| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 28, December 2024, pages 68-75
Formation mechanism of an inclusion complex of lenthionine with α-cyclodextrin and enhancement of its bioavailability
Shoichiro Shimada, Yusuke Yamaguchi, Saki Nakajo, Soichiro Mochizuki, Ryuji Hirata, Yuki Tanabe, Kyohei Yamada, Hitomi Kumagai*
Graduate School of Bioresource Sciences, Nihon University, 1866 Kameino, Fujisawa-shi 252-0880, Japan
*Corresponding author: Hitomi Kumagai, Graduate School of Bioresource Sciences, Nihon University, 1866 Kameino, Fujisawa-shi 252-0880, Japan. E-mail: kumagai.hitomi@nihon-u.ac.jp
DOI: 10.26599/JFB.2024.95028397
Received: October 11, 2024
Revised received & accepted: December 2, 2024
| Abstract | ▴Top |
1,2,3,5,6-Pentathiepane (lenthionine) is a cyclic polysulphur compound that contributes to the distinctive flavour of shiitake mushrooms (Lentinula edodes) and inhibits platelet aggregation. However, its volatile hydrophobic characteristics present a challenge for its use in food applications. In this study, lenthionine was complexed with α-cyclodextrin (α-CD) to enhance its stability and bioavailability. The inclusion ratio of lenthionine with α-CD was 1:2. The formation of a stable inclusion complex was confirmed through diffusion order spectroscopy (DOSY) and proton nuclear magnetic resonance (1H NMR) analyses. The inclusion of lenthionine in α-CD markedly suppressed its release. In vivo experiments demonstrated that the lenthionine/α-CD inclusion complex exhibited a more rapid and pronounced inhibitory effect on platelet aggregation than lenthionine alone. These findings indicate that inclusion of lenthionine in α-CD simultaneously mitigates its flavour and enhances its bioavailability, thereby expanding its potential for application in functional foods and pharmaceuticals.
Keywords: Lenthionine; Cyclic polysulphur compound; Cycrodextrin; Inclusion complex; Platelet aggregation
| 1. Introduction | ▴Top |
1,2,3,5,6-Pentathiepane (lenthionine) is a cyclic polysulphur compound comprising disulphide and trisulphide bonds. It has been identified as a component that characterises the flavour of shiitake mushrooms (Lentinula edodes) (Wang et al., 2021a). The mechanism of its formation and the properties of the enzymes involved in its production have already been elucidated, where lenthionine is produced from lenthinic acid by the action of γ-glutamyl transpeptidase and C-S lyase (Yasumoto et al., 1971; Kumagai et al., 2002; Li et al., 2012). Furthermore, due to their distinctive flavour profiles, shiitake mushrooms are used in a plethora of traditional cuisines, including Japanese and Chinese dishes. Given that lenthionine content is associated with the palatability of shiitake mushrooms, techniques to regulate lenthionine content by modulating enzyme activities and gene expression involved in its production such as the administration of citric acid or the imposition of drought stress have also been investigated (Wang et al., 2021b; Hong et al., 2022).
The physiological functions of sulphur compounds in food have also been the subject of considerable research interest. A number of components in vegetables belonging to the Allium genus and Brassicaceae family such as garlic (Allium sativum L.), onion (Allium cepa L.), broccoli (Brassica oleracea L. var. Italica), and radishes (Raphanus sativus L.) have been studied as functional ingredients (Ariga and Seki, 2006; Uto-Kondo et al., 2018; Shang et al., 2019; Vanduchova et al., 2019; Yamaguchi and Kumagai, 2020, Yamaguchi et al., 2021, 2023, 2024; Bastaki et al., 2021; Otoo and Allen, 2023; El-Saadony et al., 2024). As we previously reported, lenthionine inhibits platelet aggregation both in vitro and in vivo (Shimada et al., 2004, 2008, 2024). Additionally, it possesses a range of other physiological functions, including antibacterial and anti-inflammatory effects (Morita and Kobayashi, 1967; Kupcova et al., 2018). However, the distinctive flavour of lenthionine limits its use in food.
Cyclodextrins (CDs) are cyclic oligomers comprising multiple glucopyranose residues linked by α-1,4 glycosidic bonds. They occur naturally in three types, designated as α, β and γ, with 6, 7 or 8 glucopyranose units, respectively. The outer side of the CDs is characterised by the presence of hydroxyl groups that confer higher polarity. In contrast, the inner side exhibits lower polarity, making it possible to include hydrophobic compounds (Figure 1) (Connors, 1997). The inclusion of polysulphur compounds in food by CDs has been documented for diallyl sulphide, diallyl disulphide, and diallyl trisulphide in garlic oil (Nguyen and Yoshii, 2018; Qian et al., 2024). These sulphur compounds that are unstable under light, oxygen, heat and moisture, maintain antioxidant capacity due to their increased thermal stability and sustained release in water by inclusion with β-CD (Li et al., 2023). Additionally, glucoraphanin in broccoli juice included with α-CD enhances its stability during industrial processing (Martínez-Hernández et al., 2019).
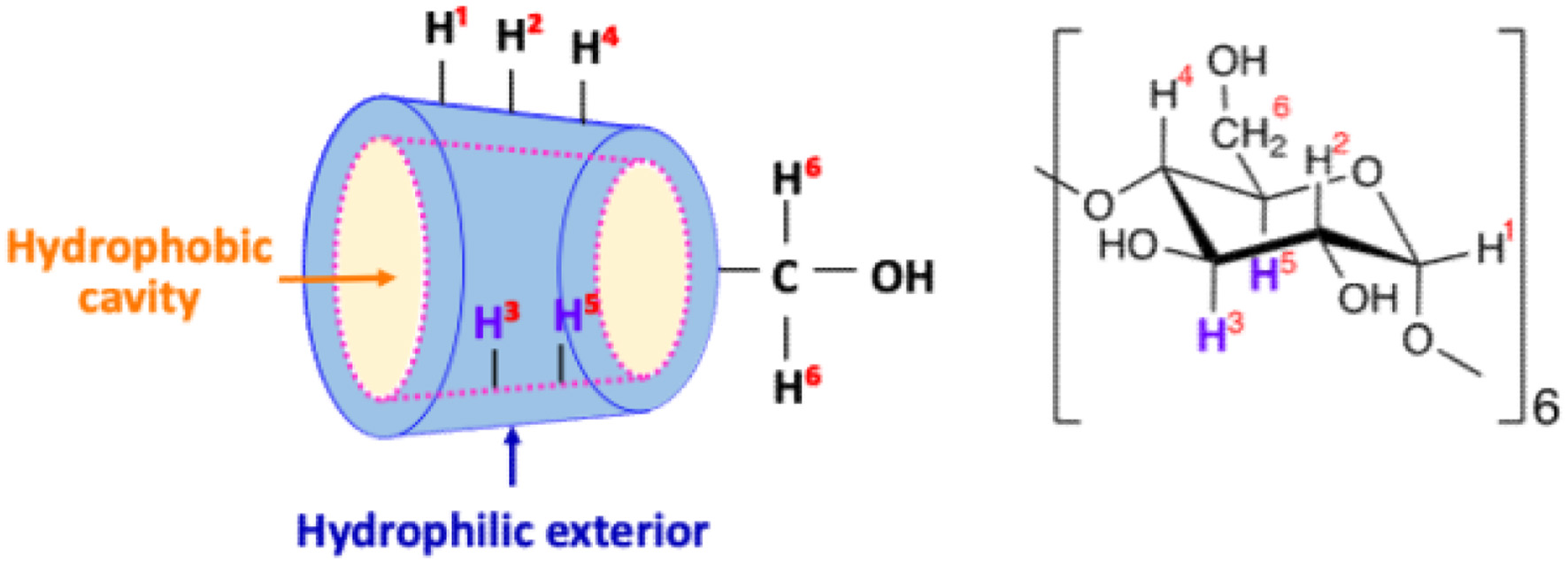 Click for large image | Figure 1. Schematic diagram of α-CD structure. |
As for inclusion of shiitake flavours in CDs, α-CD is more effective in retaining lenthionine during spray-drying than β-CD and γ-CD (Shiga et al., 2004). However, the underlying mechanism of the inclusion of lenthionine in α-CD and the bioavailability of included lenthionine remain elusive. The objective of this study was to investigate the formation mechanism of inclusion complex of lenthionine with α-CD and to evaluate the in vivo effect of lenthionine included with α-CD on platelet aggregation inhibition.
