| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 26, June 2024, pages 29-41
Substrate specificity of exopeptidases in small intestinal mucosa determines the structure of food-derived collagen peptides in rat lumen and blood
Sri Wijanartia, b, Reizeng Guc, Liang Chenc, Wenying Liuc, Muyi Caic, Ryota Suzukia, Kenji Satoa, *
aDivision of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Kitashirakawa Oiwake-cho, Kyoto 606 8502, Japan
bDepartment of Bioresources Technology and Veterinary, Vocational College, Gadjah Mada University, Sleman, D.I. Yogyakarta 55281, Indonesia
cChina National Research Institute of Food & Fermentation Industry, Bldg. 6, No. 24, Jiuxianqiao Middle Road, Chaoyang District, Beijing 100015, P.R. China
*Corresponding author: Kenji Sato, Division of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Kitashirakawa Oiwake-cho, Kyoto 606 8502, Japan. E-mail: sato.kenji.7x@kyoto-u.ac.jp
DOI: 10.31665/JFB.2024.18378
Received: March 25, 2024
Revised received & accepted: May 26, 2024
| Abstract | ▴Top |
Gly-Pro-Hyp-Gly is the most common motif in collagen. Ingestion of collagen hydrolysate (CH) has been reported to significantly increase Pro-Hyp and Hyp-Gly in human peripheral blood. However, the elevation of Gly-Pro content remains negligible. This study seeks to elucidate the underlying reasons behind the bioavailability of CH-derived peptides using in vivo and in vitro digestion. After oral administration of CH (800 mg/kg body weight) to rats, Pro-Hyp significantly increased in the lumen, small intestinal tissue, and blood from the abdominal vein, while only negligible amounts of Gly-Pro were detected. In vitro digestion of CH by digestive exopeptidases, including aminopeptidase N, leucine aminopeptidase, and carboxypeptidase A predominantly generated Gly-Pro. Interestingly, the digestion of CH with crude extract of small intestinal mucosa predominantly generated Pro-Hyp and negligible amounts of Gly-Pro. Furthermore, synthetic Gly-Pro was rapidly degraded by crude extract of the intestinal mucosa, while Pro-Hyp showed higher resistance. These facts indicate prolidase activity in the mucosa plays a crucial role in determining collagen-derived peptides in the body.
Keywords: Peptidase; Intestinal mucosa; Pro-Hyp; Gly-Pro; Bioavailability
| 1. Introduction | ▴Top |
The bioavailability of food-derived peptides is now believed to be crucial for exerting their bioactivity in vivo (Matsui, 2018; Sato, 2022). Conventionally, it was believed that ingested proteins or peptides were rapidly hydrolyzed into amino acids and smaller peptides during digestion. Certain di- and tripeptides have been demonstrated to enter enterocytes via peptide transporter 1 (PepT1) (Hanh et al., 2017; Rohm et al., 2019). However, it was also assumed that these peptides were rapidly degraded in enterocytes and blood circulation. This concept was changed by the findings that have demonstrated the increase of di- and tripeptides containing hydroxyproline (Hyp) in the human peripheral blood at µM levels after ingestion of collagen hydrolysate (CH) (Asai et al., 2019; Iwai et al., 2005; Ohara et al., 2007; Shigemura et al., 2018; Taga et al., 2014). In human blood, collagen-derived peptides are predominantly composed of Pro-Hyp, with the second most abundant being Hyp-Gly (Shigemura et al., 2011). In vitro studies using cell culture systems and animal studies have demonstrated that these collagen peptides are responsible for the beneficial effects of collagen hydrolysate ingestion (Asai et al., 2020a; Asai et al., 2020b; Jimi et al., 2021; Nakatani et al., 2009; Ohara et al., 2010; Shigemura et al., 2009; Shigemura et al., 2011). Furthermore, human clinical studies have indicated that ingestion of collagen hydrolysates exerts health benefits such as improvement of skin (Inoue et al., 2016; Oba et al., 2013; Ohara et al., 2010; Sugihara et al., 2018) and joint conditions (Nakatani et al., 2009; Zdzieblik et al., 2017), and improvement of wound healing of pressure ulcer (Sugihara et al., 2018; Yamanaka et al., 2017).
Collagen has a specific amino acid sequence in which every third residue is Gly, resulting in a repetition as Gly-Xa-Xb (Xa and Xb are any amino acids) in the triple helical domain. Pro residue at Xb is specifically changed to Hyp residue by prolyl hydroxylase during post-translational modification, producing Gly-Pro-Hyp-Gly as the most frequently observed motif (10.5% of total Hyp) in collagen (Ramshaw et al., 1998; Shoulders and Raines, 2009). Pro-Hyp, Hyp-Gly, Gly-Pro-Hyp, and Pro-Hyp-Gly, which notably increase in human peripheral blood upon ingestion of CH (Asai et al., 2019; Iwai et al., 2005; Shigemura et al., 2018), can be derived from this motif. Interestingly, ingestion of CH has shown either no significant increase or only a slight increase in Gly-Pro levels (Asai et al., 2019; Iwai et al., 2005; Ohara et al., 2007; Shigemura et al., 2018; Taga et al., 2014; Taga et al., 2019; Yamamoto et al., 2016), despite its potential derivation from the same motif. Furthermore, in vitro studies using the Caco-2 monolayer system have reported that Gly-Pro can be passed through the Caco-2 monolayer in intact form (Enjoh et al., 1996; Ganapathy and Leibachs, 1983; Rohm et al., 2019). However, these inconsistencies in bioavailability have not been solved. In this study, we clarified a crucial determining factor of the bioavailability of food-derived peptides using in vitro and in vivo digestion.
To predict peptides with high bioavailability, in vitro digestion has been carried out using a combination of some endoproteinases such as pepsin and trypsin and crude protease preparations such as gastric juice (containing pepsin and gastric lipase) and pancreatin (containing amylase, trypsin, chymotrypsin, and lipase) (Guo et al., 2014; Aléman et al., 2013; Larder et al., 2021a; Larder et al., 2021b; Feng and Betti, 2017; Yang et al., 2019; Bai et al. 2023; Brodkorb et al., 2019). However, our research has shown that most short-chain food-derived peptides resist endoproteinases but are readily degraded by exopeptidases (Chen et al., 2019). Pancreatic juice contains carboxypeptidase A (CPA), which cleaves amino acid residues at the carboxyl terminus of peptide except for Pro, Asp, Glu, Arg, Lys, and Hyp (Laethem et al., 1996). The intestinal mucosa contains various exopeptidases secreted by enterocytes such as leucine aminopeptidase (LAP), aminopeptidases N (APN), aminopeptidase A (APA), aminopeptidase B (APB), prolidase, dipeptidyl peptidase 3 (DPP3), and dipeptidyl peptidase 4 (DPP4) (Aganga et al., 2021; Endo et al., 1989; Gustafsson et al., 2011; Hiwada et al., 1981; Johansson et al., 2011; Lundquist and Artursson, 2016; Misiura and Miltyk, 2020). LAP and APN are well-known exopeptidases that release amino terminal amino acids of peptides. LAP preferentially releases Leu and other amino acids, including Pro, from the amino terminus but is unable to release Arg and Lys (Gu and Walling, 2000). APN releases most amino acids, including Arg and Pro (slow), from the amino terminus (Niven et al., 1995). Conversely, APB preferentially releases Arg and Lys from the amino terminus (Nagata et al., 1991). Prolidase preferentially cleaves Pro residue at the carboxyl terminus (Wilk et al., 2017). DPPs release dipeptides at the amino terminus (Ohara-Nemoto et al., 2002). However, their role in determining the bioavailability of food-derived peptides has not been well examined.
