300 µL of wash buffer and then blocked with 200 µL of blocking buffer for 1 h. The plate was washed 3 times between each of following steps. The 100 µL of sample and IL-8 standards were added into the wells and incubated for 2 h at room temperature. A 100 µL of secondary anti-human IL-8 antibody was added into the well for 1 h incubation, followed by 30 min incubation with 100 µL avidin-horseradish peroxidase conjugate (Av-HRP). A 100 µL of 3,3′,5,5′-tetramethylbenzidine (TMB) for colour development. This reaction was stopped by adding 100 µL of 1M H3PO4. The absorbance was measured at 450 nm using a UV/vis microplate reader mentioned above. The concentration (pg/mL) of IL-8 was extrapolated from the standard calibration curve.
2.8. Intracellular antioxidant enzyme activities
Glutathione reductase (GR), glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) activities were measured using colorimetric assay kits (Cayman Chemical,Ann Arbor, MI, USA) according to the manufacturer’s instructions. Briefly, Caco-2 Bbe1 cells in a 6-well plate were pre-incubated with or without 1.0 g HE/mL extracts for 30 min, and then H2O2 was directly added into the wells to reach a final concentration of 1 mM and incubated for 6 h at 37 °C in 5% CO2. The cells were washed twice with HBSS and then 0.5 mL of cold buffer (100 mM potassium phosphate buffer containing 1 mM EDTA, pH 7.5) was added into the wells. The cells were collected into 2 mL bead mill tubes with five 2.8 mm ceramic beads and were lysed for 20 sec at s speed of 5 m/s using a bead mill homogenizer (OMNI, Kennesaw, GA, USA). The mixture was centrifuged at 5,000×g for 10 min at 4 °C and the supernatant was collected for the assays of enzyme activities. The activity of each enzyme was calculated in milliunits per mg of protein (mU/mg). The results were reported as percent of the negative control.
2.9. In vitro digestion
Bioaccessibility of honey extracts was evaluated by a simulated gastrointestinal digestion described in our previous study (Zhang et al., 2017) with slight modifications. Briefly, 50 µL of each honey extract stock solution was mixed with 1.5 mL of artificial saliva and made up to 8 mL by adding HBSS. The mixtures were incubated for 15 min at 37 °C in a water bath shaker at 200 rev/min, and then adjusted to pH 1.5 using 6 M HCl. Porcine pepsin was added to reach a final concentration of 1.3 mg/mL. The mixture was continuously incubated for 1.5 h in a water bath shaker at 200 rev/min, and then added KH2PO4 (2 mL, 0.5 M), pancreatin (final concentration: 0.175 mg/mL) and procine bile salt (final concentration: 1.1 mg/mL). The pH of this solution was then adjusted to 8.0 using 10 M NaOH and incubated at 37 °C in a water bath shaker for another 2 h. The digestion process was terminated by adding phenylmethylsulfonyl fluoride (PMSF) at a final concentration of 1 mM. The digested mixture was acidified with 1% formic acid (for analysis of phenolic acids) or without (for analysis of flavonoids) before centrifuging at 2,000 × g for 10 min. The supernatant was cleaned by an OASIS HLB cartridge (150 mg, Waters, Milford, MA, USA). The elute was stored at −80 °C before LC-MS analysis.
2.10. Cell transport assay
Transport experiments were carried out by following a previously described procedure (Zhang et al., 2017) with slight modifications. Briefly, Caco-2 BBe1 cells were seeded onto polyethylene terephthalate (PET) membrane permeable support inserts (10.5 mm, 0.4 µm pore size, Corning Inc., Corning, NY, USA) at a density of 2 × 105 cells/mL. The culture medium (DMEM with 10% FBS and 1× Antibiotic-Antimycotic) was changed every second day until the cells became confluent monolayer. Only monolayers displaying trans-epithelial electrical resistance (TEER) values greater than 1,000 Ω·cm2 was used in the experiment. TEER values were measured using a Millicell-ERS Volt-Ohm Meter (Millipore, Bedford, MA, USA). The medium in both wells and inserts was washed twice with HBSS, and added with FBS-free DMEM and 1.0 g HE/mL honey extract in FBS-free DMEM, respectively. Cells were treated with honey extracts at 1.0 g HE/mL. After 6 h incubation at 37 °C in 5% CO2, the solution from apical and basal compartments were collected, acidified by 1% formic acid, and cleaned using Strata-X polymeric cartridges (30 mg, Phenomenex, Torrance, CA, USA). The cells with cold buffer were collected into 2 mL bead mill tubes with five 2.8 mm ceramic beads and were lysed for 20 sec at s speed of 5 m/s using a bead mill homogenizer (OMNI, Kennesaw, GA, USA). The mixture was centrifuged at 5,000 × g for 10 min at 4 °C and the supernatant was collected, acidified, and cleaned using the procedure mentioned above.
2.11. Analysis of phenolic compounds by HPLC and LC-MS/MS
Phenolic compounds were analyzed using an Agilent 1260 series HPLC system consisting of an autosampler, a degasser, a quaternary pump, a thermostatted column compartment and a diodearray detector. LC-MS/MS analysis was performed using a Thermo® Scientific Q-Exactive™ Orbitrap mass spectrometer coupled to a Vanquish™ Flex Binary UPLC System with a diode array detector (DAD) (Waltham, MA, USA). A Kinetex XB-C18 100A column (100 x 4.6 mm, 2.6 µm, Phenomenex Inc., Torrance, CA, USA) was used. The binary mobile phase consisted of solvent A (99.9% H2O/0.1% formic acid) and solvent B (94.9% methanol/5% acetonitrile/0.1% formic acid). The phenolic compounds were analyzed by using negative ionization mode. The solvent gradient was: 0–5 min, 0% to 12% B; 5–15 min, 12% to 23% B; 15–30 min, 23% to 50% B; 30 - 40 min, 50% to 80% B; 40–42 min, 80% to 100% B; 42–45 min, 100% B; 45–46 min, 100% to 0% B; 46–52 min, 0% B. The column temperature was set at 40 °C, the flow rate was 0.700 mL/min, and the injection volume was 2 µL. UV peaks were monitored at 280 nm, 320 nm, 360 nm and 520 nm. The spray voltages for both negative and positive modes were set at 4.5 kV. Mass spectrometry data were collected using DDMS2 method (TopN = 10, NCE = 30, intensity threshold was set at 1.0e5 counts) for compound identification, and with Full-MS mode for quantification. Data were visualized and analysed using Thermo FreeStyle™ 1.7PS2 software. Quantification was achieved using standard curves generated from individual compounds in serial dilutions (0.005–10 mg/L; r2 > 0.995).