| 2. Materials and methods | ▴Top |
2.1. Reagents
Lenthionine was obtained from Ogawa & Co., Ltd. (Tokyo, Japan), and α-cyclodextrin (α-CD), deuterium oxide (99.8%), and adenosine 5′-diphosphate sodium salt (ADP) were obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Ethanol-d6 (99.5%) and cycloheptane were obtained from Sigma-Aldrich (Tokyo, Japan). All the other reagents used were of a reagent special grade or equivalent.
2.2. Determination of the inclusion ratio of lenthionine to α-CD by the phenol sulphuric acid method
Lenthionine solutions (100 μL) at concentrations of 50, 100, 150, and 200 μmol/mL in dimethyl sulphoxide was added to 1 mL of α-CD solution at a concentration of 200 μmol/mL. The solution was then incubated at 40°C for 3 h. After the incubation, the inclusion material was separated from the solution by centrifugation at 1,200×g for 10 min. Following the removal of the inclusion bodies, the remaining supernatant was diluted 5,000-fold. Then, 1 mL of 5% phenol and 5 mL of sulphuric acid were both added to 1 mL of the diluted solution, and the mixture was incubated for 30 min. Subsequently, the absorbance at 490 nm was determined, and the concentration of α-CD in the supernatant was quantified using a calibration curve prepared with α-CD. The observed decrease in the concentration of α-CD in the supernatant was attributed to the inclusion of lenthionine in α-CD and the subsequent precipitation of the resulting inclusion complex. Consequently, the amount of α-CD used for the inclusion of lenthionine was calculated from the reduced amount of α-CD in the supernatant, and the inclusion ratio of lenthionine to α-CD was obtained.
2.3. 1H NMR spectroscopic analysis of the inclusion complex of lenthionine with α-CD
A solution of 396 µL of lenthionine dissolved in ethanol-d6 was added to 1 mL of α-CD dissolved in deuterium oxide, resulting in a 1:5 molar ratio of lenthionine to α-CD. Following a 1-min vortexing step, the mixture was stirred at room temperature for 1 h with a stirrer. After stirring, the mixture was refrigerated at 4°C for 15 min, and the precipitate was collected by centrifugation at 18,870×g for 5 min at room temperature. Subsequently, 1 mL of deuterium oxide was added to the precipitate, and this was diluted 10-fold with additional deuterium oxide. The samples were analysed by diffusion-ordered spectroscopy (DOSY) and proton nuclear magnetic resonance (1H NMR) at 25°C using a 500-MHz NMR spectrometer (ECA500, JEOL Ltd.). Signals are quoted as δ values in ppm using residual protonated solvent signals as the internal standard (D2O: δ 4.79 ppm, DMSO-d6: δ 2.50 ppm). Lenthionine and cycloheptane, both dissolved in DMSO-d6, and α-CD dissolved in D2O were also subjected to 1H NMR spectroscopy for comparison.
2.4. Effect of α-CD on suppressing the release of lenthionine
A 198 μL volume of lenthionine solution (final concentration 20 mM) dissolved in ethanol was added to 500 μL of α-CD solution (final concentration 20, 40, 60, and 100 mM), at molar ratios of lenthionine to α-CD ranging from 1:1 to 1:5. The solution was mixed for 1 min and allowed to stand at room temperature for 1 h. The solution was diluted 100-fold. Subsequently, the diluted solution was collected in a vial, and a SPME fibre (50/30 μm DVB/Carbon/PDMS Stable Flex, Sigma-Aldrich Japan, Tokyo) was set into the SPME syringe (Solid Phase Microextraction Holder Manual, Sigma-Aldrich Japan, Tokyo). An SPME syringe was inserted into the vial with the fibre exposed in the headspace and allowed to adsorb volatile lenthionine onto the SPME fibre for 5 min. Subsequently, the adsorbed fibre was introduced into a GC-MS injector, and lenthionine was desorbed by heating, thus allowing for the quantification of the amount of volatile lenthionine. As a control, the α-CD solution was replaced with an equal volume of ultrapure water that served as the solvent for the α-CD solution, and the amount of volatile lenthionine was measured in a similar manner.
The chromatograph was a JEOL 7890GC equipped with the JMS-Q1500 detector, and an HP-5 (30 m × 0.25 i.d.) column (Agilent Technologies, Palo Alto, California, USA). The temperature was programmed as follows: initial temperature at 60°C (0 min); program rate at 20°C/min; final temperature at 220°C (0 min); injector temperature at 250°C; detector temperature at 250°C; carrier gas was He; splitless (closed for 0.5 min); desorption time of 3 min, conditioning at 250°C (30 min); ionisation current at 300mA; ionisation voltage at 70eV; acceleration voltage at 10kV; and ionisation method was EI.
2.5. Animals
Six-week-old male Wistar SPF rats (CLEA Japan, Inc., Tokyo, Japan) were pre-housed for 8 days to allow acclimation. The rats were divided into 2 groups of five rats each and housed in wire mesh gauges maintained at a temperature of 22 ± 2°C with a 12-h light/dark cycle. A solid diet for rodents (CE-2, CLEA Japan, Inc., Tokyo, Japan) and tap water were provided ad libitum. All animal experiments were performed in accordance with the Guidelines for Animal Experiments of the College of Bioresource Science of Nihon University (Japan) (approval numbers: AP24BRS025-1).