We hypothesized that susceptibility to these exopeptidases in the intestinal mucosa could determine the structure of peptides with high bioavailability. Thus, we examined the degradation products of CH by several exopeptidase preparations, crude extracts from rat intestinal mucosa, and blood plasma. Consequently, the present study demonstrates that the exopeptidases in small intestinal mucosa generate Hyp containing di- and tripeptides with low levels of peptides containing Pro at the carboxyl terminus (X-Pro), while X-Pro peptides resisted LAP, CPA, and APN digestion. These results indicate that prolidase activity in the small intestinal mucosa, in addition to LAP, CPA, and APN, plays a crucial role in determining collagen-derived peptides with high bioavailability.
| 2. Material and methods | ▴Top |
2.1. Collagen hydrolysate (CH)
Collagen hydrolysate (CH, Marine Oligopeptide) was a kind gift from the Japan Food Peptide Institute (Osaka, Japan). According to the supplier’s information, CH was prepared by two protease preparations, and the average molecular weight of peptides in CH is approximately 500 Da.
2.2. Reagents
Acetonitrile (HPLC-grade), tris(hydroxymethyl)aminomethane (Tris), phosphate buffered saline pH 7.4 (PBS, 10×), and porcine pancreatin were purchased from Nacalai Tesque (Kyoto, Japan). 6-Aminoquinolyl-N-hydroxysccinimidyl carbamate (AccQ) was purchased from Toronto Research Chemical (Toronto, ON, Canada). A lysis buffer (CelLytic), aminopeptidase N (APN), leucine aminopeptidase (LAP) from porcine kidney, carboxypeptidase A (CPA) from bovine pancreas, L-leucine p-nitroanilide (Leu-pNA, substrate for EC 3.4.11.2) were purchased from Sigma-Aldrich (St. Louis, MO). 9-Fluorenyl methoxycarbonyl (Fmoc) amino acid derivatives, Fmoc amino acid-bound p-alkoxybenzyl alcohol (Alko) resin, proline-bound 2-chlorotrityl chloride (Barlos) resin, and stable isotope labeled Fmoc-Gly (2,2-d2)-OH were purchased from Watanabe Chemical Industries (Hiroshima, Japan). All other reagents were of analytical grade or higher.
2.3. Animal experiments
Five-week-old male Wistar/ST rats (120–140 g) were purchased from Japan SLC (Shizuoka, Japan). Every three rats were kept in a cage at 22–25°C and 40–70% relative humidity, with a 12-hour light/dark cycle. The rats were allowed free access to a normal rodent chow (solid type of certified diet MF; Oriental Yeast, Tokyo, Japan) and drinking water during an acclimatization period of one week. All the animals were treated and cared for according to the guidelines of the National Institutes of Health (NIH) for experimental animals. All experimental procedures were approved by the Animal Care Committee of the Louis Pasteur Center for Medical Research (Kyoto, Japan, approval number 20221). After the acclimatization period, rats were divided into the control group (n = 3) and the collagen hydrolysate (CH) group (n = 3). The CH groups were administered collagen hydrolysate water solution (300 μL) at a dose of 800 mg/kg body weight, while the control group was administered the same volume of distilled water. One hour after the oral administration, small intestine and blood from the abdominal vena cava were collected from rats under anesthesia using isoflurane inhalation.
For the single oral administration of Gly-Pro, eight-week-old male Wistar/ST rats were divided into three groups: control, Gly-Pro 30 min, and Gly-Pro 60 min group (n = 3). After 1 week of acclimatization, the control group was administered distilled water, and the other two groups with Gly-Pro solution at 50 mg/kg body weight. The control group was sacrificed directly after water administration, while the Gly-Pro groups were sacrificed at 30 min and 60 min after oral administration, respectively. The small intestine and the blood from the inferior vena cava were collected as described above.
To quantify the orally administered Gly-Pro in the blood, a stable isotope labeled Gly(d2)-Pro was synthesized as described in the subsequent section. The Gly(d2)-Pro solution and distilled water (vehicle) were orally administered to eight-week-old male Wistar/ST rats (n = 3) for the Gly(d2)-Pro and control groups, respectively. The rats were sacrificed 30 min after administration. The small intestine and the blood from the abdominal vena cava were collected as described above.
The inner content of the small intestines was flushed using 10 mL of cold PBS. The effluent was added with three volumes of ethanol and centrifuged at 13,000 ×g at 4°C for 10 min. The supernatant was collected and used for the following experiments as the lumen sample. The washed small intestine was cut into small pieces. Aliquots of the intestine (100 mg) were homogenized with PBS (100 µL) using a Biomasher II (Nippi, Tokyo, Japan). The homogenate was mixed with three volumes of ethanol and centrifuged at 13,000 ×g for 10 min at 4°C. The supernatant was collected and used as the small intestinal tissue extract for the following experiments. The blood was collected using a heparinized syringe and centrifuged at 1,500 ×g at 4°C for 10 min. The resultant plasma was mixed with three volumes of ethanol and centrifuged as described above. The supernatant was used as a plasma sample for the following experiments.
2.4. Preparation of crude mucosa extract from rat small intestine
To prepare the crude mucosa extract, 6-week-old male Wistar/ST rats were sacrificed by drawing blood from the abdominal vena cava under anesthesia by isoflurane inhalation. The whole small intestine was collected immediately. After flushing the inner content with cold PBS, the intestine was split open longitudinally with scissors. The mucosa was collected by scraping using a spatula and then mixed with two volumes of a lysis buffer (CelLytic) before homogenization in Biomasher II. The suspension was centrifuged at 13,000 ×g at 4°C for 15 min. The supernatant was used as a crude mucosa extract.
2.5. Leucine aminopeptidase (LAP) assay
LAP activity was measured using L-leucine-p-nitroanilide (Leu-pNA) as substrate according to the supplier test protocol (Sigma-Aldrich) with some modifications. Leu-pNA was dissolved in methanol to give a concentration of 1.2 mM. PBS (90 µL) was mixed with 5 µL of the crude extract of small intestinal mucosa and 5 µL of the substrate solution. The absorbances at 405 nm were immediately recorded before and after 5 min incubation at 37 °C. One unit was defined as the enzymes hydrolyzing 1.0 µmole of Leu-pNA to Leu and pNA per minute under assay conditions. The enzyme activity was expressed as a unit/mL, which can be obtained by the following equation: Unit/mL = ((Δabsorbance at 405/min of sample-Δabsorbance at 405/min of blank) × total volume (0.1 mL) × dilution factor (20))/(9.9 × volume of enzyme (mL)), where 9.9 was the millimolar extinction coefficient of pNA at 405 nm.
2.7. Prolidase activity assay
Prolidase activity of crude extract of small intestinal mucosa was measured using synthetic Gly-Pro as the substrate. Aliquots (180 µL) of 0.5 mM Gly-Pro solution in PBS were reacted with 20 µL of crude extract of mucosa (25 dilution using PBS) and incubated at 37 °C for 10 min. The reaction was terminated with the addition of three volumes of ethanol. The content of free Pro in the supernatant (10 µL) liberated from Gly-Pro was quantified by amino acid analysis as described in our previous study (Miyauchi et al., 2022) without HCl hydrolysis. One unit was defined as the enzymes liberating 1.0 µmole of Pro from Gly-Pro per minute under assay conditions. The enzyme activity was expressed as a unit/mL and was calculated using the following equation: Unit/mL = (free Pro at 10 min (µmol) – free Pro at 0 min (µmol)) × total volume (0.2 mL) × dilution factor (250)/(10 min × volume of enzyme (0.02 mL)).
2.8. In vitro digestion using commercial enzymes
In vitro digestion was carried out using the method previously described (Ejima et al., 2018) with some modifications. Briefly, CH was dissolved in 1 mL of PBS to give a 2.5 mg/mL concentration. The resulting CH solution (1 mL) underwent digestion with a mixture containing pancreatin (100 μg), CPA (7.7 U), and LAP (2.45 U) at 37 °C for 4 h. The reaction was halted by freezing at −20°C. Similarly, another CH solution (1 mL) was digested with APN (0.8 U).