2.12. Statistical analysis
Results were expressed as mean ± standard deviation of then mean of at least triplicated measurements unless otherwise specified. A one-way analysis of variance (ANOVA) followed by Tukey’s Honest Significant Difference (HSD) test was performed using SigmaPlot 15.0 (Palo Alto, CA, USA) to analyze the difference of mean values of antioxidant activities, cell viability, CAA unit, released IL-8 concentration, and antioxidant enzyme activities. Significant differences were considered at p < 0.05.
| 3. Results and discussion | ▴Top |
3.1. In vitro antioxidant activities
The SPE extracted phenolics of honeys were assessed for their antioxidant activities using FRAP, DPPH and ORAC assays. As shown in Table 1, among the four honeys, buckwheat honey showed the highest antioxidant activity in all three in vitro methods, followed by orange, clover and alfalfa honeys in FRAP and DPPH values, and clover, alfalfa and orange in ORAC value. The difference in antioxidant activity depended on the assays used; however, the results corresponded closely to the total phenolic content (TPC) of the same honeys that we reported recently (Zhu et al., 2024). While the phenolic profiles varied significantly, and unique compounds were identified as chemical markers for the four floral honeys (Zhu et al., 2024), the TPC of honeys of the present study were similar except for buckwheat honey which was three times higher than the rest, i.e., 379, 415, 425 and 1,269 μg GAE/g of alfalfa, orange, clover and buckwheat honey, respectively (Zhu et al., 2024). Buckwheat honeys also had the highest TPC and antioxidant among the 39 different honey batches in another study (Shen et al., 2019).
 Click to view | Table 1. Antioxidant activities of honey extracts and their major phenolic compounds |
While it is evident that collectively phenolic compounds are the principal antioxidants of honeys as demonstrated in the present as well as other studies, to better understand the role of individual honey phenolics in the total antioxidant activity, we examined the antioxidant activity of major phenolic compounds common to all four selected honeys, i.e., PHBA, CA, PCA, IFA, P5ME, PBK, KAE AND PCB (Zhu et al., 2024), using the same in vitro assays. As shown in Table 1, cinnamic acids, particularly caffeic and isoferulic acid, and kaempferol had higher FRAP, DPPH and ORAC values. p-Hydroxybenzoic acid which had the highest concentration of all honeys, especially in buckwheat honey (Zhu et al., 2024), showed very little antioxidant activities in all three assays. P5ME and PBK (flavanonols), and PCM, a flavanone, the three flavonoids unique to honey also had relatively lower in vitro antioxidant activity (Table 1). In the meantime, PHBA was 12.5 μg/g in buckwheat honey, 2–11 fold higher than the other three honeys (Zhu et al., 2024), yet it had the weakest FRAP and DPPH activities, and moderate ORAC values among the compounds tested; conversely, the highest FRAP and DPPH values were found in CA which was only 1.1 μg/g in buckwheat honey, but 6.9 μg/g in Alfalfa honey (Table 1). These suggest that phenolic compounds with high concentrations in honey may not be the major contributors to the total antioxidant activities, and vice versa, compounds at low concentrations may contribute more to the in vitro antioxidant activities. Our result suggests that these in vitro results need to be verified in vivo or by other more biologically relevant method. It also necessitates further studies focusing on the roles of other polyphenols found in relatively lower concentrations in honeys (Zhu et al., 2024). Antioxidant activities have been reported for other honey phenolics using similar in vitro assays (Ruiz-Ruiz et al., 2017; Sun et al., 2020; Tanleque-Alberto et al., 2020), and our result from the DPPH assay (0.365–0.918 μmol TE/g honey) was in agreement of that of Mozambique honeys (Tanleque-Alberto et al., 2020); however, most studies derive their conclusions by associating the total antioxidant activity of honey with TPC or concentrations of individual phenolics, rather than verifying the antioxidant activity of individual compounds using the same in vitro assays (Shen et al., 2019; Tanleque-Alberto et al., 2020). Since the most prevailing phenolics did not show
3.2. Cell-based antioxidant activity (CAA)
Caco-2 BBe1 cells were used to evaluate the CAA of the phenolic extracts of honeys. Extracts of honey showed no toxicity to the cells at and below 1.0 g HE/mL (Figure S1), therefore the following CAA assays were conducted at 0.1, 0.2, 0.5 and 1.0 g HE/mL. Major honey phenolic compounds PHBC, CA, PCA, IFA, P5ME, PBK, KAE, and PCB also had no significant effect on cell viability at 1mM (Figure S1). The results showed a clear dose-dependent CAA for all honeys. CAA of alfalfa, buckwheat and clover honeys were generally similar, but that of orange was significantly lower (Figure 1). However, at 0.1 g HE/mL, extract of buckwheat honey had significantly higher CAA compared to other honeys at the same concentration, suggesting the former is a stronger antioxidant at lower concentration. This is in agreement with other studies which also showed that buckwheat honeys had the highest CAA among different honeys (Shen et al., 2019). The lower CAA of orange honey might be due to the significantly lower total concentration of all detected phenolic compounds compared to other three honeys of this study. The sum of all detected phenolic compounds was 11 μg/g of orange honey, compared to 40, 40 and 44 μg/g of alfalfa, clover and buckwheat honeys (Zhu et al., 2024). Phenolic extracts of different honeys showed significant differences in FRAP, DPPH and ORAC values, and that of buckwheat was substantially higher (Table 1), but this was not true for CAA. Considering there might be compounds other than phenolics in honey extracts that may react to reducing agents or free radicals in chemical based assays, giving overestimated values, CAA is a result of activity in live cells after phenolic compounds are transported across the cell membrane, therefore, only those enter the cytosol of the cells will exert antioxidant activity. CAA is also more relevant to in vivo activity than chemical based antioxidant assays (Furger, 2021). In the meantime, nearly all phenolics detected in honeys of this study were aglycones which are most likely absorbed into the cytosol via passive transport (Zhang et al., 2020). Honeys containing higher concentrations of phenolic compounds will therefore have higher CAA, and those with lower concentrations, lower CAA. This explains the significantly lower CAA of orange honey than the other three honeys (Figure 1a). CAA of the major honey phenolics were also obtained, among them caffeic acid, kaempferol and pinocembrin had the highest CAA and were significantly higher than other phenolics at the concentration tested (Figure 1b). P5ME also showed similar CAA to other major honey phenolics of this study. It is worth noting that pinocembrin and P5ME, particularly the former possesses CAA, despite its very FRAP, DPPH and ORAC activities. Exactly how these major phenolics of honey contribute to the overall antioxidant activity of honey needs future investigation. Shen et al. suggested that honey phenolic acids might have synergistic CAA activity (Shen et al., 2019).