2.6. Time-course and concentration-dependent inhibition of platelet aggregation by the lenthionine/α-CD inclusion complex
To evaluate the time-course effect of the lenthionine/α-CD inclusion complex, lenthionine included in α-CD was orally administered via gastric sonde at a dose of 100 mg/kg body weight of lenthionine at a molar ratio of lenthionine to α-CD of 1:2 per rat. At 2, 4, 8, 12, 16, 20, and 24 h after the intragastric administration, 5 mL of blood was collected from the inferior vena cava into a test tube containing 3.8% sodium citrate (blood : citrate = 9:1). Blood from rats before lenthionine administration was used as a control.
To evaluate the concentration-dependent effect of the lenthionine/α-CD inclusion complex, lenthionine included in α-CD was orally administered via gastric sonde at doses of 0, 0.01, 0.1, 1, 10, and 100 mg lenthionine/kg body weight at molar ratio of lenthionine to α-CD of 1:2 per rat. Subsequently, 6 h after oral intragastric administration, 5 mL of blood was collected from the inferior vena cava into a test tube containing 3.8% sodium citrate (blood: citrate = 9:1). Blood from untreated rats that had not received the lenthionine/α-CD inclusion complex was used as a control.
Citrated blood obtained was centrifuged at 100×g for 10 min, and the upper layer was designated as platelet-rich plasma (PRP). The remaining lower layer from which PRP was prepared was further centrifuged at 300×g for 15 min, and the upper layer was designated as platelet-poor plasma (PPP).
Inhibition of platelet aggregation was measured using an aggregometer (NKK HEMA TRACER 1 MODEL PAT-4, SSR Engineering Corporation, Tokyo, Japan) with a CHROMATOPAC recorder (C-R4A, Shimadzu Corporation, Kyoto, Japan) or a platelet agglutination measuring Device PA-20 (Kowa Company, Ltd., Japan). A stir bar was placed in a silicone-coated cuvette, to which 300 μL of PPP or PRP was added, and the mixture was preheated at 37°C. Transmittance was recorded while stirring at 37°C and 1,000 rpm. The transmittance of PPP was set to 0%, whereas that of PRP was set to 100%. Span adjustment was performed for each measurement. After span adjustment, 18 μL of ADP was added as an agonist to induce platelet aggregation (the final concentrations was 50 μM), and the change in transmittance was recorded for 10 min.
The inhibition rate of platelet aggregation was determined by analysing the changes in the transmittance (T%) of the PRP. The change in transmittance is attributed to a change in the platelet aggregation rate (Agg. %), and the highest value was defined as the maximum aggregation rate (Agg. max %). The inhibition rate (inhibition%) was calculated as (1-T′/T)×100, where T is the maximum transmittance of PRP from the control rats and T′ is that of PRP from the lenthionine-administered rats.
2.7. Statistical analysis
The results are presented as mean ± SE from three or five experiments. Statistical analysis was conducted using the Tukey-Kramer test.
| 3. Results | ▴Top |
3.1. Inclusion ratio of lenthionine to α-CD
To ascertain the inclusion ratio of lenthionine to α-CD, a series of lenthionine solutions of varying concentrations were added to α-CD. The amount of α-CD consumed by the inclusion reaction with lenthionine was quantified (Figure 2). Of the initial 200 μmol α-CD, approximately 10 μmol α-CD was consumed with the addition of 5 μmol lenthionine, approximately 20 μmol α-CD was consumed with the addition of 10 μmol lenthionine, approximately 30 μmol α-CD was consumed with the addition of 15 μmol lenthionine, and approximately 40 μmol α-CD was consumed with the addition of 20 μmol lenthionine. At all lenthionine concentrations, the amount of α-CD consumed was twice that of lenthionine. This indicates that lenthionine is included in α-CD at a 1:2 ratio.
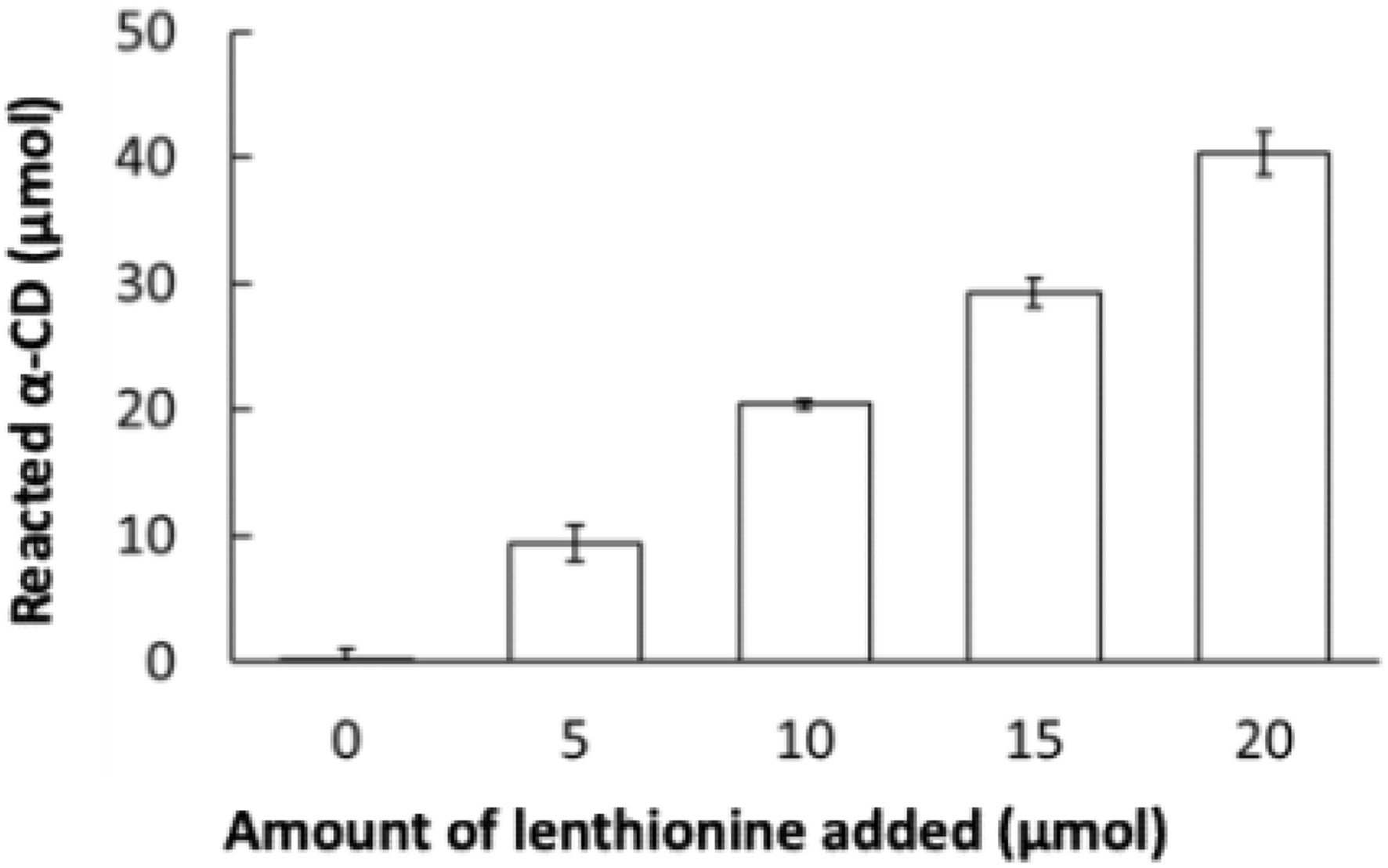 Click for large image | Figure 2. Reaction of lenthionine with α-CD. Values are means ± SE of 3 different experiments. |
3.2. Change in 1H NMR spectra by inclusion of lenthionine in α-CD
1H NMR spectral data for α-CD were assigned as follows: 1H NMR (500 MHz, D2O); δ = 3.54 (6H, t, J = 18.6 Hz), 3.58 (6H, dd, J = 13.5, 3.4 Hz), 3.79–3.86 (18H, m), 3.93 (6H, t, J = 18.9 Hz), and 5.01 (6H, d, J = 3.44 Hz).