2.9. In vitro digestion using crude extracts of mucosa and blood plasma
CH (0.2 mg) was digested by crude extract of mucosa and blood plasma with 0.08 U leucine aminopeptidase (LAP) activity in 100 µL of PBS at 37°C for 4 h. Crude extract of mucosa was diluted with PBS to give suitable LAP activity. The reaction was terminated by adding 300 μL of ethanol. The supernatant was collected after centrifugation at 13,000 ×g at 4°C for 15 min and used as crude mucosa and blood plasma digests of CH. For the blank, crude extracts without CH were incubated and treated in the same manner as the samples.
2.10. Digestion of synthetic Gly-Pro and Pro-Hyp by partially purified crude extract of the mucosa
To remove low molecular weight compounds, especially amino acids and peptides, exopeptidases in the crude extract of mucosa were partially purified using size exclusion chromatography (SEC). The crude extract was clarified by passing through a centrifugal filter (5.0 µm pore size, Ultrafree-MC UFC30SV00, Merck Millipore) at 3,000 ×g for 5 min. Two hundred microliters of the clarified sample were injected into a Superdex 75 (GE Healthcare, Chicago, IL, USA) equilibrated with PBS at 0.5 mL/min flow rate. Seven fractions were collected. The LAP and prolidase activities of each fraction were measured. Fraction A, with the highest LAP and prolidase activities, was selected for the following experiment.
Synthetic Gly-Pro and Pro-Hyp (400 µM) in 400 µL of PBS were digested with the partially purified mucosa exopeptidase preparation with LAP and prolidase activity 0.01 U. The mixture was incubated at 37 °C. Aliquots (50 µL) were withdrawn at 0, 30, 60, 90, and 240 min of incubation, and released free Pro was quantified as described previously. The degradation (%) was calculated using the following equation: Degradation rate (%) = free Pro content in the supernatant (nmol)/peptide content (20 nmol) × 100.
2.11. Identification of peptides in CH
Aliquot (10 µL) of the 2.5 mg/mL CH in PBS solution was directly injected into a liquid chromatography-tandem mass spectrometer (LC-MS/MS, LCMS 8040, Shimadzu, Kyoto, Japan) equipped with an Inertsil ODS-3 column (2.1 mm i.d. × 250 mm, GL Science, Tokyo, Japan), which was maintained at 40°C. The elution was performed using a binary linear gradient using 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B) at 0.2 mL/min. The gradient program was as follows: 0–20 min, 0–30% B; 20–25 min, 30–100% B; 25–30 min, 100% B; 30–30.1 min, 100–0% B, and 30.1–40 min, 0% B. Peptides were detected by total ion scan at positive mode at scan ranges of mass to charge ratio (m/z), 100–200, 200–225, 225–250, 250–275, 275–300, 300–350, 350–400, 400–500, and 500–1,000. Precursor ions in some major peaks were further analyzed by product ion scan mode with collision energy at −15, −25, and −35 eV.
2.12. Identification of peptides in the in vitro and in vivo digests of CH
CH was digested in vitro using the 4 different methods described above. Furthermore, the inner content of the small intestine, intestinal tissue extract, and blood plasma were collected from rats 1 h after administration of CH or vehicle. Ethanol-soluble fractions of these digests and extracts were prepared as described above. Amino/imino groups of peptides and amino acids were derivatized with AccQ to improve the resolution of short-chain peptides. Aliquots of the ethanol-soluble fraction (20 μL and 100 μL for digests and extracts, respectively) were dried in a microcentrifuge tube (1.5 mL) under vacuum and added with 20 μL of water, 60 μL of 50 mM sodium borate buffer, pH 8.8, and 20 μL of 0.3% AccQ-acetonitrile solution. The reaction was carried out at 50 °C for 10 min. The reactant was clarified using a Cosmonice filter. An aliquot (10 μL) of the filtrate was injected into the LC-MS/MS equipped with the Inertsil ODS-3 column. The same elution condition as described above was used. The AccQ-derivatives were specifically detected by precursor ion scan at positive mode targeting AccQ-derived b1 ion with m/z = 171.1 in the scan ranges at m/z = 325–350, 350–375, 375–400, 400–450, and 450–500. To estimate peptide sequences, the precursor ions in major peaks were further subjected to product ion scan with collision energy at −15, −25, and −35 eV.
2.13. Peptide synthesis
The estimated peptides were synthesized using an Fmoc strategy with a solid-phase automatic peptide synthesizer (PSSM-8, Shimadzu), following the supplier’s protocol. For the synthesis of peptides with Pro at the carboxyl terminus, proline-bound Barlos resin rather than Alko resin was used to suppress the formation of diketopiperazines. The synthesized peptides were purified by RP-HPLC using a Cosmosil MS-II (10 mm i.d. × 250 nm; Nacalai Tesque). The binary gradient was used to elute the peptides using 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B) at 2.0 mL/min flow rate. The gradient program was as follows: 0–20 min; B 0–50%, 20–30 min; B 50–100%, 30–35 min; B 100%, 35–35.1 min; B 100–0%, 35.1–45 min; B 0%. The column was maintained at 40°C. The concentration of each peptide was determined by amino acid analysis after HCl hydrolysis, as described in our previous study (Miyauchi et al., 2022), and used as a standard for peptide quantification.
2.14. Peptide quantification
The content of each peptide in the in vitro digests of CH and samples from the animal experiments was determined using the LC-MS/MS in multi-reaction monitoring (MRM) in positive ion mode following the AccQ derivatization described above. The AccQ derivatives were resolved by the Inertsil ODS-3 column using the same eluents as described above. The elution program was as follows: 0–15 min, 0–50% B; 15–20 min, 50–100% B; 20–25 min, 100% B; and 25–35 min, 0% B. MRM conditions were optimized by LabSolutions LCMS Ver. 5.5 (Shimadzu) using the synthetic peptides.
2.15. Statistical analysis
The data were analyzed using SPSS version 28 (IBM, New York, USA). Studentˋs t-test was used to determine the difference between the means of the two groups. One Way ANOVA with Duncan post hoc test was used to compare more than two groups. A p-value less than 0.05 was considered a significant difference.
| 3. Results | ▴Top |
3.1. Identification of peptides in collagen hydrolysate (CH)
The total ion chromatograms of peptides in the CH are shown in Figure 1. Compounds in major peaks were estimated based on precursor and product ions, as shown in Table S1. Identified amino acids and peptides are indicated by one-letter abbreviation of amino acid on the peaks (Figure 1). Pro-Hyp (PO), of which elution position is indicated by asterisks, was not detected in the CH before digestion, while some amino acids and dipeptides were detected in the scan range of m/z 100–300. Most dipeptides consisted of hydrophobic amino acids such as Leu, Ile, Phe, and Met at the carboxyl terminus. In addition, many peaks were detected in the scan range of m/z 300–1,000 (Figure 1), corresponding to tripeptides, tetrapeptides, pentapeptides, and larger ones. These results reveal that the CH before digestion mainly consists of peptides larger than tripeptide and does not contain Pro-Hyp.
