| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 28, December 2024, pages 59-67
Lifespan extension and suppression effects of fermented botanical product on the impairment of locomotor activity and accumulation of aggregates containing ubiquitinated proteins in the muscle of Drosophila aging-accelerated models
Dat Tuan Lea, Takumi Suizub, Kotaro Fujiokab, Hideto Toriib, Yoshihiro H. Inouea, *
aBiomedical Research Center, Kyoto Institute of Technology, Matsugasaki, Gosyokaido-cho, Sakyo-ku, Kyoto 606-0962, Japan
bManda Fermentation Co., Ltd., Onomichi, Hiroshima, Japan
*Corresponding author: Yoshihiro H. Inoue, Biomedical Research Center, Kyoto Institute of Technology, Matsugasaki, Gosyokaido-cho, Sakyo-ku, Kyoto 606-0962, Japan. E-mail: yhinoue@kit.ac.jp
DOI: 10.26599/JFB.2024.95028396
Received: November 20, 2024
Revised received & accepted: December 20, 2024
| Abstract | ▴Top |
Although many health foods and their active ingredients have been identified based on their antioxidant activities using biochemical and cell culture analyses, the potential activities are easily lost during gastrointestinal digestion. The lifespan of Drosophila adults who consumed the diet supplemented with diluted fermented botanical product (FBP) was significantly extended. FBP consumption partially suppressed the oxidative stress-induced reduction in viability and epithelial disorders in the flies’ digestive tracts. Although locomotor activity gradually declined with age, FBP significantly suppressed the age-dependent impairment of activity. Accumulation of ubiquitinated protein aggregates containing damaged and no longer needed proteins was significantly suppressed in flies continuously fed FBP after eclosion. Suppression was also observed in flies that were aged to a certain extent, equivalent to middle-aged humans. Our results suggest that FBP exerts antioxidant and antiaging effects in Drosophila. Hyperactivation of the transcription factor Nrf2 is not involved in the antioxidant effects of FBP.
Keywords: Functional food; FBP; Antioxidant effect; Antiaging effect; Drosophila
| 1. Introduction | ▴Top |
Although many foods and their ingredients have been argued to have antiaging and life-extending effects, some claims are based on the fact that they have antioxidant properties that can reduce oxidative damage, which is considered a factor in the progression of aging (Miyazawa et al., 2022). Their antioxidant activity and capacity are often lost during gastrointestinal digestion. Biological assays using living organisms are indispensable for assessing the effects of aging and extension of life. In this study, we investigated whether a fermented food product called fermented botanical product (FBP) can extend the lifespan of living organisms and delay their aging phenotypes. FBP is a fermented supplemental food available commercially, which is made from the following 53 botanic raw materials that have been fermented for more than three years; fruits (apples, persimmons, bananas, pineapples, grapes, chocolate vine fruits, figs, silvervine, crimson glory vine, red bayberries, strawberries, Japanese-apricots), cereals (rice, brown rice, glutinous rice, foxtail millet, common millet, barley, corn), citrus fruits (oranges, citrus-hassaku, navel oranges, citrus-iyokan, lemons, citrus-natsumikan, citrus-kabosu, kumquats, pomelos, citrus-ponkan, citrus-yuzu), pulses (soybeans, black sesame seeds, white sesame seeds, black soybeans), root vegetables (carrots, garlic, burdock roots, lily bulbs, lotus roots), seaweed (seaweed hijiki, sea mustard, laver seaweed, green laver seaweeds, seaweed kelp), honey paste, walnuts, potato starch, cucumbers, celery, perilla leaf, and sugars (brown sugar, fructose powder, glucose powder) (Ashida et al., 2002; Shimada et al., 2004). Not only the FBP effect that inactivates the influenza virus in the culture medium (Nomura et al., 2017), but also the ability of FBP to improve animal and human health through oral consumption has been previously described. For example, the diet supplemental FBP improves growth without harmful effects on broilers (Lokaewmanee et al., 2012) and alleviates weaning stress in piglets (Sukemori et al., 2021). Furthermore, FBP consumption improved emotional stress-induced stomach ulcers and oxidative stress-induced neuronal damage in rats (Kawai et al., 1998; Yang et al., 2015). FBP consumption activates innate immunity and prevents hemolysis and lipid peroxidation in cultured fish (Ashida et al., 2002). It also attenuated allergic symptoms in a mouse model of cedar pollinosis (Fujimura et al., 2018). FBP consumption has been shown to have significant antioxidant effects in mice (Kim et al., 1998). Anticarcinogenic effects of FBP on human breast cancer cells have also been reported (Marotta et al., 2009). Although FBP is expected to have various health-promoting effects and improve immune function, its effects on aging and lifespan have not been investigated. Bioassays using mammals such as mice and rats are time-consuming and incur considerable experimental costs. Therefore, this study used Drosophila as an experimental animal model to analyze whether FBP has antioxidant and antiaging effects. We investigated whether it could extend the lifespan of living organisms and delay the appearance of age-related phenotypes.