1H NMR spectral data for α-CD including lenthionine were assigned as follows: 1H NMR (500 MHz, D2O); δ = 3.54 (6H, t, J = 18.6 Hz), 3.58 (6H, dd, J = 13.5, 3.4 Hz), 3.75–3.88 (18H, m), 3.94 (6H, t, J = 18.9 Hz), 4.41 (4H, s), and 5.01 (6H, d, J = 3.44 Hz).
1H NMR spectral data for lenthionine were assigned as follows: 1H NMR (500 MHz, DMSO-d6); δ = 4.46 (4H, s).
1H NMR spectral data for cycloheptane were assigned as follows: 1H NMR (500 MHz, DMSO-d6); δ = 1.15 (14H, s).
Diffusion coefficients for α-CD, α-CD including lenthionine, and lenthionine included in α-CD were measured as 0.335 m2/Gs (Figure 3).
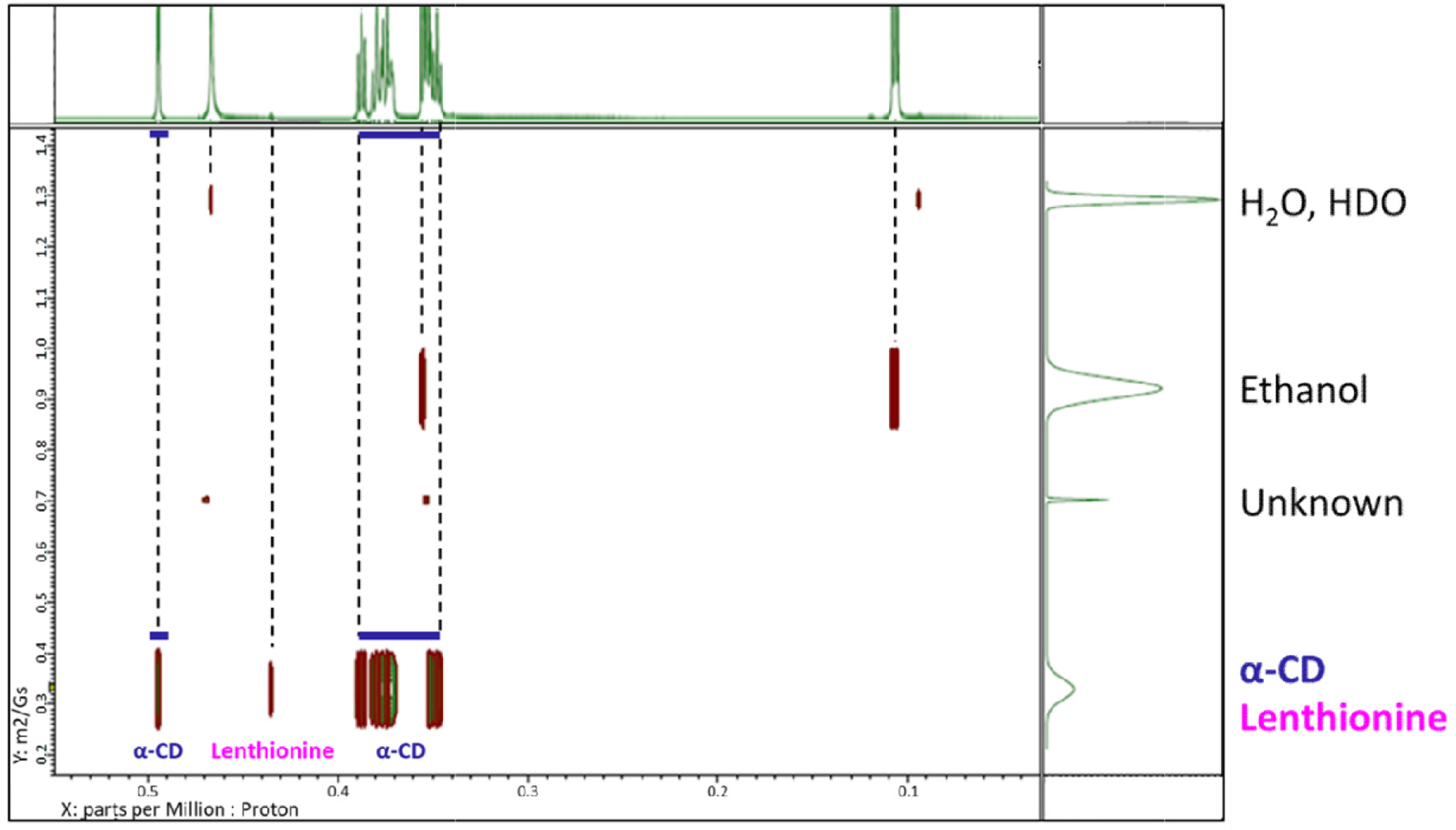 Click for large image | Figure 3. DOSY-NMR spectra of the lenthionine/α-CD inclusion complex. |
The differences in chemical shifts between α-CD and the lenthionine/α-CD inclusion complex were as follows: 0.002 ppm for H1, 0.001 ppm for H2, 0.012 ppm for H3, 0.001 ppm for H4, 0.022 ppm for H5, and 0.005 ppm for H6 (Figure 4, Table 1).