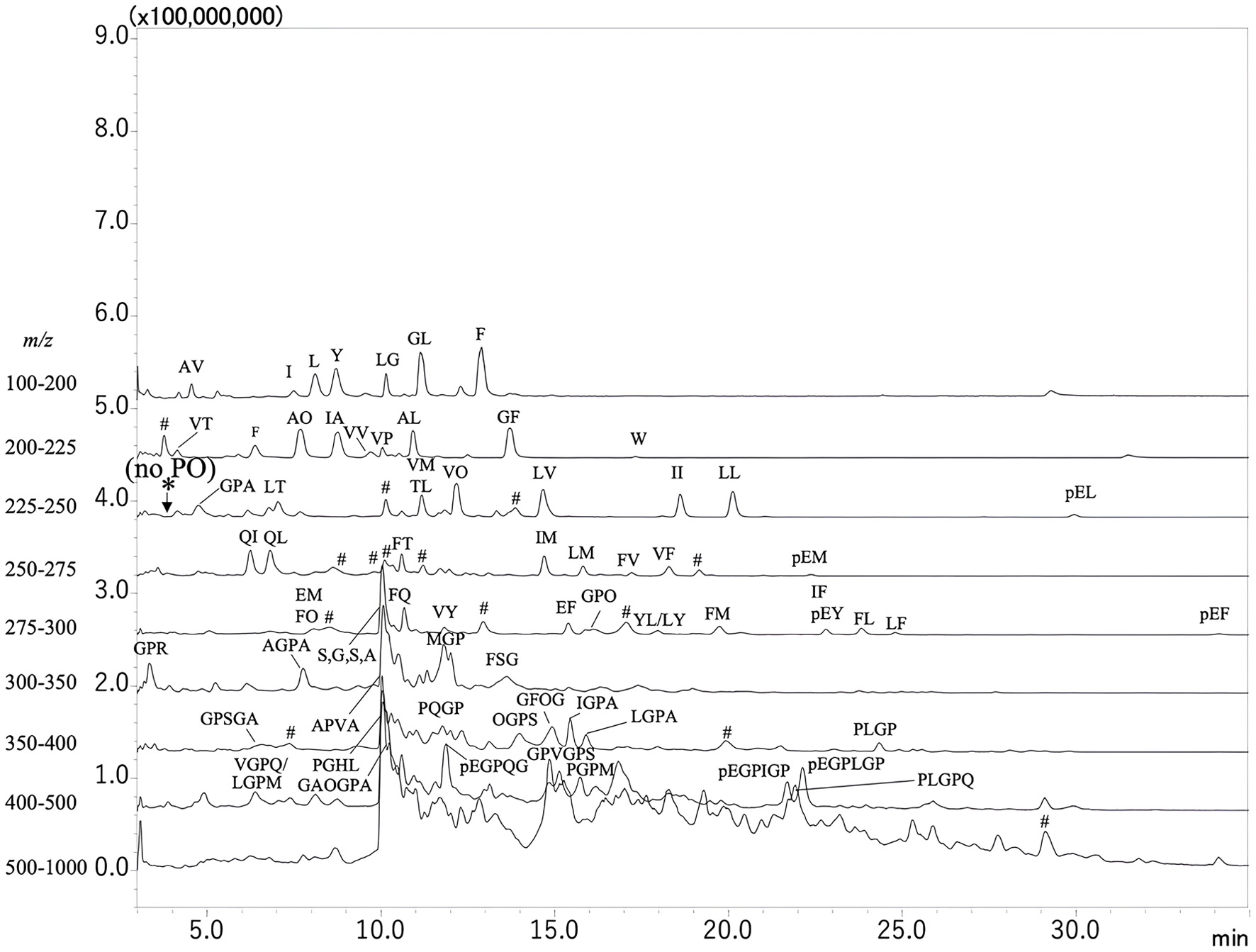 Click for large image | Figure 1. Mass spectrometry chromatograms of compounds in collagen hydrolysate (CH) before digestion. Non-derivatized compounds were resolved by reversed-phase high-performance liquid chromatography (RP-HPLC) and detected by mass spectrometer (LC-MS/MS) in total ion scan at positive mode at different scan ranges across mass-to-charge ratio (m/z) 100–200, 200–225, 225–250, 250–275, 275–300, 300–350, 350–400, 400–500, and 500–1,000. Amino acids and peptides are indicated by a one-letter abbreviation for amino acids. O and pE represent hydroxyproline and pyroglutamic acid, respectively. *Represents the position of the Pro-Hyp (PO) peak. #Represents the peaks that could not be assigned to peptides with any combination of amino acids. |
3.2. Exopeptidases activity of crude extract of small intestinal mucosa
The crude extract of mucosa had both LAP and prolidase activities at 5.80 and 5.85 U/mL, respectively.
3.3. Peptides in the in vitro digests of CH
CH was further digested with four different protease preparations: APN, a mixture of pancreatin, CPA, and LAP, rat small intestinal mucosal extract, and rat blood plasma. Amino compounds in the digests were derivatized with AccQ and analyzed by LC-MS/MS in the precursor ion mode targeting the b1 ion of AccQ moiety, as shown in Figure 2. The one-letter abbreviations indicate the amino acid peaks. Amino acid derivatives less than m/z 325 (amino acids less than 154 Da) were also observed but not shown in Figure 2. In addition to amino acids, several peptide peaks were observed in all digests marked with numbers. Conversely, minimal peptide peaks were detected with m/z values exceeding 500 in all digests (data not illustrated). While some amino acid peaks were present in the mucosa and plasma blanks (Figures 2d and f), no peptide peaks were detected. Sequences of peptides in the peaks marked with numbers were estimated based on the precursor and product ions, as shown in Table 1. Unexpectedly, a large peak of Gly-Pro (peak 1) was observed in the digest of CH with APN and the mixture of pancreatin, CPA, and LAP (Figure 2a and b), while Gly-Pro could not be observed in the digests of CH with rat small intestinal mucosa extract and blood plasma (Figure 2c and e). Pro-Hyp (peak 7) was observed in all digests at different levels.
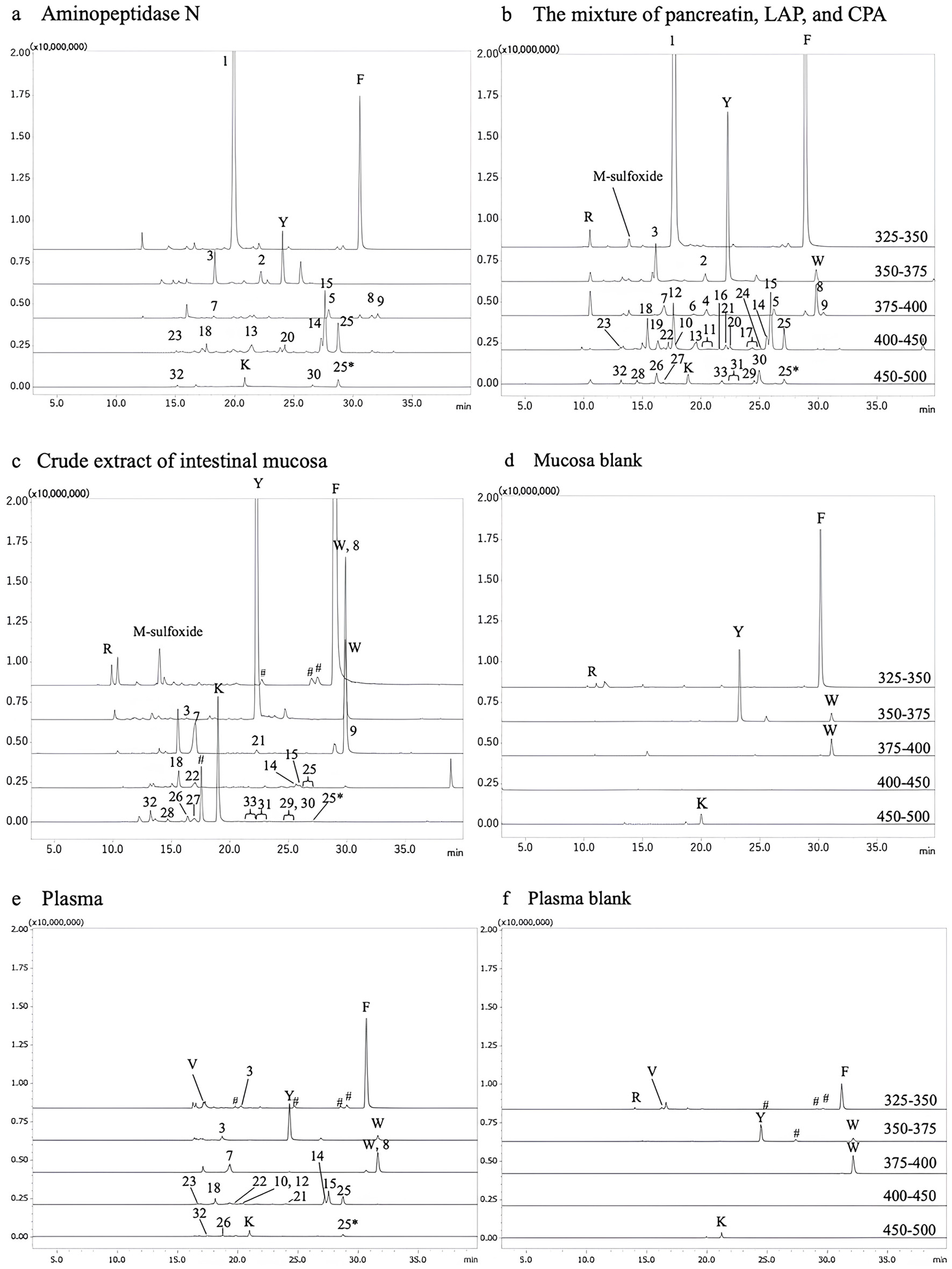 Click for large image | Figure 2. Mass spectrometry chromatograms of AccQ derivatives in digests of collagen hydrolysate (CH). (a) In vitro digest using aminopeptidase N; (b) In vitro digest using a mixture of pancreatin, leucine aminopeptidases, and carboxypeptidases A; (c) In vitro digest using crude extract of small intestinal mucosa of rats; (d) Crude extract of mucosa blank; (e) In vitro digest using blood plasma of rats; (f) Blood plasma blank. The AccQ-derivatives were specifically detected by LC-MS/MS in precursor ion scan targeting b1 ion of AccQ moiety (m/z = 171) in positive mode across m/z range of 325–250, 350–375, 375–400, 400–450, and 450–500. #Represents the peaks that could not be assigned to peptides with any combination of amino acids. |