The vinegar fly Drosophila melanogaster has been used as the most useful genetic model owing to its short life cycle, high fecundity, and availability of advanced genetic techniques (Ashburner et al., 2005). Drosophila also has numerous advantages in discovery of foods and medicines good for health, particularly as a model for studies on antiaging foods and substances (Staats et al., 2018; Piper and Partridge, 2018). Our Drosophila assay is a useful and sensitive method that allows us to monitor the antioxidative and antiaging effects that suppress the aging progression of functional foods and medicines (Oka et al., 2015; Le et al., 2019; Zheng et al., 2020; Le and Inoue, 2021; Suzuta et al., 2022; Tsuji et al., 2024). We have previously characterized a hypomorphic mutant of Sod1 encoding Cu/Zn superoxide dismutase that eliminates superoxide radicals (Oka et al., 2015). This Drosophila senescence-accelerated model, the Sod1n1 mutant, was used to examine the lifespan extension and antiaging effects of FBP. This mutant showed considerably reduced superoxide dismutase (SOD) activity due to amino acid substitutions, without reduced mRNA levels. Our previous studies demonstrated that this fly model displays several aging phenotypes earlier than wild-type flies. This useful tester strain allows for the identification of antiaging substances contained in functional foods (Oka et al., 2015; Le et al., 2019; Zheng et al., 2020; Le and Inoue, 2021; Suzuta et al., 2022). We used this homozygous mutant for the lifespan and climbing assays. To examine the phenotypes of certain adult tissues under oxidative stress conditions, we performed depletion of the Sod1 gene at the adult stage. We used the Gal4/UAS system, which is an ectopic gene expression system used in Drosophila (Brand and Perrimon, 1993).
Using these fly stocks, we established an accelerated aging model that allowed us to analyze aging-related phenotypes even in the earlier adult stages. As flies age, they display impaired locomotor activity, accumulation of abnormal protein aggregates containing ubiquitinated proteins in the muscle, and loss of dopaminergic neurons in the brain (Le et al., 2019; Le and Inoue, 2021). By examining these aging phenotypes, it is possible to estimate the extent of aging progression in these tissues. Aging begins when adults reach adulthood (eclosion) and ends their lifespan. The adult lifespan can be modified by genetic changes or drugs in this organism (Oka et al., 2015; Le et al., 2019; Le and Inoue, 2021; Suzuta et al., 2022). As the adult lifespan of Drosophila is shorter than that of mice (several years in mice vs. 70 days in Drosophila), the effect of lifespan extension can be evaluated over a shorter period. In the current study, we used a Sod1 mutant that is sensitive to oxidative stress. It has an even shorter adult lifespan (two weeks on average) and exhibits an aging phenotype early in adulthood (Oka et al. 2015). In this study, we fed test flies a diet supplemented with diluted FBP and compared their loss of viability to that of control flies fed a diet without FBP. In addition, we evaluated the antiaging effects of FBP on adult Drosophila. We examined whether it could suppress age-dependent impairment of locomotor activity and the muscle aging phenotype, in which ubiquitinated protein aggregates containing damaged and no longer needed proteins accumulate with aging. In particular, we investigated whether FBP has an antimuscle-aging effect in middle-aged flies. This is a novel supplemental food showing antiaging effect on animals that have already aged. Furthermore, we examined whether it could suppress aging-and oxidative damage-induced phenotypes in midgut epithelia. Finally, to understand the mechanism by which FBP exhibits antiaging and antioxidative stress effects in Drosophila adults, we investigated whether FBP consumption influences the activation of the Nrf2 transcription factor, which is critical for the elimination of oxidative stress.