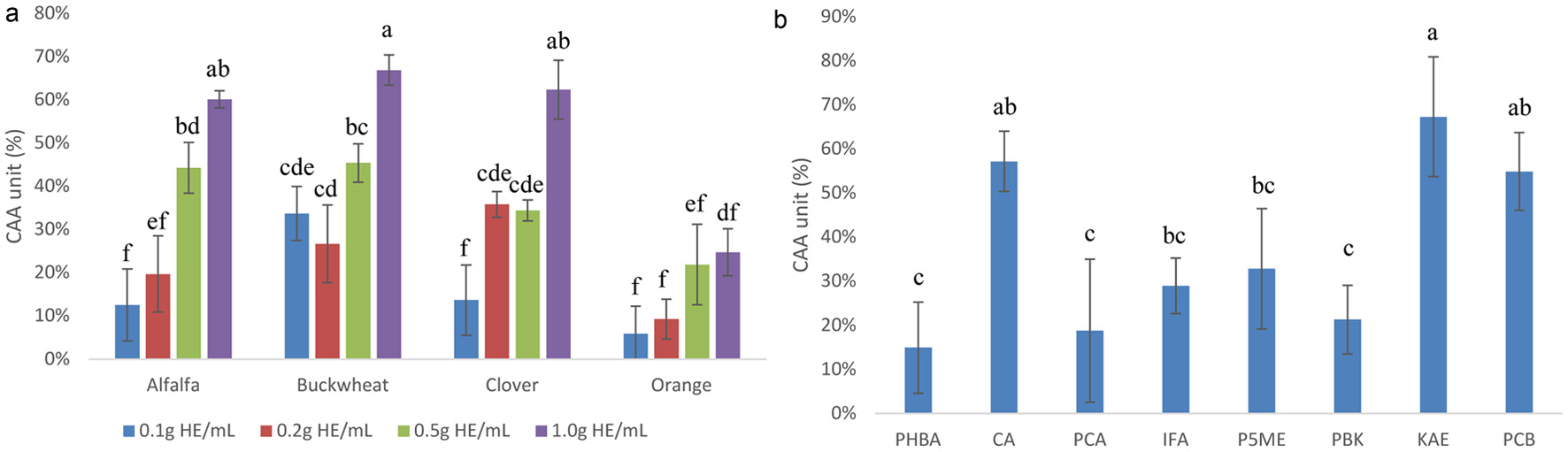 Click for large image | Figure 1. CAA of Caco-2 BBe1 cell treated with 0.1, 0.2, 0.5 and 1.0 g HE/mL of the phenolic extracts of alfalfa, buckwheat, clover, and orange honeys (a), and 1 mM of major phenolic compounds in honeys (b). Value are expressed as CAA Unit (%) and presented as mean ± SD, n = 3. Shared letters indicate no significant difference (p < 0.05). |
3.3. Phenolic extracts of honeys on antioxidant enzymes
Phenolic extracts of honeys at 1.0 g HE/mL were also evaluated for their effect on endogenous antioxidant enzymes, i.e., GR, GPx, SOD and CAT, in a Caco-2 BBe1/H2O2 cell model. In general, exposure to H2O2 significantly reduced the activity of all enzymes over the normal control; however, treatment by phenolic extracts lowered the enzyme activities. Only extract of buckwheat honey significantly restored the H2O2-induced reduction of GR, SOD and CAT (Figure 2). These results indicate that honey phenolics can also provide protection against reactive oxygen species such as H2O2. The stronger activity of buckwheat honey is highly likely due to its higher TPC and sum of all phenolic compounds (Zhu et al., 2024). Effect of honey phenolics on antioxidant enzymes was studied on Manuka honey, not the phenolic extract, in a different cell model (Gasparrini et al., 2018), which showed that the increased antioxidant enzyme activities were through enhanced NF-E2-related factor 2 (Nrf2) expression. Although the authors hinted that phenolics may be responsible for the activity. Our results suggest that honey phenolics contribute to the antioxidant defense by enhancing the activity of the endogenous antioxidant enzymes, but the exact mechanism, especially how collectively or individually these phenolics modulate the Nrf2/antioxidant responsive element (Nrf2/ARE) pathway which regulates the antioxidant enzymes needs to be confirmed (Furger, 2021).
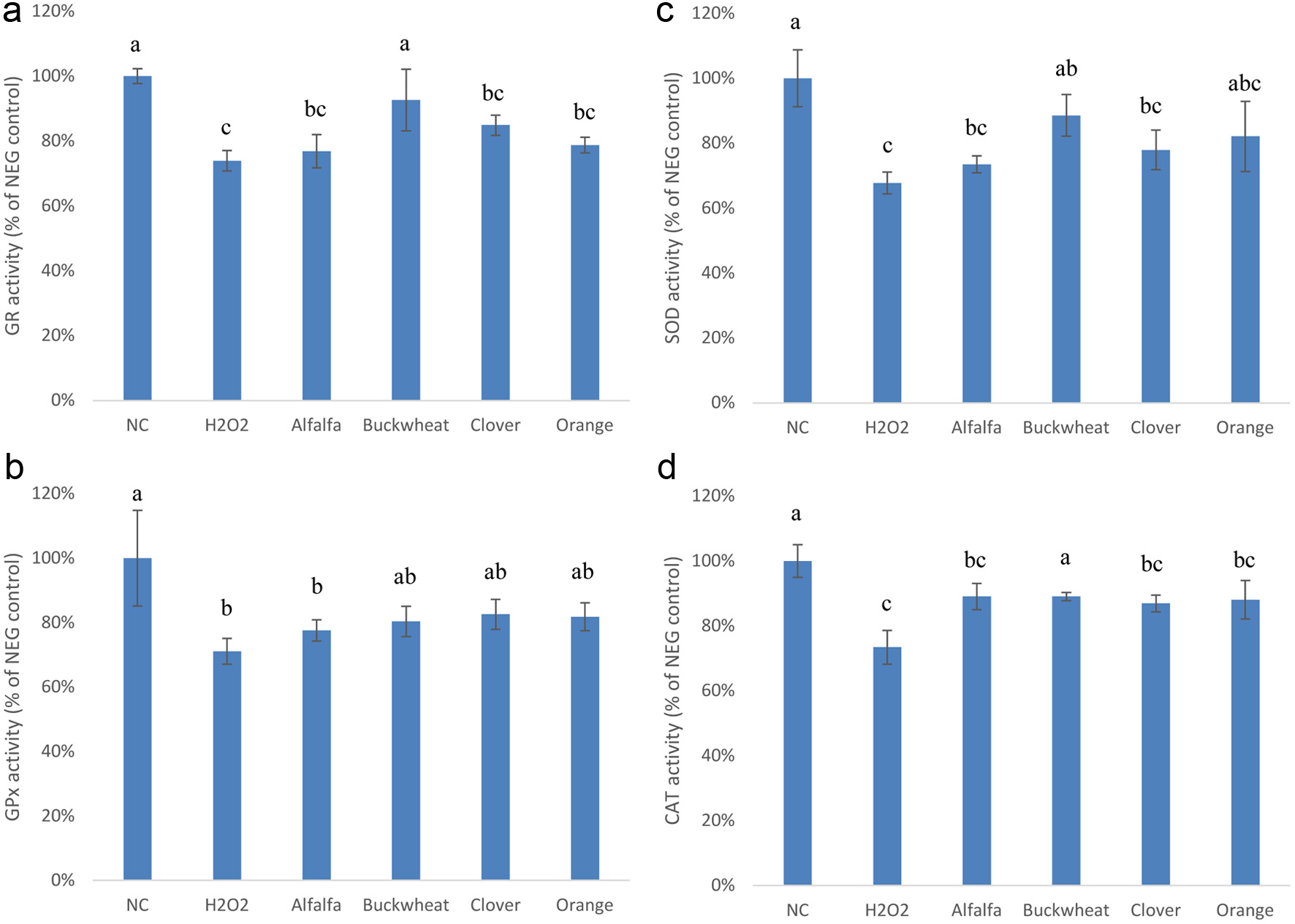 Click for large image | Figure 2. Effect of the phenolic extracts of alfalfa, buckwheat, clover, and orange honeys on endogenous antioxidant enzyme activities (a: GR; b:GPx; c: SOD; d: CAT) in H2O2-stimulated Caco-2 BBe1 cells. The negative control (NC) represents untreated cells. Positive control (H2O2) represents cells treated with H2O2 only. Values are presented as mean ± SD, n = 3. Shared letters indicate no significant difference (p < 0.05). |
3.4. Phenolic extracts of honeys on IL-8 secretion