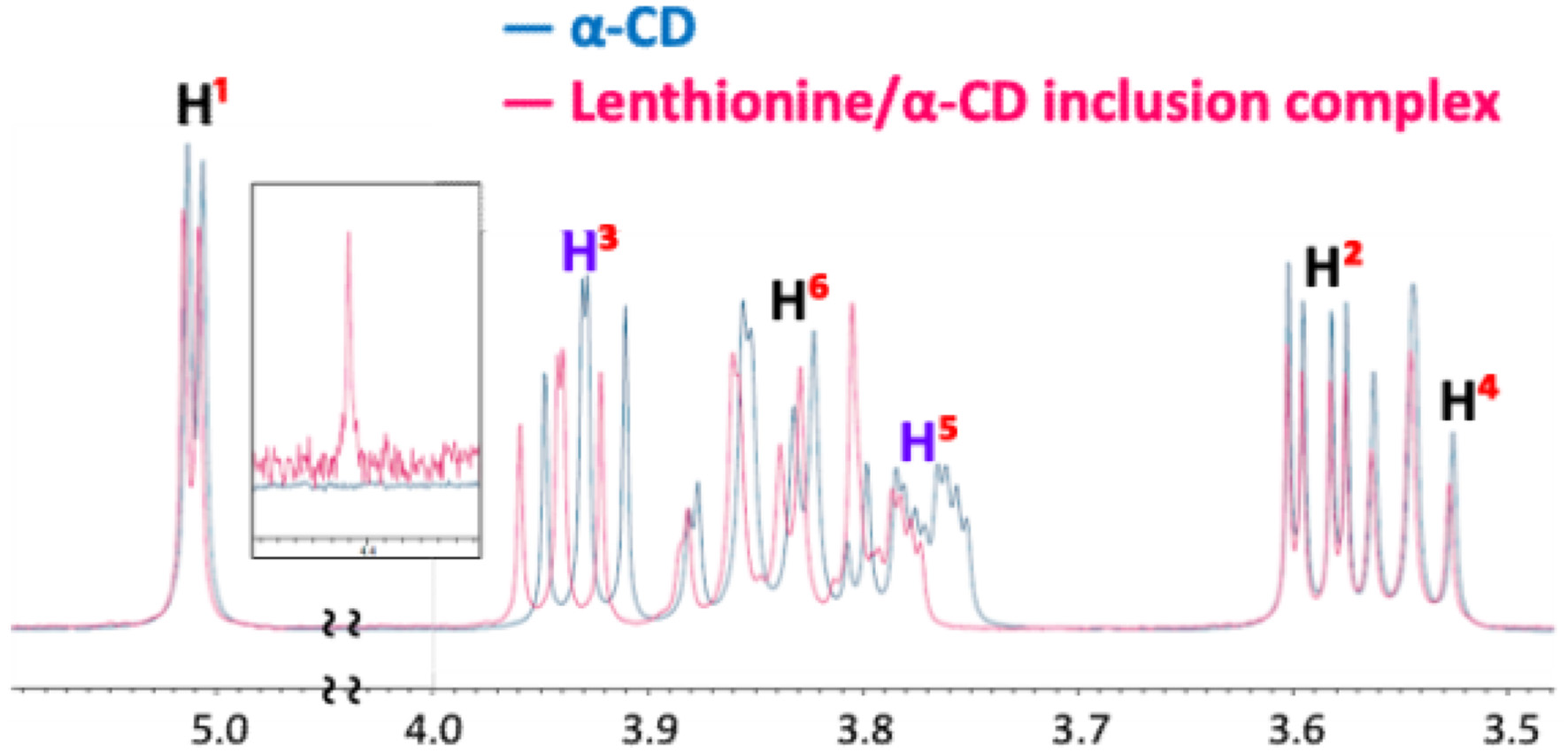 Click for large image | Figure 4. 1H NMR spectral data of α-CD and the lenthionine/α-CD inclusion complex. |
 Click to view | Table 1. Chemical shifts (δ) of α-CD and the lenthionine/α-CD inclusion complex |
3.3. Effect of α-CD on suppressing the release of lenthionine
The release of lenthionine included in α-CD was determined by GC/MS. The volatile amount of lenthionine alone without the addition of α-CD was 0.99 ± 0.05 μmol, while that with the addition of α-CD at a molar ratio of 1:1 was 1.14 ± 0.08 μmol, indicating that α-CD did not suppress lenthionine release. Conversely, when the molar ratio of α-CD : lenthionine was 1:2 or higher, its release was significantly reduced to 0.51 ± 0.20 μmol at 1:2, 0.37 ± 0.08 μmol at 1:3, and 0.40 ± 0.12 μmol at 1:5. Therefore, the addition of α-CD at a molar ratio of 1:2 was effective enough to suppress the release of lenthionine (Figure 5).
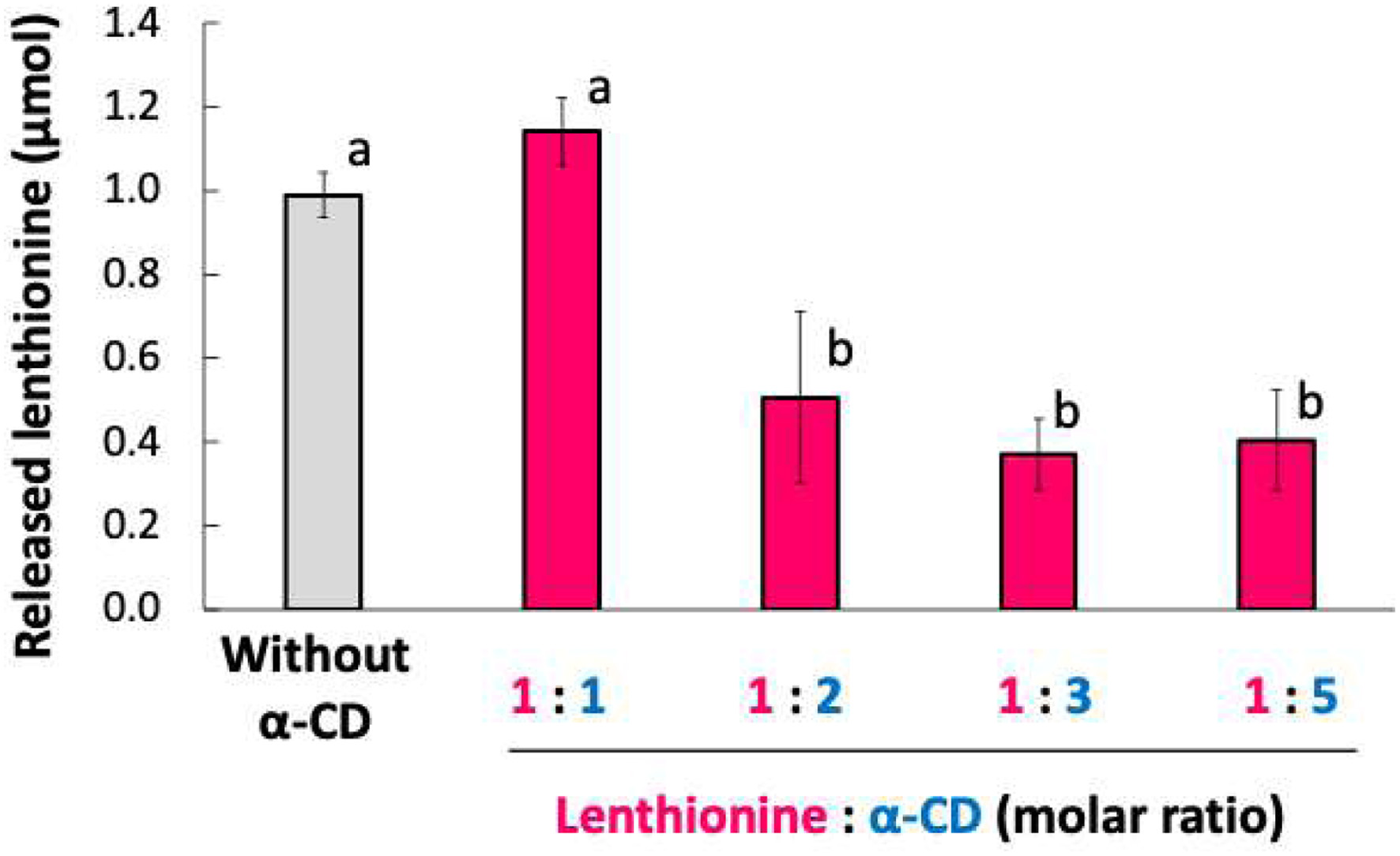 Click for large image | Figure 5. Amount of lenthionine released from the inclusion complex with α-CD. Values are presented as means ± SE of 3 different experiments. Bars with different letters indicate significant differeneces at p < 0.01 according to Tukey-Kramer test. |
3.4. Inhibition of platelet aggregation by the lenthionine/α-CD inclusion complex
Lenthionine included in α-CD was orally administered to rats, and the inhibitory effect against platelet aggregation was measured. The administration of 100 mg/kg body weight of lenthionine at a molar ratio of lenthionine to α-CD of 1:2 to rats over time revealed that the inhibitory effect of the lenthionine/α-CD inclusion complex was the highest between 4 and 8 h after the oral administration (Figure 6). In our previous study, the administration of lenthionine alone resulted in the highest inhibitory levels at 8 to 16 h after administration (Shimada et al., 2008). These findings suggest that the