| 2. Materials and method | ▴Top |
2.1. Fly stocks and husbandry
w was used as the normal control stock (Le et al., 2019). As the Drosophila senescence-accelerated model, the Sod1n1 mutant, was used to examine the lifespan extension and antiaging effects of FBP (Oka et al., 2015, Le et al., 2019). To deplete Sod1 mRNA in adult muscles, UAS-Sod1RNAiF103 stock (Oka et al., 2015) and Mef2-Gal4 as a Gal4 driver stock (Le et al., 2019; Suzuta et al., 2022) were used and designated as Mef2 > Sod1RNAi (muscle-specific Sod1 depletion). To monitor ARE-dependent transcription by Nrf2 transcription factor, ARE-GFP reporter (ARE-GFP), in which GFP cDNA was inserted after the ARE sequences, was used (Le and Inoue, 2021, Tsuji et al., 2024).
A standard fly diet, which contained 7.2 g agar, 100 g glucose, 40 g dried yeast, and 40 g cornmeal per liter were prepared as described previously (Ozaki et al., 2022). All fly stocks were maintained on a regular cornmeal diet at 25°C, with the exception of depletion and overexpression experiments that were performed at 28°C.
2.2. FBP feeding
Young adults from the assay stock were collected within 24 hours of eclosion, as previously described (Le et al., 2019; Le and Inoue, 2021; Suzuta et al., 2022). Twenty flies were reared in a single plastic vial containing Drosophila instant medium (Formula 4-24® Instant Drosophila medium, Blue; Carolina Biological Supply Company, Burlington, NC, USA). FBP was fully diluted and added to at the low concentrations, final concentrations of 0.05, 0.5, and 2 mg/mL in the instant medium. The diet prepared in the instant medium contained sufficient nutrients. To feed the flies on the instant medium supplemented with diluted FBP solution at 25°C, the vials with the diet were replaced every 2–3 day. To raise the flies at 28°C and induce ectopic expression via the UAS-Gal4 system, food vials were changed every 2 day.
2.3. Lifespan assay
The lifespan-extension effect of FBP was confirmed in Sod1n1 mutant fly, which is a Drosophila senescence-accelerated model. The lifespan assay was performed as previously described (Oka et al., 2015; Le et al., 2019; Zheng et al., 2020; Suzuta et al., 2022). Young adults were collected within 24 hours after eclosion. Twenty flies were reared in a single plastic vial containing the Drosophila instant medium. The instant medium without FBP was used as a control. The assay was repeated five to seven times (using to 100-140 flies from each assay stock). Dead adults in each vial were scored every 12 hour. Food vials were changed every three day. Survival curves were analyzed using the Kaplan-Meier test as described previously (Le et al., 2019; Zheng et al., 2020; Suzuta et al., 2022).
2.4. Climbing assays
Climbing assays utilizing the fly’s instinct characteristics of negative geotaxis were performed according to a previously described protocol with slight modifications (Oka et al., 2015; Le et al., 2019; Ozaki et al., 2022). Here, we investigated the rapid loss of locomotor activity in adult Drosophila under oxidative stress. Newly eclosed Mef2>Sod1RNAi adult males, which harbor the reduced level of mRNA encoding Cu/Zn SOD in their muscle, were collected and raised on a diet containing 0.5 mg/mL FBP or without FBP (control). Twenty flies of the same age were placed in a plastic culture tube and the climbing activity of each fly was quantified as described previously (Le et al., 2019). The mean scores of the five repeated trials (100 flies) are presented.
2.4. Immunostaining procedures in muscle and midgut
For muscle immunohistochemistry, young flies were collected within 24 hours after eclosion and raised on diets supplemented with or without diluted FBP as a control. A thin thoracic block containing indirect flight muscles was collected from the abdomen (Oka et al., 2015; Ozaki et al., 2022) and fixed in paraformaldehyde. To identify abnormal protein aggregates, FK2 monoclonal antibody against ubiquitinated proteins (Enzo Life Sciences, Farmingdale, NY, USA) and Alexa Fluor 488-conjugated secondary antibody (Thermo Fisher Scientific, Tokyo, Japan) were used. One fluorescence image per fly was observed, and the pixels in one optic field (4.0 × 10−2 mm2) were measured using a confocal microscope (Fv10i, Olympus, Tokyo, Japan).