There is sufficient in vitro and in vivo evidence that honey, mainly due to its phenolic content, possesses anti-inflammatory activity (Ranneh et al., 2021; Silva et al., 2021). While extensive experiment was not conducted in the present study, the phenolic extracts of honeys at 0.25, 0.5 and 1.0 g HE/mL were examined for their effect on the release of the proinflammatory cytokine IL-8 in a Caco-2 BBe1/TNF-α cell model. The anti-inflammatory effect of honey phenolic extracts as expressed in IL-8 inhibition appeared to be dose-dependent. All honeys showed significant inhibition of TNF-α-induced IL-8 at 1.0 g HE/mL, but phenolic extract of buckwheat showed significant inhibition at 0.5 1.0 g HE/mL, suggesting its stronger anti-inflammatory effect compared to other honeys (Figure 3). This is in agreement with the above CAA results, which showed that buckwheat honey also had stronger antioxidant activity, especially at lower concentration (Figure 1a). Phenolics of honey have been found to contribute to the anti-inflammatory effects in a different cell model (Yu et al., 2023); however, roles of individual honey phenolics in anti-inflammatory activity and the underlying mechanisms need to be further studied.
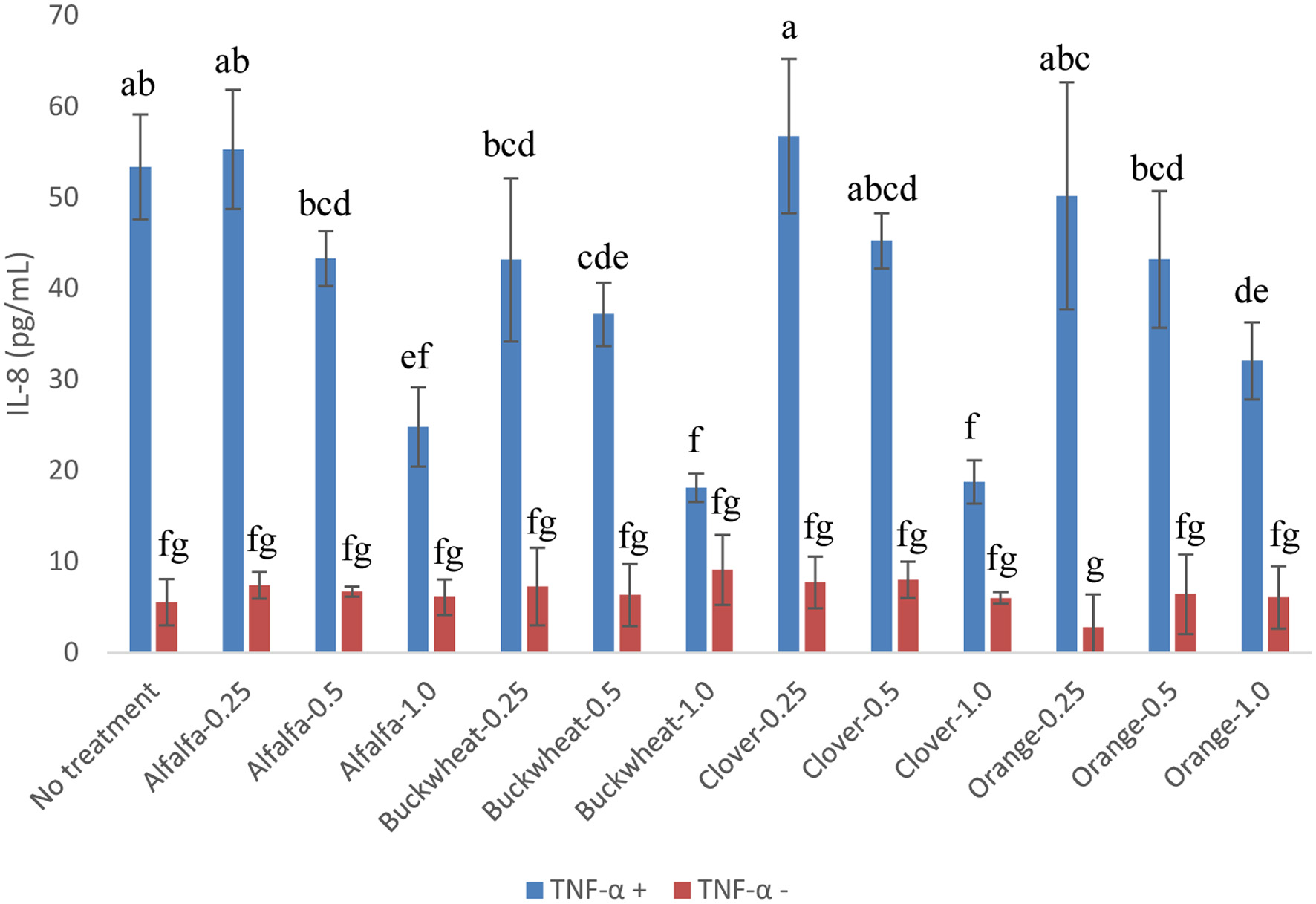 Click for large image | Figure 3. Released IL-8 of Caco-2 BBe1 cell treated with 2 ng/mL of TNF-α in combination with 0.25, 0.5 and 1.0 g HE/mL of the phenolic extracts of alfalfa, buckwheat, clover, and orange honeys. Value are expressed as IL-8 concentration and presented as mean ± SD, n = 4. Shared letters indicate no significant difference (p < 0.05). |
3.5. Bioaccessibility of honey phenolics
It is understood that the in vivo activity of any food bioactives depends on its bioaccessibility and bioavailability. The bioaccessibility of honey phenolics of the present study was evaluated using a simulated upper gut digestion system, and overall, all phenolics except kaempferol showed good stability after in vitro digestion (Table 2). The bioaccessibility of the three propolis-derived flavonoids pinobanksin, pinocembrin and P4ME was between 79–95%. Very limited number of studies have assessed the bioaccessibility of honey phenolics, but results of the present study generally agree with those of similar studies. For example, in vitro gastric digestion did not affect the TPC of honey thus phenolics of honey were considered to have high bioaccessibility, although the effect on FRAP, DPPH activity was mixed (O’Sullivan et al., 2013). In another study, not only TPC, FRAP and DPPH values were not significantly affected by in vitro gastric and duodenal phase digestions, but individual phenolic compounds, including the major honey phenolic of the present study, such as PBK, KAE, CA, also had high bioaccessibility (Seraglio et al., 2017). One study, however, showed mixed effect of in vitro digestion on the antioxidant activities and anti-inflammatory activity of honey phenolics (Alevia et al., 2021). Because of the high bioaccessibility of the honey phenolics, in vitro antioxidant and anti-inflammatory activities of digested honey extracts were not tested in the present study.