lenthionine/α-CD inclusion complex resulted in a more rapid effect than lenthionine alone. When lenthionine included in α-CD was orally administered to rats at doses of 0, 0.01, 0.1, 1, 10, and 100 mg lenthionine/kg body weight, the inhibition percent significantly increased at doses of 1 mg lenthionine/kg body weight or greater compared to that of the control. The inhibition percent of the lenthionine/α-CD inclusion complex against platelet aggregation was approximately 5 to 10% higher than that of lenthionine alone (Shimada et al., 2008) (Figure 7).
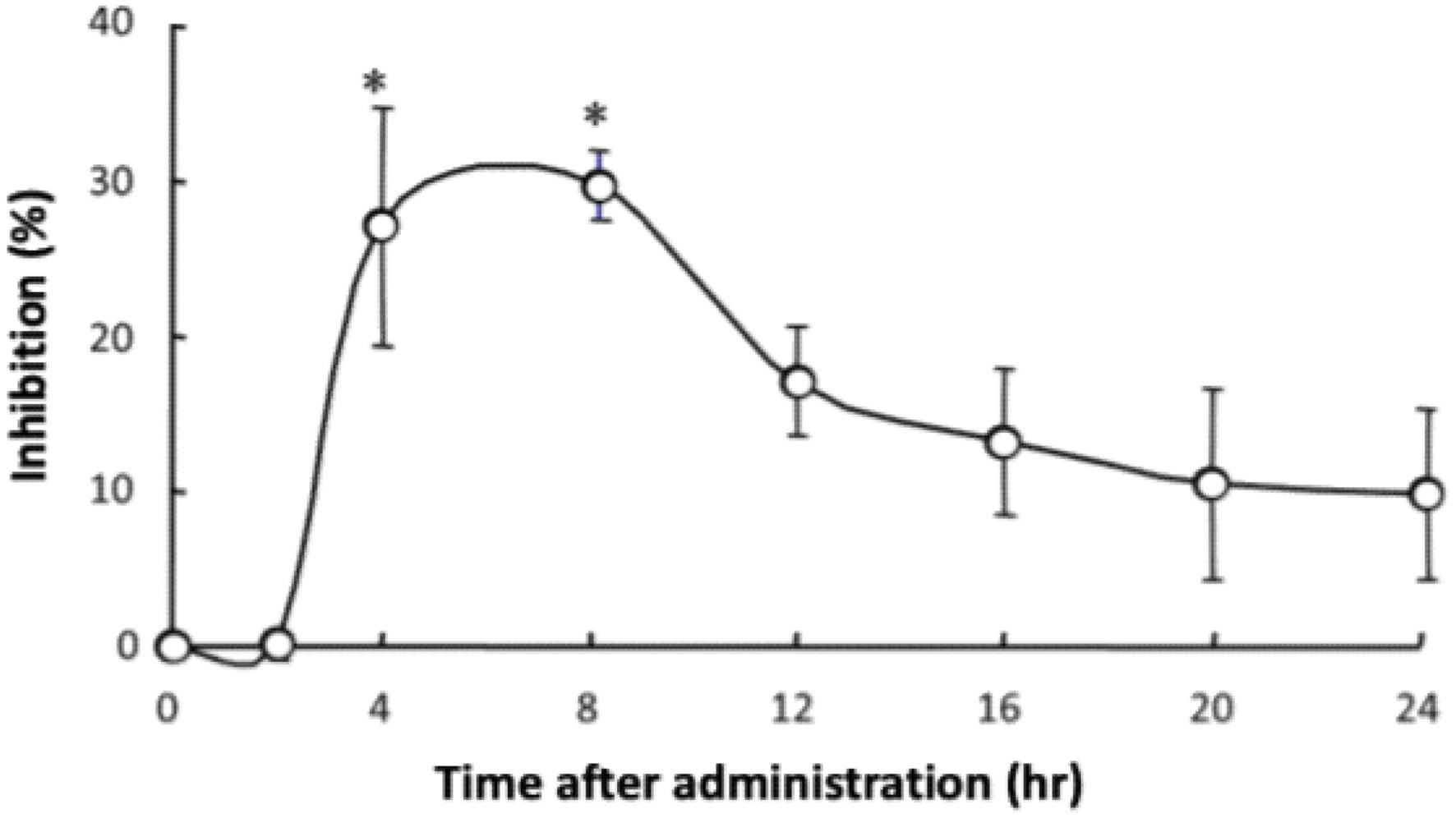 Click for large image | Figure 6. Time-dependent changes in the inhibitory effect of the lenthionine/α-CD inclusion complex on platelet aggregation after oral administration. Values are presented as means ± SE of 5 different experiments. * represents a significant difference from the 0-h group at p < 0.01. |
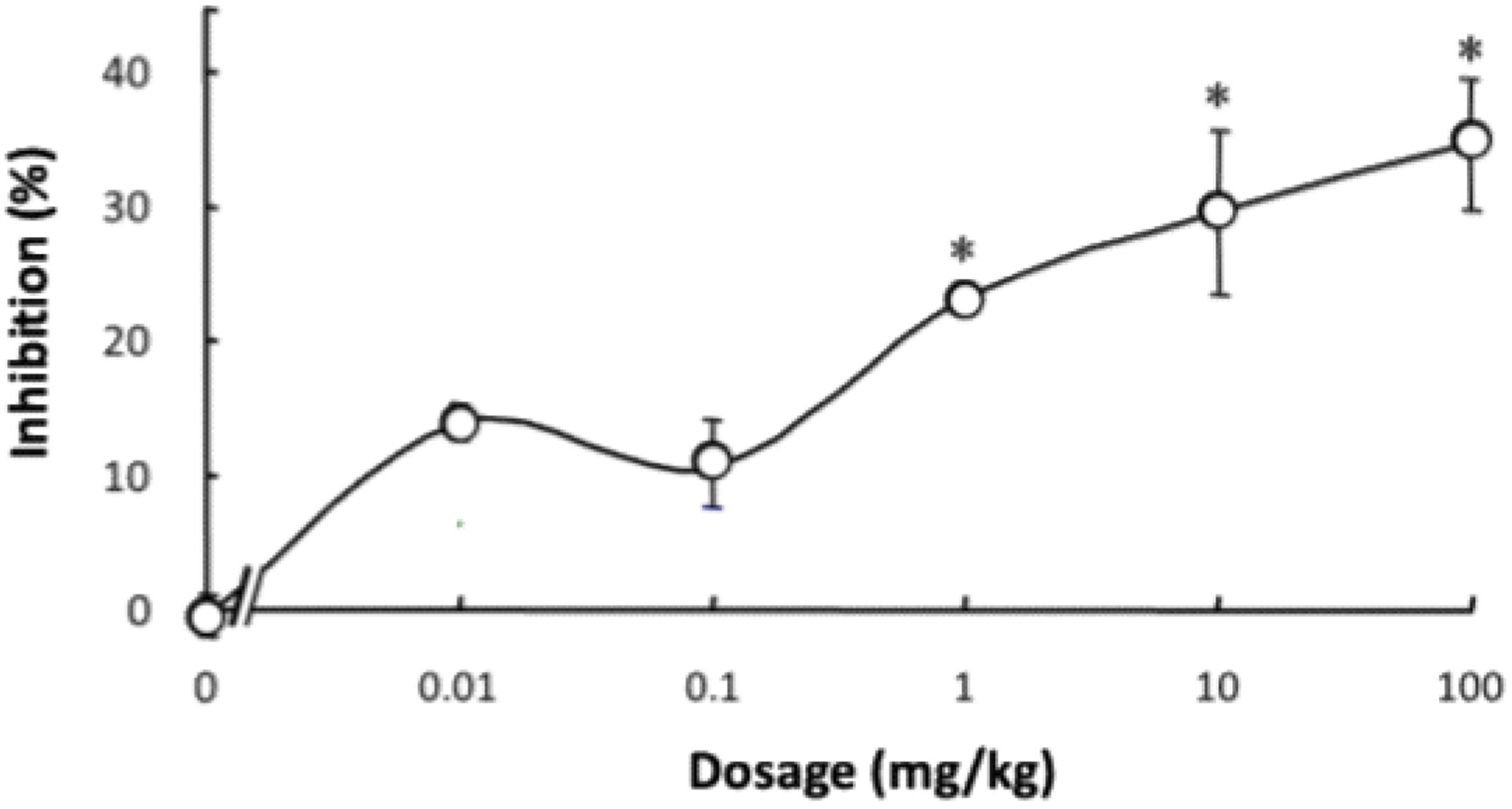 Click for large image | Figure 7. Dose-dependent changes in the inhibitory effect of the lenthionine/α-CD inclusion complex on platelet aggregation after oral administration. Values are presented as means ± SE of 5 different experiments. * represents a significant difference from the 0-mg/kg group at p < 0.01. |
| 4. Discussion | ▴Top |
Shiitake mushrooms, known for their distinctive flavours, are widely used in traditional Japanese and Chinese cuisine. Lenthionine, a cyclic polysulphur compound with disulphide and trisulphide, is a key component replicating the flavour of shiitake mushrooms. Certain sulphuric compounds in food are known to exhibit physiological activities. Sulphides and sulphoxides in the Allium genus such as garlic and onion (Ariga and Seki, 2006; Yamaguchi and Kumagai, 2020, Yamaguchi et al., 2021, 2023) and isothiocyanates in the Brassicaceae family such as broccoli and radishes (Kumagai et al., 1994; Fahey et al., 1997; Yamaguchi et al., 2024) exert