To assess the extent of oxidative damage in the adult midgut, extra intestinal stem cells (ISC), which were generated to regenerate damaged epithelial cells, was assessed. Normal flies were subjected to extrinsic oxidative stress by feeding them paraquat. Young flies expressing GFP in the ISC were collected, aged on a standard fly diet for 22 days, and fed instant medium supplemented with 2 mg/mL FBP or without FBP (control) for 10 days at 28°C. The flies were incubated in 10 mM paraquat for 24 hours. Midguts from adult flies were prepared and fixed, as previously described (Le et al., 2019). For quantitative analysis of cell numbers in the adult midgut, the number of DAPI-positive (total cells) and esg-positive cells (corresponding to ISCs) in an area of the posterior midgut were counted using ImageJ software (NIH, Bethesda, MD, USA). Samples were observed under an Olympus laser scanning confocal microscope (Fv10i; Olympus, Tokyo, Japan).
To monitor the Nrf2 activation dependent on the ARE (Antioxidative Responsible Element) by GFP fluorescence in the whole bodies (Chatterjee and Bohmann, 2012), the young ARE-GFP flies within a day after eclosion were fed with the instant medium supplemented with 0.5 mg/mL FBP, or without FBP for 10 days. GFP fluorescence was observed under a stereofluorescence microscope (SZX7; Olympus, Tokyo, Japan).
2.5. Quantitative reverse transcription polymerase chain reaction
For quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis, total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) from the whole bodies of adult flies fed diets supplemented with or without 0.5 mg/mL FBP for 10 days. cDNA synthesis and qRT-PCR were performed as described previously (Le and Inoue, 2021). The following primers were used: RP49-Fw, 5′-TTCCTGGTGCACAACGTG-3′, RP49-Rv, 5′-TCTCCTTGCGCTTCTTGG-3′, GFP-Fw, 5′-CCACGCTGGATAGGAGTTGAG-3′, GFP-Rv, 5′-ACAAGATCCTTCTGATGGCCG-3′, Nqo1-Fw, 5′-ATCTGGCCACTGACTTTCGG-3′, Nqo1-Rv, 5′-GATGCACGGAACACAACACC-3′, Gclm-Fw, 5′-AGGATTCCAACGTCAGCAGG-3′, Gclm-Rv, 5′-AATCTGCTGCTTGAGGGCAT-3′. All qRT-PCR experiments were performed in triplicate, and the average of the three replicates in each group was considered. The ΔΔCt method was used to determine differences in target gene expression relative to the reference Rp49 gene expression (Tsuji et al. 2024).
2.6. Statistical Analysis
The significance of the differences in the survival curves between the groups was analyzed using the log-rank test. For comparisons between two groups, we used the Student’s t-test. One-way ANOVA followed by the Bonferroni post-hoc test was used to assess the differences between more than two groups. Two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test was performed to compare the mean differences between groups that were split into two independent variables. Data were considered significant at p < 0.05. Statistical analyses were performed using GraphPad Prism, version 9 (GraphPad Software, San Diego, CA, USA).
| 3. Results and discussion | ▴Top |
3.1. The lifespan extension effects of the diluted fermented botanical product (FBP) on Drosophila adults
To investigate whether the consumption of FBP influences the lifespan of living animals, we fed young adults of the aging-accelerated mutant Sod1n1, collected within 24 hours after eclosion, on instant medium supplemented with FBP at lower concentrations of 0.05, 0.5, and 2 mg/ml and examined the survival rate every 12 hour. Adults of this bioassay strain have a short lifespan. The mean time required for 50% lethality in the absence of the diluted FBP was an average of 5.5 days. By contrast, the times of the adults fed on the diets supplemented with FBP at concentrations of 0.05 and 0.5 mg/ml extended to 8.5 and 6.5 days on average, respectively (Figure 1). Adults fed the most concentrated FBP (2 mg/ml FBP) required 7.5 days. Adults with the longest lifespans survived for 26, 23, and 18 days in the case of 0.05, 0.5, and 2 mg/ml FBP consumption, respectively, whereas the longest-lived non-FBP-feeding controls survived for only 16 days. Comparison of lifespan curves showed that FBP consumption at concentrations of 0.05, 0.5, and 2 mg/ml significantly extended the mean lifespan compared to that of the diet without FBP. The differences in lifespan extension were statistically significant in every concentration (p<0.01, p<0.001, and p<0.001, respectively, log-rank test) (Figure 1), although dose dependence of FBP on lifespan extension was not observed. We mainly applied FBP at 0.5 mg/ml concentration for future experiments.