 Click to view | Table 2. Bioaccessibility of major phenolic compounds in honeys through in vitro digestion |
3.6. Transmembrane transport of major honey phenolics
To assess the potential bioavailability of the major honey phenolics, extracts of the four honeys were applied to the apical side of the Caco-2 BBe1 cell monolayer. Concentrations and percent distribution of major honey phenolics in the apical and basolateral compartments and the monolayer cells were analyzed after 6 h incubation using LC-MS (Figure 4). In general these phenolic compounds showed different stability in cell medium and varied ability to cross the Caco-2 BBe1 cell monolayer. PHBA and PCA were most stable in the apical medium regardless of its honey origin, with 98.4–109.5% and 93.6–95.7% remaining, 2.5–3.0% taken up by the cells, and 6.2–7.3% and 3.6–4.2% transported to the basolateral side, after 6 h, respectively. Stability and rate of transmembrane transport of IFA varied significantly depending on the origin of honey. IFA of alfalfa, buckwheat and clover honey was relatively stable in the apical medium (75.4–105.7%) (Figure 4a–c), but only IFA from the alfalfa honey was permeated to the basolateral medium at 13.1% (Figure 4a). No IFA was detected in cells for all treatments. For the monolayer treated phenolics of orange honey, IFA was not detected after 6 h (Figure 4d). Contrary to these phenolic acids, KAE, the major flavonol aglycone of honeys, was not stable in the cell medium, and showed minimum cellular uptake and transmembrane transport. Phenolics unique to honey, i.e. P5ME, PBK and PCB, showed higher potential bioavailability than other compounds, as seen in higher percentage of these flavanonol/flavanone polyphenols permeated to the basolateral side after 6 h incubation. Among them, P5ME had the highest transmembrane transport rate, at 22.0, 23.9, 25.3 and 20.1% for alfalfa, buckwheat, clover and orange honeys, respectively, and ca. 4.1–4.6% of P5ME was taken up by the cells. These numbers suggest that P5ME was stable in cells and cell medium under the experimental conditions. PBK and PCB were less stable, especially the latter, with ca. 9.0–19.1% and 9.0–21.4% transported across the Caco-2 BBe1 cell monolayer, and 4.1–4.9% and 0–6.9% taken up by the cells, respectively (Figure 4). These data clearly demonstrate that honey phenolics are generally stable, and can be absorbed by the Caco-2 BBe1 cells and permeated through the monolayer, particularly the honey specific, propolis-derived flavonoids P5ME, PBK and PCB.
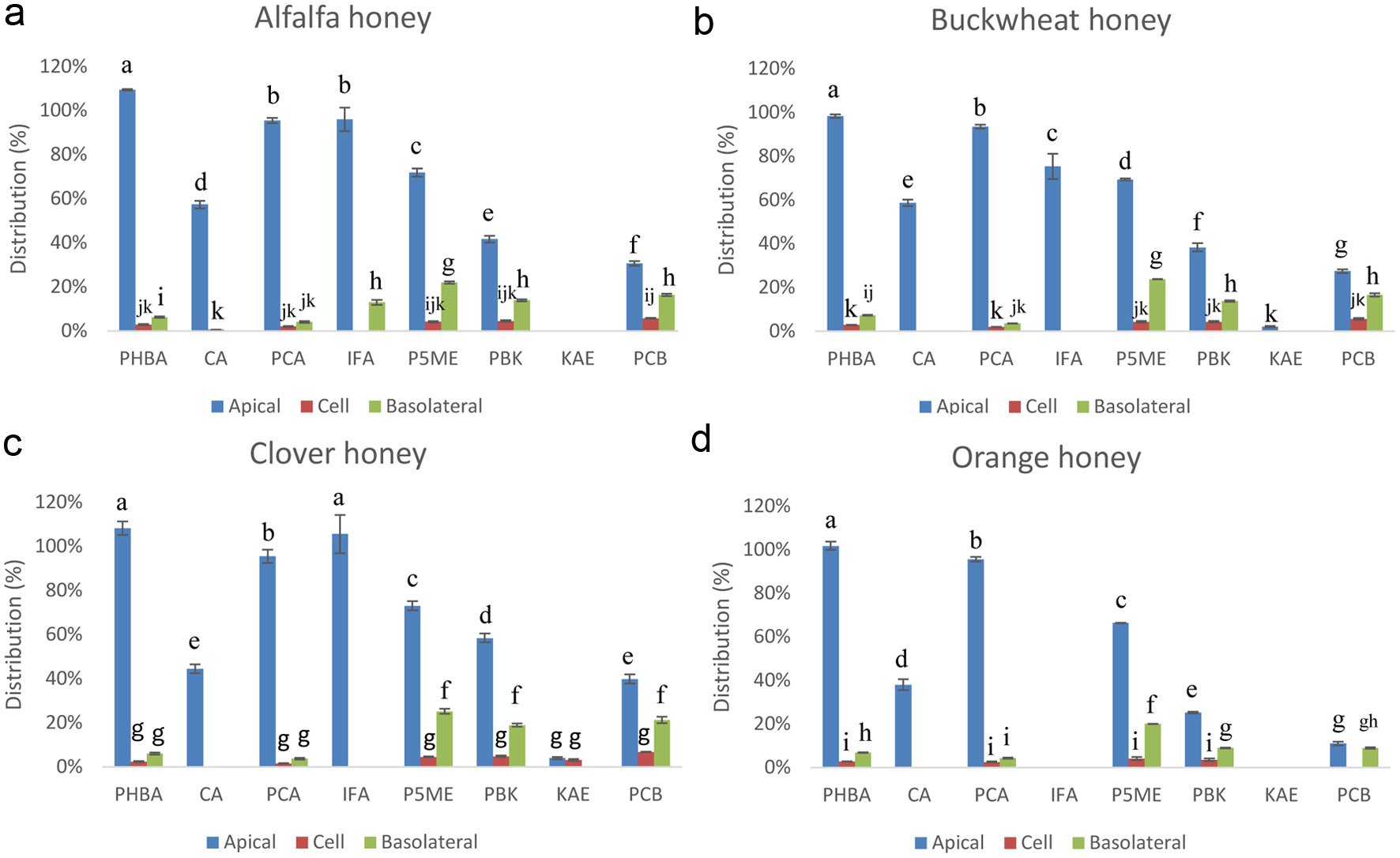 Click for large image | Figure 4. Apical, basal, and Caco-2 BBe1 cell uptake fractions of major phenolic compounds in the extract from alfalfa (a), buckwheat (b), clover (c), and orange honeys (d) after 6 h incubation. The percentage was determined by the ratio of the concentration of each compartment to the original extract (1.0 g HE/mL). Values are presented as mean ± SD, n = 3. Shared letters indicate no significant difference (p < 0.05). |