various functions in disease prevention. We reported that lenthionine inhibits platelet aggregation both in vitro and in vivo by suppressing the αIIBβ3 activation (Shimada et al., 2004, 2008, 2024) and induces phase II detoxification enzymes (Katayama et al., 2012). However, its distinctive flavour sometimes limits its use in food.
CDs, cyclic oligosaccharides with 6–8 glucopyranoside units, facilitate the formation of non-covalent host–guest inclusion complexes in their hydrophobic inner cavities (Figure 1). Such host–guest inclusion complexes may enhance solubility and bioavailability, augment the physicochemical stability of the guest molecule, diminish or eliminate disagreeable taste or odour, prevent drug–drug or drug–excipient interactions, and convert liquid drugs to microcrystalline or amorphous powders compared to the attributes of guest molecules alone (Carneiro et al., 2019). This study investigated the mechanism of lenthionine inclusion in α-CD, the suppressive effect of inclusion on release of lenthionine, and the in vivo effect of lenthionine included in α-CD on inhibition of platelet aggregation.
The observed decrease in the concentration of α-CD in the supernatant was attributed to the inclusion of lenthionine in α-CD and the subsequent precipitation of the resulting inclusion complex. To ascertain the inclusion ratio of lenthionine and α-CD, solutions comprising α-CD and lenthionine at various ratios were prepared. The concentration of α-CD that was not utilised in the inclusion was then determined through the phenol-sulphuric acid method. The amount of α-CD remaining in the supernatant decreased in proportion to the amount of lenthionine added, and the quantity of α-CD used for inclusion was twice the amount of lenthionine introduced, irrespective of the quantity of lenthionine employed. These results demonstrated that the inclusion ratio of lenthionine and α-CD was 1:2 (Figure 2).