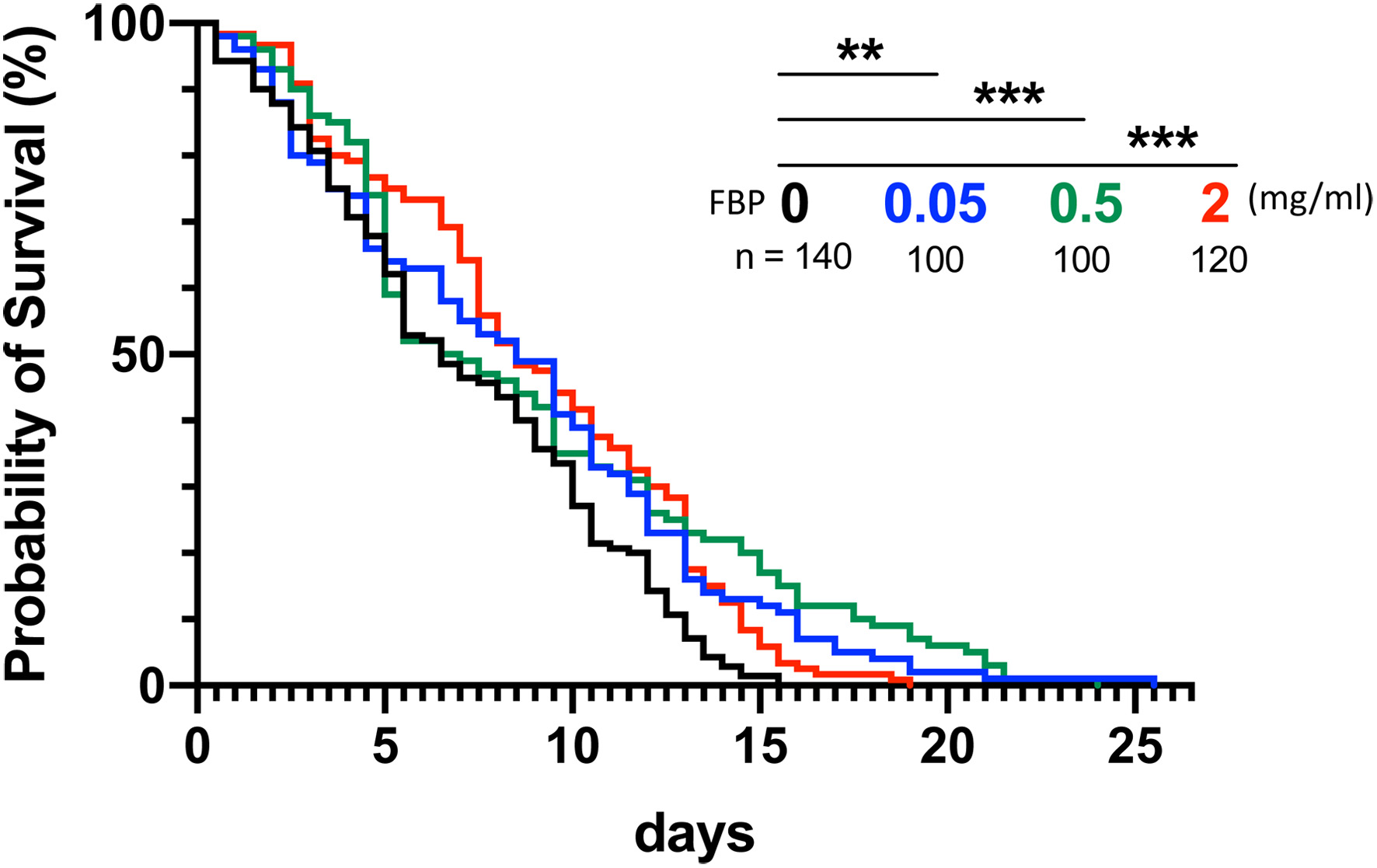 Click for large image | Figure 1. Extension of the lifespan in the Drosophila senescence-accelerated model flies by FBP administration. (A) Lifespan curves in the adult flies fed on the Drosophila instant medium without Fermented Botanical Product (FBP) (0 mg/mL, control, black) or that supplemented with FBP 0.05 mg/mL (blue), 0.5 mg/mL (green), 2 mg/mL (red) of FBP, n ≥ 100 for each. Among twenty young flies collected within 24 hours after eclosion in each vial, dead adults were scored every 12 hour. The results represent the average of five or more than five repeated experiments. Curves were plotted using Kaplan-Meier survival analysis. A log-rank test was performed for adults fed with the control diet and the FBP-containing diet. Note that a significant lifespan extension was observed in every FBP concentration, ns not significant, * p < 0.05, ** p < 0.01, *** p < 0.001, log-rank test. |
Similarly, the hot water extract of Chlorella pyrenoidosa (WEC) extends the lifespan of the Drosophila aging-accelerated model, Sod1n1 flies, at low concentrations (Zheng et al., 2020). However, it remains unclear whether WEC delayed the aging phenotype in this Drosophila model. The substrate responsible for the lifespan-extending effect was purified using biochemical methods, and one substrate was identified as phenethylamine. Administration of this substance at concentrations comparable to those present in WEC extended the lifespan of Drosophila adults. Moreover, the oral administration of low amounts of WEC and phenethylamine to mice alleviated high fat diet-induced liver damage by regulating methylglyoxal via an increase in glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Zheng et al., 2021). It is plausible that FBP may contain a substance that exerts a similar effect. In the future, it will be possible to identify the responsible substance(s) using this Drosophila assay system. It would also be interesting to investigate whether FBP exerts similar lifespan-extension and antiaging effects in mammalian models.
3.2. The FBP consumption resulted in a tolerance against oxidative stress in Drosophila adults
Next, to investigate whether FBP has antioxidant effects that reduce oxidative stress in Drosophila adults, we fed young adults from the test strain, which were collected within 24 hours after eclosion, with a diet containing 0.1 mM and 1 mM paraquat (under oxidative stress) and examined adult survival rates every 12 hour (Figure S1). When reared in the presence of 0.1 mM paraquat, half of the control adults fed no FBP died at 7 days, and all until 13.5 days after the start