Absorption of phenolic compounds both in transmembrane transport and cellular uptake depends on the structural feature when other conditions are the same. Percent flavonoids transported from the apical to basolateral compartment of the Caco-2 monolayer was found to be higher than phenolic acids (Hithamani et al., 2017). In another study, quercetin 3-glucoside and glucuronide had very low to no permeability in both apical-basolateral and basolateral- apical directions, but quercetin and cinnamic acids of hibiscus extract were transported across the monolayer from apical to basolateral; however, only quercetin aglycone was detected in the cell, suggesting the aglycone reaches its molecular targets in a more effective way (Borrás-Linares et al., 2015). The authors also suggested the existence of a transport efflux mechanism of these compounds (Borrás-Linares et al., 2015), but such mechanism does not appear to involve transporter proteins (Rastogi and Jana, 2016). The mechanism of absorption of flavonoids has been revealed in our recent study, which showed that absorption of flavonoids depended not only on the structural features, but also the number and type of the sugar moieties attached to the flavonoid backbone (Zhang et al., 2020). We further discovered that two intestinal hexose transporters, SGLT1 and GLUT2, were key players in the active transport of flavonoid glycosides, particularly diglycosides, but not of aglycones which may be by passive diffusion (Zhang et al., 2020).
In the present study, while PCB was relatively unstable, it had the highest permeability rate (Figure 4). Moreover, further examination using LC-MS of the phenolic profile of the basolateral compartment showed that PCB was transformed into two phase II metabolites, PCB glucuronides peaks M1 (Rt = 28.5 min) and M2 (Rt = 28.7 min), possibly conjugated at 7- and 5- positions, respectively (Figures 5 and 6). These two metabolites were detected only in the basolateral side of Caco-2 BBe1 cell membrane treated with pure PCB and the alfalfa, buckwheat and clover honey phenolic extracts, not the orange honey extract, possibly due to the relatively very low PCB concentration in the orange honey (Figure 5) (Zhu et al., 2024). The two PCB glucuronides were not detected in the apical side and the cell (Figure 7), suggesting it was conjugated in the cell and immediately effluxed into the basolateral medium. The tentative assignment of the two glucuronosyl positions was based on the eluding order of two similar isomers, apigenin 7-O-glucoside and apigenin 5-O-glucoside separated on a C18 column similar to the one used in the present study (Beszterda and Frański, 2021). Further study using other instrumental analysis can help identify the exact position of the two PCB glucuronide isomers of the present study. No such metabolites were detected for other honey phenolics (Figure 6).
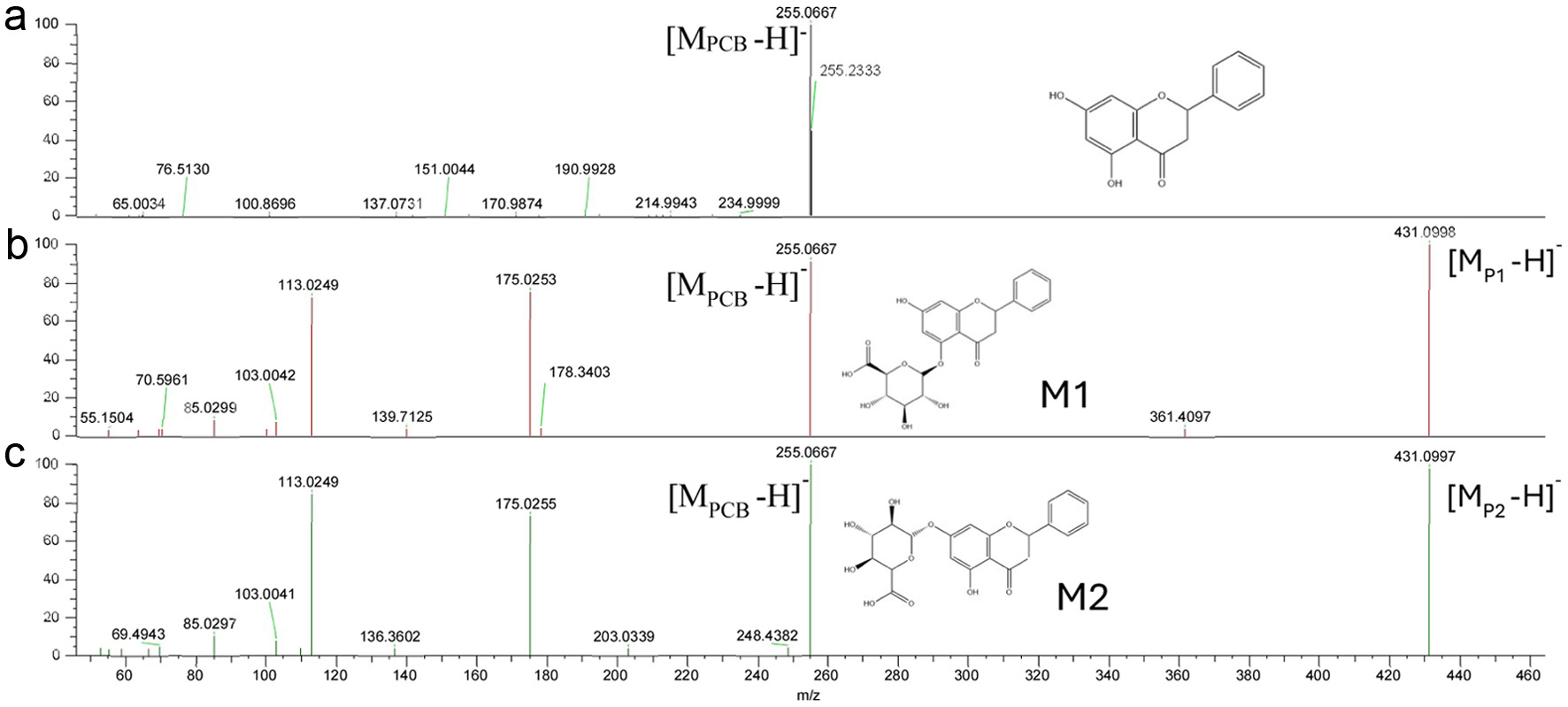 Click for large image | Figure 5. MS/MS spectra of PCB (a) and its metabolites (P1, b; P2, c) extracted from basolateral side of Caco-2 BBe1 cells after 6 h incubation. |
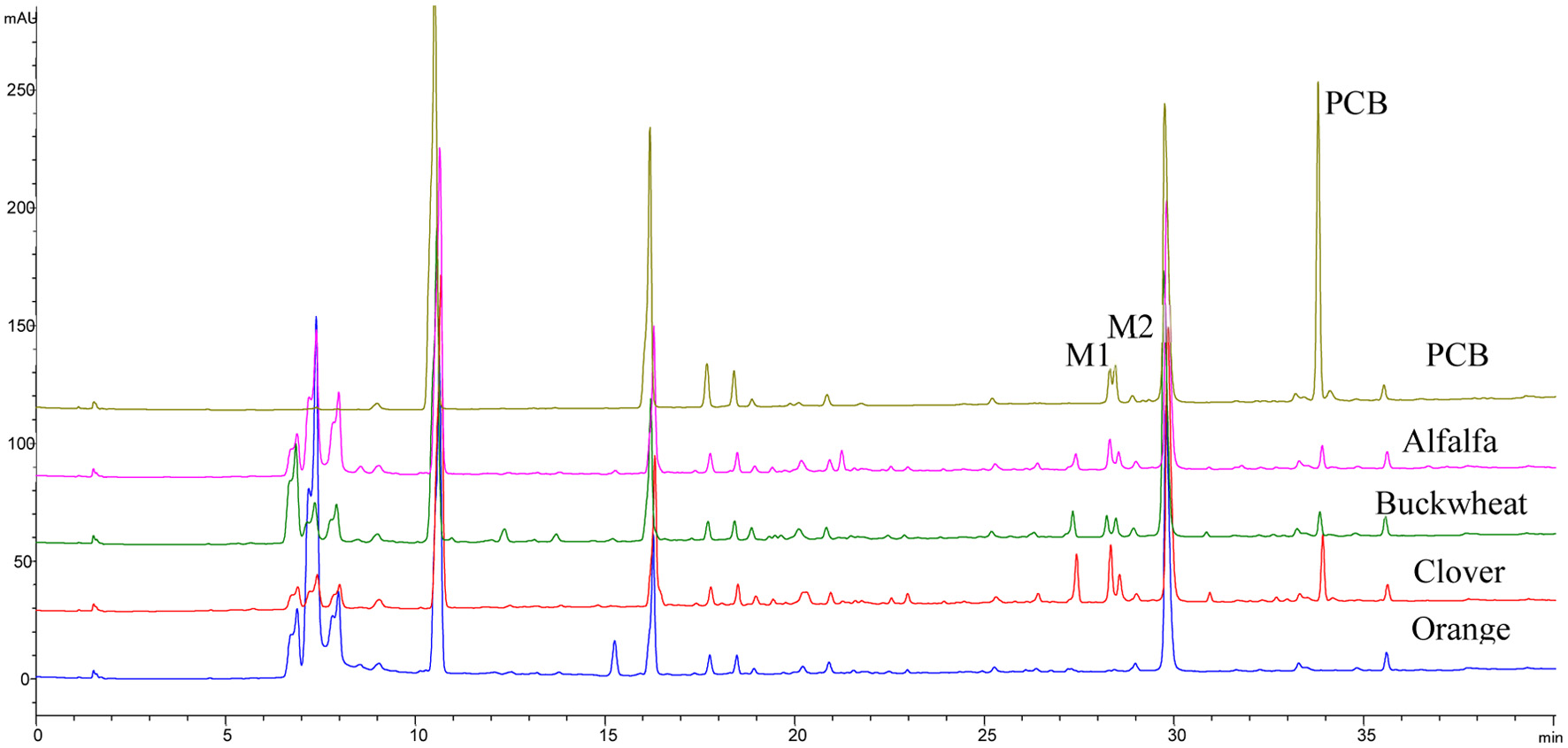 Click for large image | Figure 6. HPLC chromatograms of phenolic compounds extracted from basolateral side of Caco-2 BBe1 cells after 6 h incubation with 100 μM PCB, 1 g HE/mL alfalfa, buckwheat, clover, and orange honey extracts. |