 Click to view | Table 1. Summary of identified peptides in all digests of CH as shown in Figure 2 |
All estimated peptides were quantified using LC-MS/MS in MRM mode. The peptide contents in CH digests are shown in Figure 3. In addition to peptides listed in Table 1, Hyp-Gly and Glu-Hyp were detected by MRM mode. After in vitro digestion of CH using four different protease preparations, di- and tripeptides containing Hyp (O) and Pro (P) were generated. Digestion with APN and the mixture of pancreatin, CPA, and LAP predominantly generated Gly-Pro with smaller contents of Hyp-containing peptides such as Pro-Hyp and some non-Hyp-containing dipeptides with a proline residue at the carboxyl terminus (Glu-Pro, Pro-Pro, Asp-Pro, etc.) (Figure 3a and b). Conversely, digestion using crude mucosa extract yielded a negligible amount of Gly-Pro but predominantly Pro-Hyp (Figure 3c), along with smaller quantities of Hyp-containing di- and tripeptides. Minimal amounts of non-Hyp-containing peptides with a proline residue at the carboxyl terminus (X-Pro) were observed. Digestion of CH with plasma preparation also predominantly resulted in Pro-Hyp and smaller amounts of other Hyp-containing peptides, resembling mucosa digestion. However, small but detectable quantities of non-Hyp peptides with Pro at the carboxyl terminus, such as Gly-Pro, Glu-Pro, and Asp-Pro, were generated (Figure 3d). Additionally, the digestion with plasma preparation generated a larger content of Pro-Hyp than that with mucosa extract.
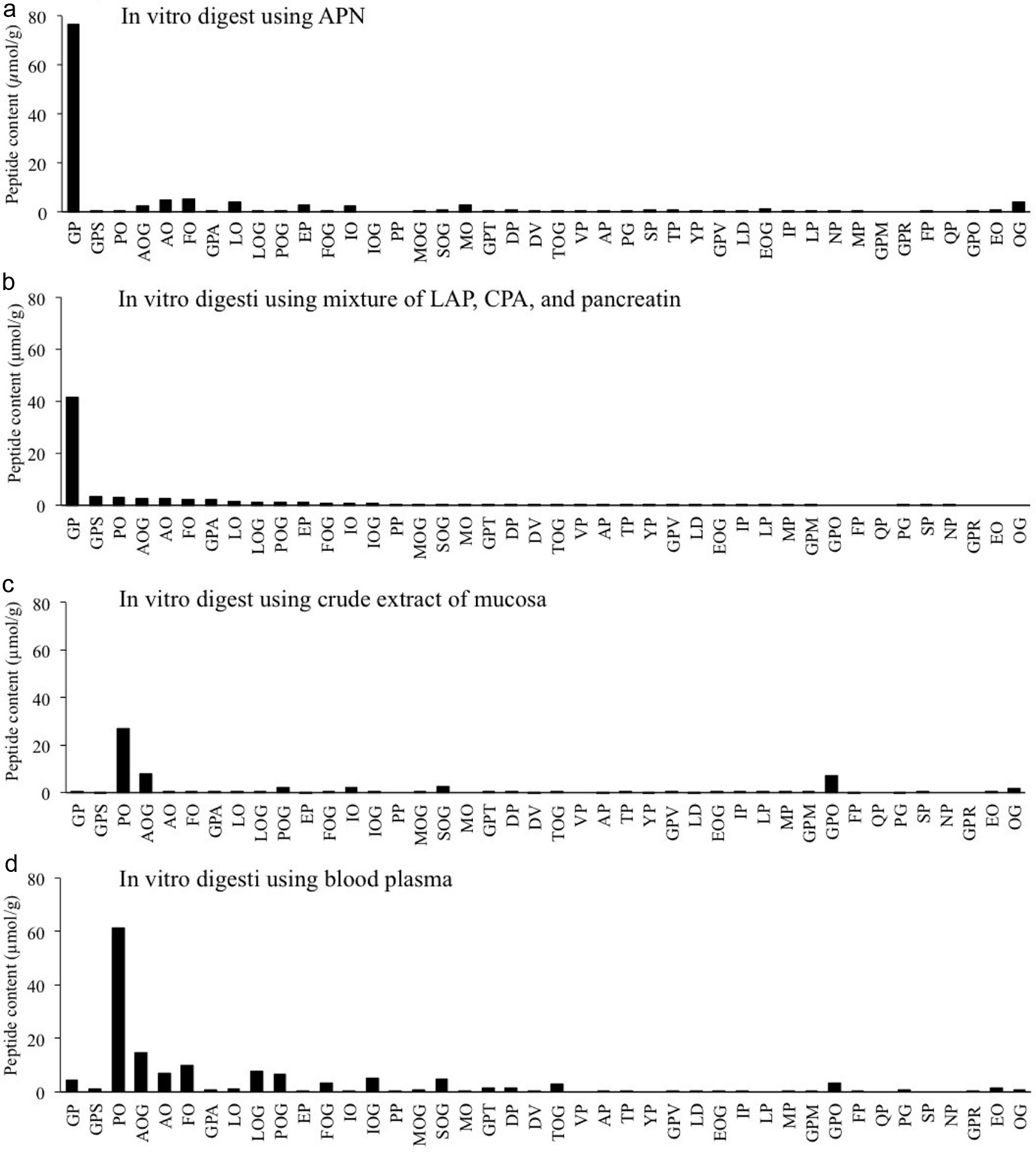 Click for large image | Figure 3. Peptide contents in different in vitro digests of CH. (a) CH digest using APN; (b) CH digest using mixture of pancreatin, LAP, and CPA; (c) CH digest using crude extract of small intestinal mucosa of rat; (d) CH digest with blood plasma of rat. All peptides were quantified by LC-MS/MS in multi-reaction monitoring mode (MRM). Estimated peptides were chemically synthesized and used for the optimization of MRM and external standards. |
3.4. Food-derived peptides in rats after CH ingestion
CH was administered to rats by oral gavage. Peptides in the lumen, small intestinal tissue, and plasma were analyzed by LC-MS/MS in MRM mode. As shown in Figure 4, Pro-Hyp was predominantly generated in the lumen and small intestinal tissue. Some Hyp- and Pro-containing peptides, including Gly-Pro, significantly increased in the lumen 1 h after ingestion. However, the non-Hyp containing dipeptides with Pro at the carboxyl terminus, such as Gly-Pro, did not significantly increase in the intestinal tissue. Furthermore, in blood from the inferior vena cava, only Pro-Hyp, Ala-Hyp, Leu-Hyp-Gly, Glu-Hyp, and Hyp-Gly significantly increased after ingestion of CH. Pro-Hyp was not only the predominant peptide in the blood before the administration of CH but also increased most significantly after the administration compared to other Hyp-containing peptides. Increased collagen peptide profiles in rats’ small intestinal tissue and blood are similar to peptide profiles in CH digest with mucosal extract.