of feeding. In contrast, half of the adults fed 0.05 mg/ml FBP survived until 9.5 days and half of the adults fed 2 mg/ml FB survived until 8 days. In the presence of a higher concentration of paraquat (1 mM), half of the adults died by 2.5 days and all died by 7 days after the start of feeding. Half of the adults fed 0.05 mg/ml FBP survived for 3.5 days and the longest survivors survived for 7.5 days. When 0.5 mg/ml and 2 mg/ml FBP were administered, several adults among >100 adults survived for up to 9 and 10 days. These oxidative stress assays suggested that FBP consumption at 0.05 mg/ml and 2 mg/ml has an antioxidant effect of reducing oxidative stress in the presence of 0.1 mM and 1 mM paraquat. The difference in survival rates between the flies fed 2 mg/ml FBP in 0.1 mM paraquat and those fed the diet only in 0.1mM paraquat was statistically significant (Figure S1A). The difference between the flies fed 0.05 mg/ml FBP and those fed the diet only in 1 mM paraquat was also significant (Figure S1B). In contrast, the intermediate concentration, 0.5 mg/ml, of FBP did not show this effect.
The fact that FBP consumption at these concentrations resulted in increased tolerance to paraquat is consistent with a previous report that FBP contains antioxidant substance(s) that scavenge free radicals (Kawai et al., 1998). However, the observed tolerance to paraquat was not concentration-dependent. This requires further investigation, and the antioxidant effect of FBP should be re-examined using wild-type Drosophila strains, as well as the optimal concentration of paraquat for this experiment.
3.3. Suppression effect of FBP on the age-dependent decline in the locomotor activity of Drosophila adults accumulating excess oxidative stress in their muscle
The locomotor activity of the assay flies accumulating excess oxidative stress in their muscle due to tissue-specific depletion of superoxide dismutase (Mef2>Sod1) was quantified using the climbing assay immediately after eclosion (within 24 hours) to 35 days, when 50% survival was reached. The diet supplemented with 0.5 mg/ml FBP was provided. The score for quantifying the locomotor activity was calculated for each trial (containing 20 flies, n > 100 flies in total), as shown (Figure 2). In both the control and FBP-feeding experiments, adult locomotor activity gradually decreased over time compared to immediately after eclosion; the decrease was less pronounced in the FBP-fed group than in the non-FBP-fed group. There were also significant differences in the time (p < 0.0001) and processing-time interactions (p < 0.0001) (two-way ANOVA with Tukey’s multiple comparisons).