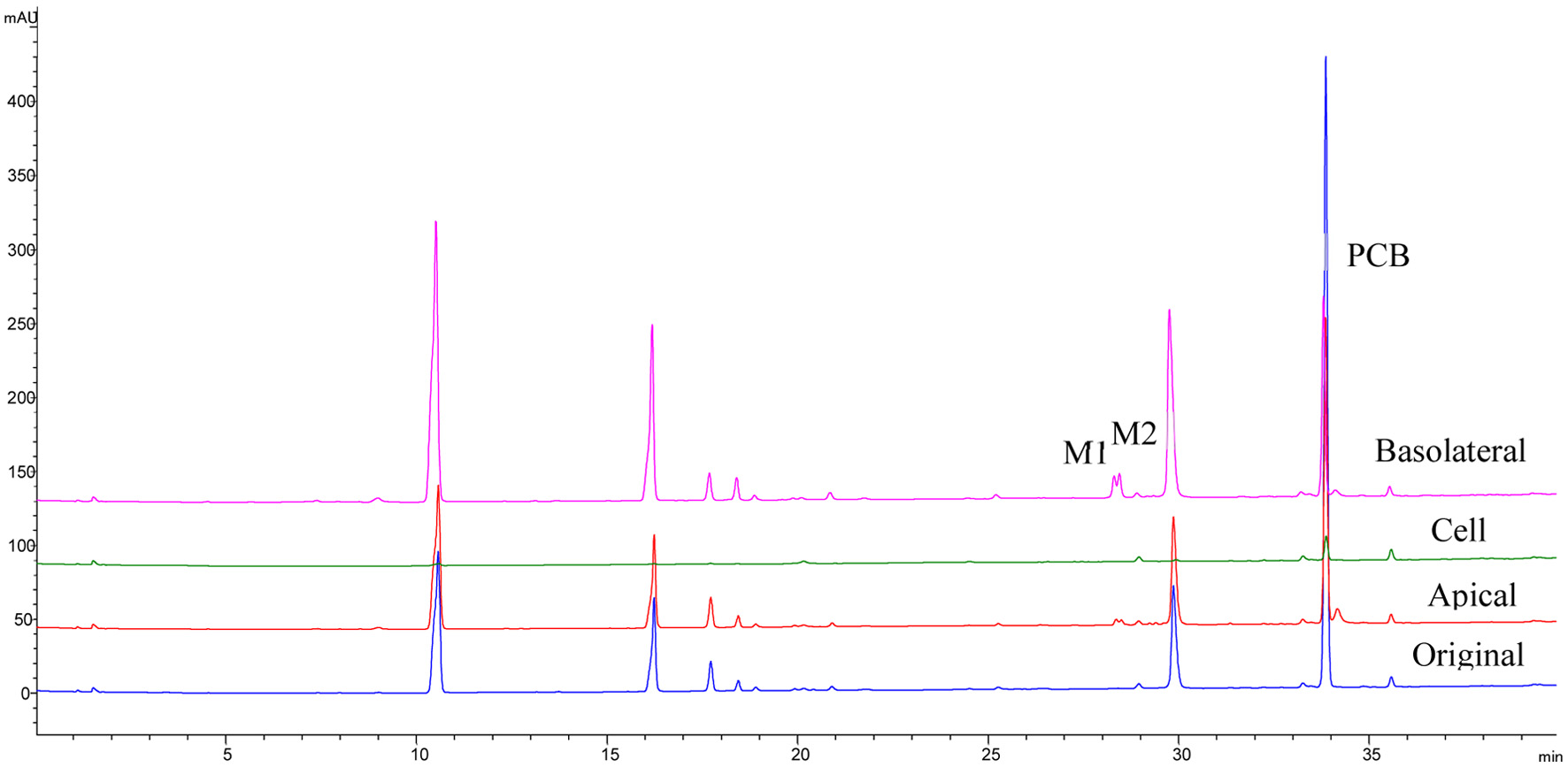 Click for large image | Figure 7. HPLC chromatograms of PCB and its metabolites P1 and P2 extracted from basolateral, apical, cells of Caco-2 BBe1 cells after 6 h incubation with 100 μM PCB. |
Flavonoids such as flavan-3-ols and their phase II metabolites were found to be relatively stable in the cell medium (Rodriguez-Mateos et al., 2014). For epicatechin, a flavan-3-ol, incubation in the Caco-2 monolayer system produced mainly phase II metabolites, including 3’- and 4’-methyl epicatechin sulphates and glucuronides at 5- and 7- positions of the A ring, and overwhelming majority of epicatechin and its metabolites were found in the apical compartment. Only 3’-methyl epicatechin 7-glucuronide and 3’-methyl epicatechin 7-sulphate were also detected in the basolateral side and in cells (Rodriguez-Mateos et al., 2014); however, epicatechin glucuronides were not found in other studies (Redan et al., 2017; Sanchez-Bridge et al., 2015). This may be due to the method and sensitivity of the analytical methods used, cell culture conditions, concentrations and length of treatment times (Redan et al., 2017).
Cellular uptake and transport mechanism and metabolism of honey phenolic compounds have not been well studied. Propolis-derived hydroxy cinnamic acid esters and a set of aglycone flavonoid compounds, mainly chrysin, galangin, PCB and PBK were found to be transformed into phase II metabolites in the form of glucuronides and sulphates in plasma after oral ingestion, but dominantly as 7-O-glucuronides (Bloor and Mitchell, 2021). No PCB 5-, but only PCB 7-O-glucurodide was detected as a phase II metabolite of PCB in humans (Bloor and Mitchell, 2021). Despite the difference, the Caco-2 BBe1 cell monolayer model used in the present study is helpful in understanding the cellular uptake and transmembrane transport mechanism and detection of potential phase II metabolites. Certain co-existing polyphenols could significantly increase the transmembrane efflux of epicatechin, thus potentially enhances the bioavailability (Sanchez-Bridge et al., 2015), therefore, adsorption of honey phenolics may also differ between using pure compounds versus extracts.
In summary, four honeys collected in North America were extracted and assessed for their antioxidant and anti-inflammatory activities using chemical-based and cell culture based models. Buckwheat showed the highest FRAP, DPPH and ORAC values. This corresponds to the TPC of buckwheat. Eight major phenolic compounds of the studied honeys also showed varied in vitro antioxidant activities, among them, CA and KAE and IFA were most strongest; however, they are not the most prevalent phenolics of honey. Honey phenolics with higher concentrations such as PHBA did not show higher antioxidant activity in chemical-based assays, suggesting the other factors than major phenolic compounds contribute to the overall antioxidant potential of honeys. The higher antioxidant activity of buckwheat honey was also found in CAA. CAA is considered