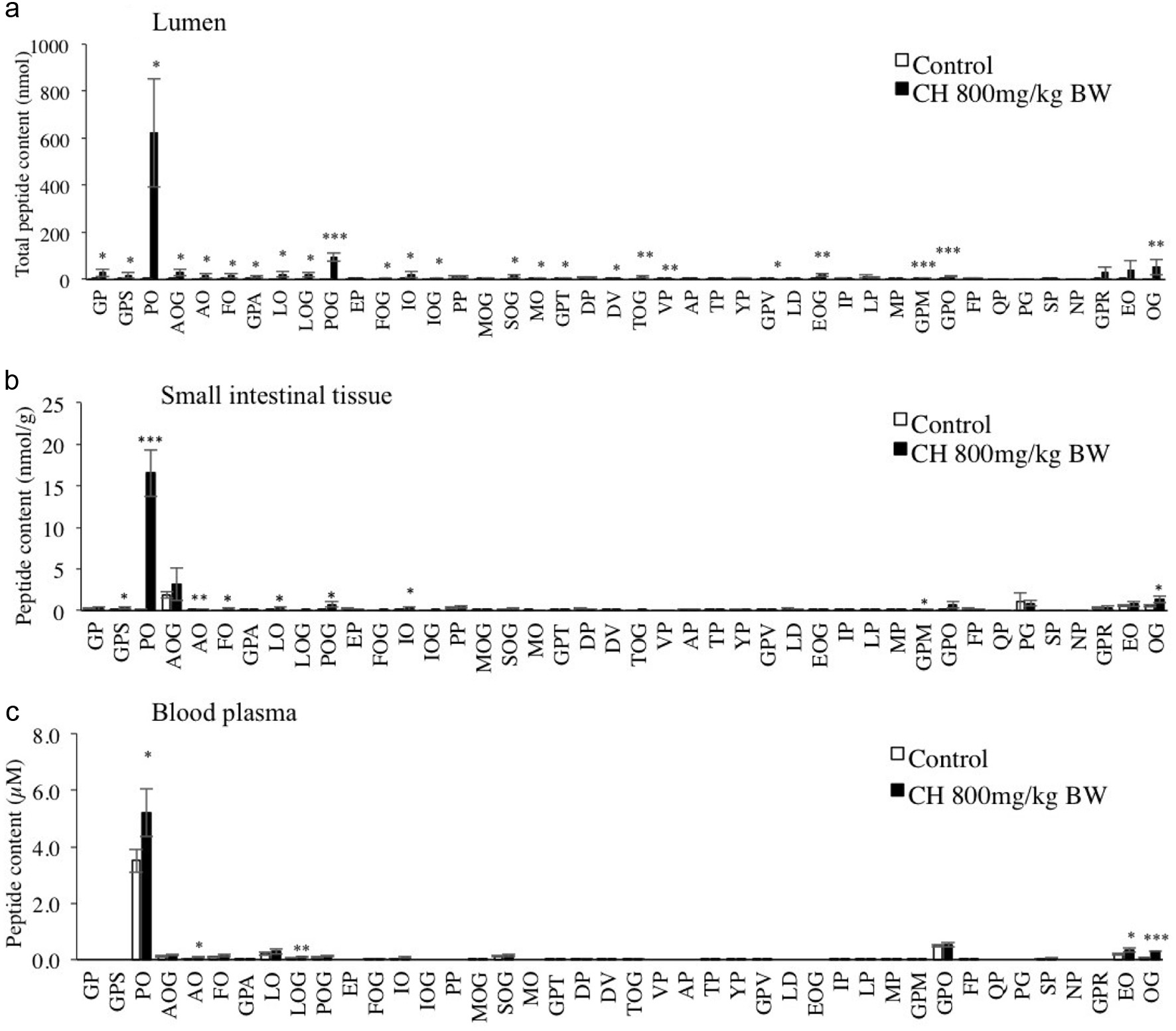 Click for large image | Figure 4. Peptide contents in the lumen (a), small intestinal tissue (b), and blood plasma of rats (c) after ingestion of CH. The collagen group was administered CH solution at 800 mg/kg BW, and the control group with 300 µL water. After 1 h, the rats were sacrificed, and the peptides were extracted from the lumen, small intestinal tissue, and plasma. All peptides were derivatized using AccQ and quantified by LC-MS/MS in MRM mode. The results are presented as the mean ± SD. Asterisks (***), (**), and (*) represent significant differences, p < 0.001, p < 0.01, and p < 0.05, respectively, between the collagen group and control group by Studentˋs t-test (n = 3). |
3.5. Single oral administration of synthetic Gly-Pro in rats
Synthetic Gly-Pro was orally administered to rats at a dose of 50 mg/kg body weight. Gly-Pro did not significantly increase in the lumen or blood plasma (Figures 5a and b). To quantify the orally administered Gly-Pro in the blood plasma, a stable isotope labeled Gly(d2)-Pro was administered to rats at the same dose. Although Gly(d2)-Pro was detectable in the blood plasma, its level was very low (<15 nM) compared to the baseline level, with most of it being degraded into amino acids (Figures 5c and d).