We investigated the interaction of the lenthionine molecule with α-CD using diffusion-ordered spectroscopy (DOSY) and proton nuclear magnetic resonance (1H NMR) spectroscopy. DOSY NMR is a powerful tool for characterising host–guest interactions, and its application to cyclodextrin inclusion complexes has been well established in both food and pharmaceutical research (Pessine et al., 2012). DOSY NMR measures diffusion coefficients alongside 1H NMR spectra, and identical diffusion coefficients for both host and guest molecules indicate the formation of a strong inclusion complex. In this study, we observed identical diffusion coefficients for α-CD and lenthionine, suggesting that α-CD forms a stable complex with lenthionine when mixed in aqueous solution (Figure 3). The inclusion of lenthionine to α-CD was further investigated by analysing changes in the chemical shift of protons of α-CD. The inclusion of a guest molecule within the hydrophobic cavity of α-CD leads to changes in the chemical shift values of the protons in the α-CD molecule as observed in the 1H NMR spectrum. This change was attributed to the interactions between α-CD and the guest molecule. In the structure of α-CD, protons H3 and H5 are located within the hydrophobic cavity, with H3 positioned near the wider rim and H5 near the narrower rim of the α-CD molecule. In contrast, the remaining protons (H1, H2, and H4) are situated on the exterior of the α-CD molecule. Therefore, upon the inclusion of a guest molecule in the α-CD cavity, interactions occur between the guest molecule and the inner cavity protons, particularly H3 and H5, resulting in a change in their chemical shifts (Saha et al., 2016). The chemical shifts in the NMR spectrum can be influenced by both shielding and de-shielding effects. A high-field shift of H3 and H5 occurs due to shielding, while a low-field shift is caused by de-shielding effects from the guest molecule (Wood et al., 1977). Sulphur atoms such as those present in lenthionine are known to induce de-shielding effects on nearby protons. This is evidenced by comparing the 1H NMR spectra of cycloheptane and lenthionine. Both molecules possess a similar cyclic structure; however, in lenthionine, sulphur atoms replace some of the carbon atoms present in cycloheptane. This substitution leads to the protons in lenthionine exhibiting downfield shifts relative to those in cycloheptane, highlighting the de-shielding effect caused by the sulphur atoms. In our study, we observed that the H3 and H5 protons of α-CD were shifted downfield, while the other protons exhibited minimal shifts (Figure 4, Table 1). These results suggest that upon inclusion, the sulphur atoms of lenthionine are in close proximity to the inner cavity protons of α-CD, causing de-shielding effects and supporting the formation of the inclusion complex.
Furthermore, the inclusion of lenthionine in α-CD was substantiated by the release of lenthionine. Release of lenthionine was significantly suppressed when the ratio of lenthionine to α-CD was 1:2 or greater (Figure 5). The lack of change in the amount of release observed with the ratio of lenthionine to α-CD = 1:1 would be due to the presence of free lenthionine diffused into the headspace being uninhibited. Thus far, the results have demonstrated that a ratio of 1:2 between lenthionine and α-CD is optimal, and this result supports the aforementioned conclusion.
According to the World Health Organization’s Global Health Estimates for 2019, cardiovascular diseases, including ischaemic heart disease and stroke, are identified as the primary causes of mortality globally. These diseases are frequently associated with thrombosis, which is precipitated by platelet aggregation (Li et al., 2010; Bledzka et al., 2013; Estevez and Du, 2017; Shin et al., 2017; Chen and Ju, 2020; Lordan et al., 2021; Harishkumar et al., 2022). Consequently, inhibiting platelet aggregation has been recognised as a crucial strategy for the prevention and management of atherothrombotic disorders (Jackson, 2007). Our previous research has demonstrated that lenthionine inhibits platelet aggregation both in vitro and in vivo (Shimada et al., 2004, 2008, 2024). However, it has not been confirmed whether lenthionine included in α-CD exerts the same antiplatelet effects as those of unincluded lenthionine. Therefore, we administered the lenthionine/α-CD inclusion complex to rats and investigated its inhibitory effect on platelet aggregation. The orally administered lenthionine/α-CD inclusion complex demonstrated notable inhibitory activity against platelet aggregation between 4 and 8 h following administration, with the greatest efficacy observed at the 4-h mark (0.1 ± 1.0% at 2 h, 27.3 ± 7.7% at 4 h, 30.0 ± 2.3% at 8 h, 17.3 ± 3.5% at 12 h, 13.3 ± 4.7% at 16 h, 10.6 ± 6.3% at 20 h and 9.8 ± 5.6% at 24 h) (Figure 6). Previous research has demonstrated that when lenthionine is administered alone, a significant inhibitory effect on platelet aggregation is observed between 8 and 20 h after administration, with a peak at 12 h (Shimada et al., 2008). The inhibition percent of lenthionine alone against platelet aggregation was −1.1 ± 3.5% at 2 h, 6.7 ± 1.3% at 4 h, 21.6 ± 1.6% at 8 h, 27.4 ± 1.5% at 12 h, 19.2 ± 2.4% at 16 h, 13.2 ± 1.7% at 20 h and 7.9 ± 1.6% at 24 h. In comparison, lenthionine included in α-CD exerted its inhibitory effect more rapidly indicating that the lenthionine/α-CD inclusion complex was absorbed from the small intestine faster than lenthionine alone. As the enhanced solubility and dispersibility of compounds with low solubility can facilitate improved bioavailability (Sarabia-Vallejo et al., 2023), the inclusion of lenthionine in α-CD may have improved its dispersibility and enhanced its absorption from the intestinal tract. Furthermore, the effective dose of the lenthionine/α-CD inclusion complex was determined by orally administering it to rats at doses of 0, 0.01, 0.1, 1, 10, and 100 mg/kg and measuring the inhibitory effect on platelet aggregation. The data demonstrated that the lenthionine/α-CD inclusion complex exerted a significant inhibitory effect on platelet aggregation at doses of 1 mg/kg or greater compared to the control (−0.5 ± 1.6% at 0 mg/kg, 13.9 ± 1.4% at 0.01 mg/kg, 10.5 ± 3.7% at 0.1 mg/kg, 23.3 ± 1.2% at 1 mg/kg, 29.6 ± 6.2% at 10 mg/kg and 34.7 ± 4.9% at 100 mg/kg) (Figure 7). This effect was observed to be 5–10% higher than the effect of unincluded lenthionine (−1.2 ± 0.8% at 0 mg/kg, 4.9 ± 0.7% at 0.01 mg/kg, 4.6 ± 1.5% at 0.1 mg/kg, 12.8 ± 0.9% at 1 mg/kg, 20.0 ± 3.0% at 10 mg/kg, and 27.4 ± 1.5% at 100 mg/kg) (Shimada et al., 2008). These findings demonstrate that the inclusion of lenthionine in α-CD is effective for improving its bioavailability.
| 5. Conclusion | ▴Top |
In conclusion, lenthionine forms a stable inclusion complex with α-CD at a molar ratio of 1:2, as evidenced by the phenol-sulphuric acid method. DOSY and 1H NMR spectroscopy revealed that lenthionine molecule interacted with protons of α-CD in the hydrophobic inner cavity. The inclusion of lenthionine in α-CD resulted in enhanced stability and reduced release, and this is essential for its utilisation in food products. Moreover, the lenthionine/α-CD inclusion complex exhibited a more rapid and potent inhibitory effect on platelet aggregation compared to lenthionine alone, the maximum inhibitory effect being observed 4 to 8 h after administration. This indicates that the inclusion of lenthionine in α-CD enhances bioavailability and facilitates faster absorption. Furthermore, the lenthionine/α-CD inclusion complex exerted a notable inhibitory effect on platelet aggregation at doses as low as 1 mg/kg, with an overall enhancement of 5–10% in efficacy compared to unincluded lenthionine. These findings illustrate the potential of α-CD to augment the functional attributes of lenthionine, thereby rendering it a promising strategy for enhancing the bioavailability and stability of bioactive compounds in food and pharmaceutical applications.
| References | ▴Top |