a more biologically relevant marker because compounds must be transported across the live cell membrane to exert antioxidant activity. The higher antioxidant potential of buckwheat honey was also demonstrated in the different antioxidant enzymes in Caco-2 BBe1 cells. Only the phenolic extract of buckwheat honey showed significant activity in restoring H2O2-induced reduction of GR, SOD and CAT, key defense enzymes against oxidative stress. Similar effect was found for phenolics of buckwheat honey in reducing TNF-α-induced IL-8 secretion, a proinflammatory biomarker in Caco-2 BBe1 cell. It is not clear on what phenolic compound or combinations of them in buckwheat might have caused the significantly higher antioxidant and anti-inflammatory activities; however, results from the cellular uptake and transmembrane transport of the phenolic compounds of the different honeys may provide some insight. The three propolis-derived flavonoids P5ME, PBK and PCB, showed similar and higher ability than other phenolics to permeate through Caco-2 BBe1 cell monolayer, particularly P5ME which had the highest transmembrane transport cellular take up rate. These three unique phenolics were similar in all honeys studied except Orange honey, suggesting they may present higher potential of in vivo activity. While only PCB glucuronides were found as phase II metabolites in the basolateral medium of the Caco-2 BBe1 cell monolayer, the role of metabolites are not clear. Further research is needed to focus on the metabolism of honey phenolics and their role in antioxidant and anti-inflammatory activities. Another factor that might affect the potential in vivo activity may be the bioaccessibility of honey phenolics. While most phenolics were stable during in vitro digestion, KAE was not. It also was not stable in cell culture medium. Results from the present study demonstrate that honey phenolics are the underlying contributor to the antioxidant and anti-inflammatory activities; however, at individual phenolic compound level, and when bioaccessibility, potential bioavailability and metabolism are taken into consideration, more research is needed. In particular, bioavailability of honey phenolics and their metabolites, and the molecular mechanism of the antioxidant, anti-inflammatory activities should be investigated.
| Supplementary material | ▴Top |
Suppl 1. Cell viability of honey phenolic extracts and individual phenolics. Caco-2 BBe1 cell were treated with 0.25, 0.5, 1.0 and 2.0 g HE/mL of the phenolic extracts of alfalfa, buckwheat, clover, and orange honeys (A); and major phenolic acids (). Value are expressed as relative viability (%) and presented as mean ± SD, n = 4. p-hydroxybenzoic acid (PHBA), caffeic acid (CA), p-coumaric acid (PCA), isoferulic acid (IFA), pinobanksin-5-methyl ether (P5ME), pinobanksin (PBK), kaempferol (KAE), pinocembrin (PCB).
Acknowledgments
We thank the National Honey Board of the United States of America and Glycemia Consulting Inc. (Toronto, Ontario, Canada) for providing honey samples and financial support. This is Project #J-002712 of Agriculture & Agri-Food Canada.
| References | ▴Top |