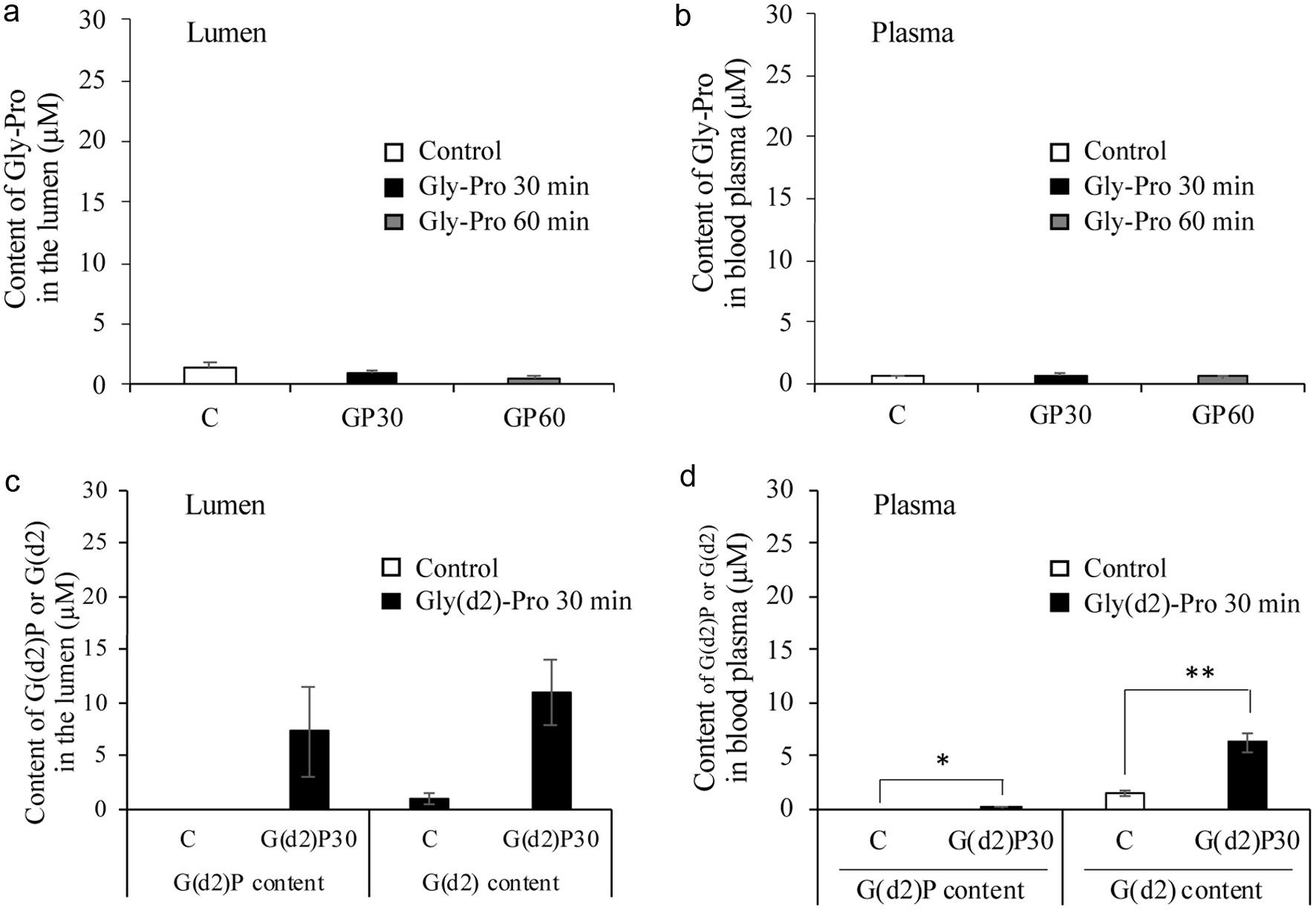 Click for large image | Figure 5. Gly-Pro contents in the small intestinal lumen (a) and blood plasma (b) of rats 30 and 60 min after oral administration of synthetic Gly-Pro at a dose of 50 mg/kg body weight. The contents of stable isotope labeled Gly-Pro (G(d2)P) and Gly (G(d2)) in the small intestinal lumen (c) and blood plasma (d) at 30 minutes after oral administration at the same dose. Asterisks (**) and (*) represent significant differences, p < 0.01, and p < 0.05, respectively, between the G(d2)P30 group and control group by Studentˋs t-test (n = 3). |
3.6. Digestion of synthetic Gly-Pro and Pro-Hyp by partially purified crude extract of the mucosa
Peptidases in the crude extract of mucosa were partially purified by SEC, as illustrated in Figure 6a. Seven fractions were collected. Fraction A showed the highest LAP (0.24 U/mL) and prolidase (0.25 U/mL) activities. Thus, it was used as partially purified mucosa exopeptidase.
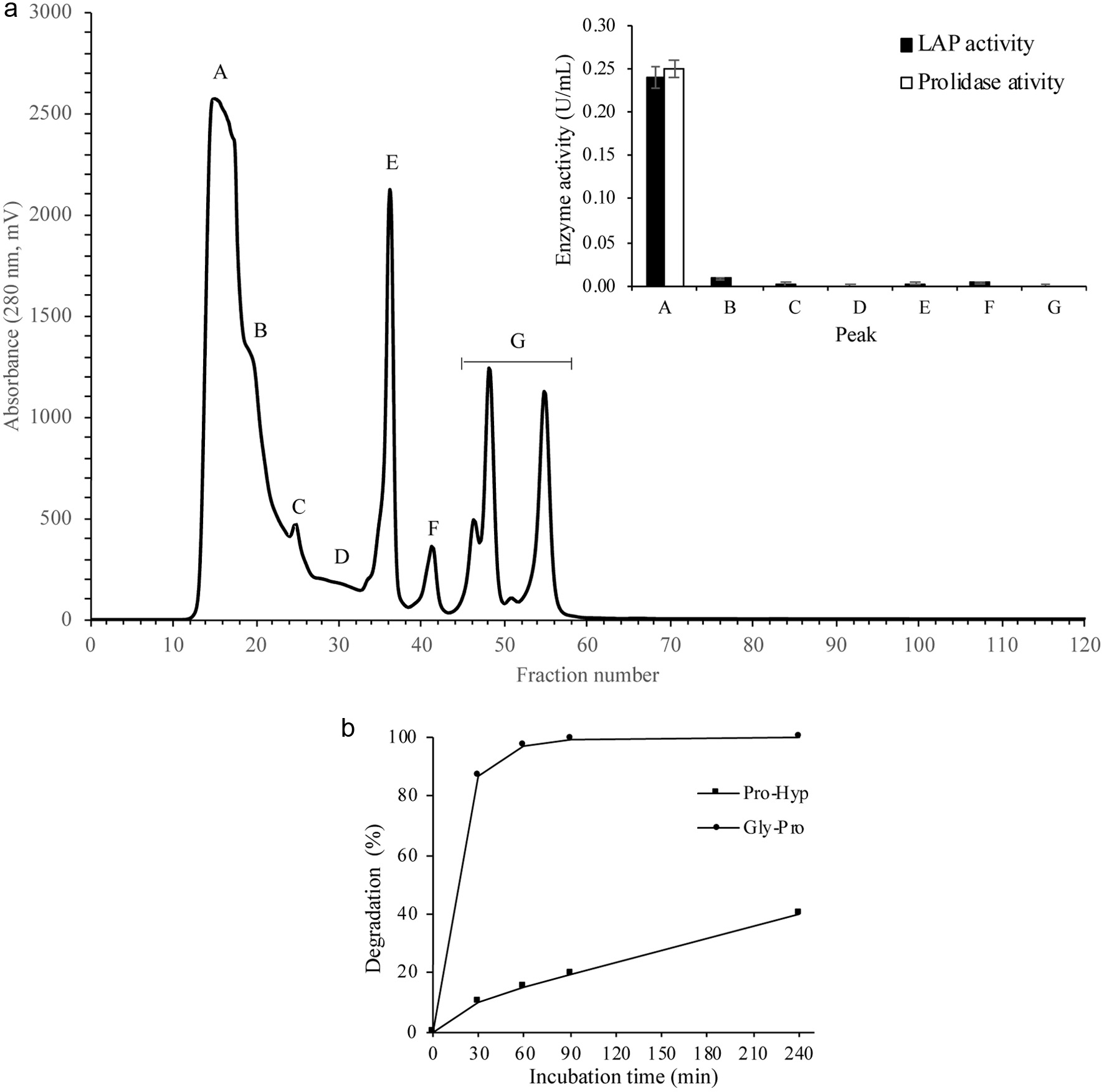 Click for large image | Figure 6. Degradation of Gly-Pro and Pro-Hyp by partially purified crude extract of mucosa. Upper: Partial purification of exopeptidases in the crude extract of the mucosa by size exclusion chromatography using Superdex 75 10/300 GL column. Peak A showed the highest LAP and prolidase activities. Thus, it was selected to digest synthetic peptides. Lower: Synthetic peptides (Gly-Pro and Pro-Hyp) were digested with the partially purified mucosal enzymes. The degradation rate (%) was calculated by quantifying the liberated Pro. The results are presented as the average of duplicate analysis. |
Synthetic Gly-Pro and Pro-Hyp were digested using partially purified mucosa exopeptidases. Gly-Pro was completely degraded after 60 min (Figure 6b). LC-MS/MS analysis also confirmed the absence of Gly-Pro after 60 min digestion. On the other hand, Pro-Hyp was slowly degraded, and around 60% remained even after 240 min incubation.
| 4. Discussion | ▴Top |
It has been demonstrated that food-derived collagen peptides such as Pro-Hyp, Hyp-Gly, etc. are circulating in human blood at relatively high concentrations (>µM levels) for a few hours after ingestion of CH at 80–400 mg/kg body weight (Asai et al., 2019; Iwai et al., 2005; Ohara et al., 2007; Shigemura et al., 2018; Taga et al., 2014). Compared to humans, a lower concentration of collagen-derived peptides, including Pro-Hyp, was detected in rat blood 1h after ingestion of a higher dose of CH at 800 mg/kg (Figure 4), which is consistent with other animal experiments (Hanh et al., 2017; Kawaguchi et al., 2012; Wang et al., 2015). However, collagen peptide composition in the rat lumen and small intestinal tissue is similar to that observed in human blood. These facts indicate that peptides in CH are similarly digested by exopeptidases in the small intestinal lumen of humans and rats. In rats, the remaining peptides are, however, rapidly degraded when passing through the enterocyte to blood circulation and incorporated into cells from blood circulation, which may rapidly decrease blood levels of collagen-derived peptides in rats compared to humans.
Pro-Hyp and Hyp-Gly, the major food-derived collagen peptides in human blood, can be derived from the Gly-Pro-Hyp-Gly motif in larger collagen peptides by gastrointestinal digestion. As mentioned above, CH ingestion has little impact on Gly-Pro levels in human blood plasma, while Gly-Pro is potentially generated from the same motif. In the present study, we also observed that oral administration of CH did not impact Gly-Pro plasma levels in the rats (Figure 4). In addition, only a slight increase of Gly-Pro was observed in the rat lumen. Furthermore, a single administration of Gly-Pro also did not impact the blood level of Gly-Pro (Figure 5). Ejima et al. (2018) also reported that peptides with Pro residue at the carboxyl terminus (X-Pro) are degraded during the in vivo digestion and absorption process, while these X-Pro peptides resist digestion with APN, CPA, and LAP. The present in vitro digestion of CH by crude extract of small intestinal mucosa generated only negligible amounts of X-Pro peptides, including Gly-Pro, while LAP, CPA, and APN predominantly generated Gly-Pro, which is consistent with the results by Ejima et al. (2018). These facts indicate that X-Pro peptides, including Gly-Pro, are degraded by exopeptidases in the small intestinal mucosa except for LAP, APN, or CPA. The digestion with blood plasma generated a peptide composition similar to that of mucosa digest with detectable amounts of Gly-Pro. The composition of the remaining peptides in the mucosa and plasma digests of CH is similar to that of collagen-derived peptides in human and rat blood (Asai et al., 2019; Iwai et al., 2005; Shigemura et al., 2011; Shigemura et al., 2018; Yamamoto et al., 2016).
Prolidase is the only exopeptidase that can release Pro from dipeptide with Pro at the carboxyl terminus (Aganga et al., 2021; Namiduru, 2016). As Gly-Pro is degraded by digestion with mucosa extract, prolidase is present in the intestinal mucosa. It has also been reported that the prolidase can cleave dipeptides with Hyp at the carboxyl terminus. The present study revealed that Pro-Hyp was slowly degraded by mucosa extract compared to Gly-Pro (Figure 6). Hyp is a post-translationally modified amino acid. Hyp-containing peptides such as Pro-Hyp were partially resistant to both prolidase as well as LAP, APN, and CPA in the digestive tract and mucosa. On the other hand, Gly-Pro was rapidly degraded by prolidase but was resistant to LAP, APN, and CPA. These results could explain why Hyp-containing peptides increase in blood after CH ingestion, but Gly-Pro does not.
The combination of LAP, CPA, and APN proved effective in degrading most peptides in CH (Figures 2a and b) and other protein hydrolysates (Ejima et al., 2018; Chen et al., 2019), except for X-Pro dipeptides. However, X-Pro dipeptides are degraded by prolidase. Thus, most peptides in the lumen are degraded into amino acids through sequential cleavage of exopeptidases. The combination of these exopeptidases can act as an intestinal blood barrier to food-derived peptides. Therefore, to get a reliable prediction of the structure of food-derived peptides, carefully designed in vitro digestion using exopeptidases of small intestinal mucosa with different substrate specificities, such as LAP, CPA, APN, and prolidase, is crucial.
In addition to Hyp-containing peptides, Pro-Gly can resist the digestion of these peptidases and increase in human blood more than µM levels after ingestion of an elastin hydrolysate (Shigemura et al., 2012), while Gly-Pro cannot. The reason why Pro-Gly resists the exopeptidases remains to be solved. Other modified peptides, such as pyroglutamyl and beta aspartyl-isopeptides, have been reported to significantly increase in the intestinal lumen and blood upon ingestion of protein hydrolysates (Ejima et al., 2019; Miyauchi et al., 2022) due to resistance to intestinal peptidases (Miyauchi et al., 2022).
Until now, the Caco-2 monolayer system has been used for in vitro simulation of intestinal absorption. However, Gly-Pro has been reported to pass through the Caco-2 cell monolayer as an intact form (Enjoh et al., 1996; Ganapathy and Leibachs, 1983; Rohm et al., 2019). Enterocytes secrete various exopeptidases, which accumulate in the brush border membrane and mucus layer for digestive purposes (Gustafsson et al., 2011; Hiwada et al., 1981; Johansson et al., 2011; Lundquist and Artursson, 2016; Rodríguez-Piñeiro et al., 2013). However, previous studies reported that compared to enterocytes in the human small intestine, the Caco-2 monolayer has a lower expression level of endoproteinases and exopeptidases in the brush border membrane (Lundquist and Artursson, 2016) and a lack of mucus layer (Frey et al., 1996). Taken together, some Caco-2 monolayers lack some exopeptidases, especially prolidase. Thus, such a Caco-2 mono-cell layer cannot be used for the prediction of the bioavailability of peptides. Careful consideration regarding the transporter and the expression of exopeptidases is necessary for all in vitro absorption models. Similarly, for the in-silico digestion approach, the substrate specificity of exopeptidases, especially prolidase, should be taken into consideration to predict the bioavailability of peptides. Neglecting these factors may lead to erroneous results.
| 5. Conclusion | ▴Top |
In the rat lumen, LAP, APN, and CPA degrade most food-derived peptides into amino acids but remain X-Pro dipeptides. Notably, X-Pro dipeptides, including Gly-Pro, are preferentially degraded by prolidase in the lumen. In contrast, Hyp-containing peptides resist both types of exopeptidases, which can explain the high bioavailability of Hyp-containing peptides such as Pro-Hyp and Hyp-Gly. To predict the bioavailability of food-derived peptides, both types of exopeptidases must be taken into consideration. The in vitro and in silico systems, without considering both types of exo peptidases, may lead to fallacious results.
| Supplementary material | ▴Top |
Table S1. Estimated peptides in collagen hydrolysate (CH) before digestion.
Acknowledgments
This work was financially supported by Commissioned Research between Kyoto University and Japan Food Peptide Institute (00201000017). S. Wijanarti is grateful to the Indonesia Endowment Fund for Education (LPDP), Ministry of Finance Indonesia Republic, for financial support during the study (grant number SKPB-6826/LPDP/LPDP.3/2023).
| References | ▴Top |