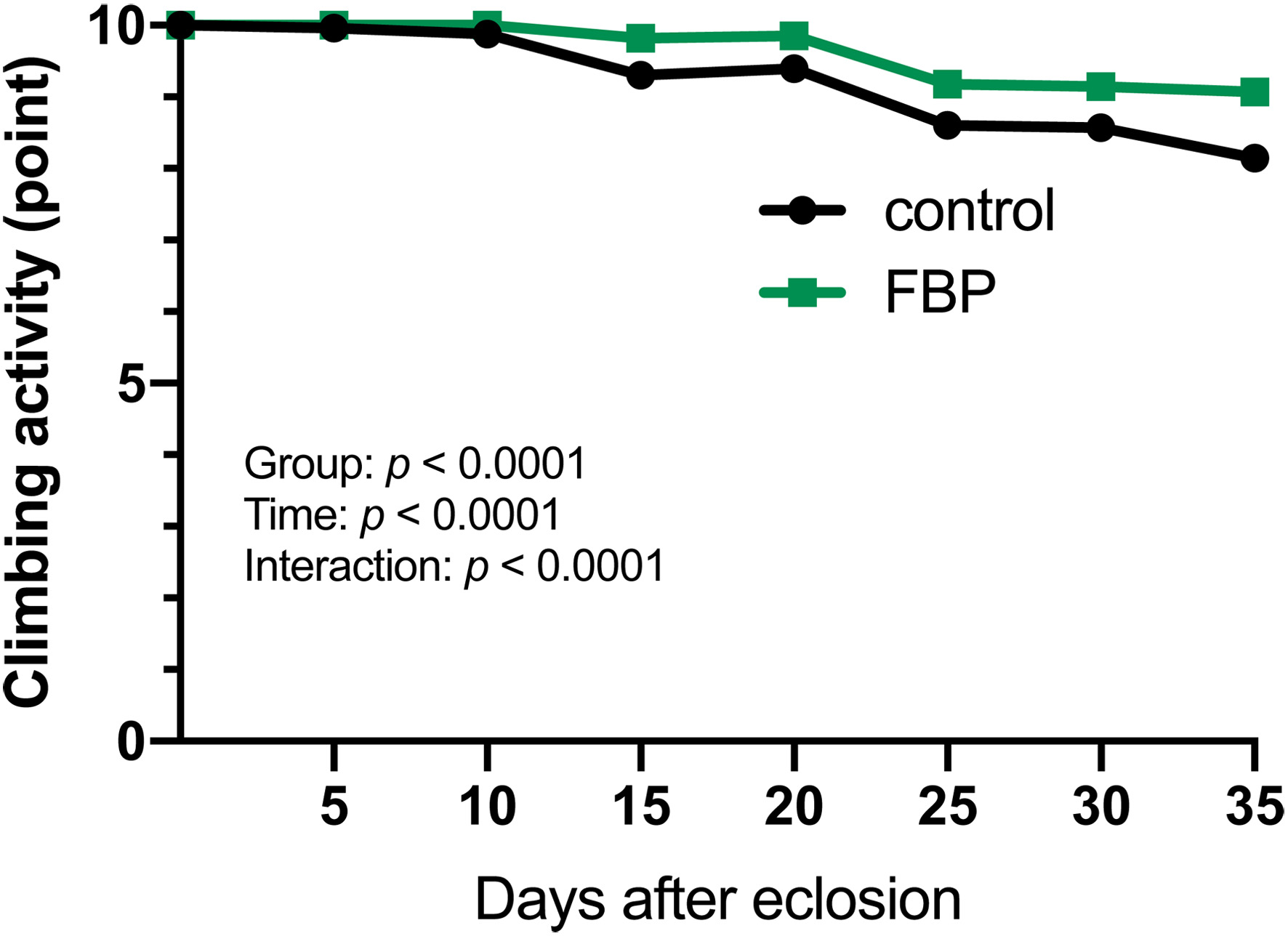 Click for large image | Figure 2. Climbing assay of adults harboring muscle-specific accumulation of oxidative stress by depletion of Sod1 mRNA. Climbing assay to quantify the locomotor activity of adults harboring muscle-specific silencing of mRNAs encoding Superoxiside dismutase 1 (Sod1) (Mef2>Sod1RNAi). Young adults were raised on a diet without FBP (control, black line) or 0.5 mg/mL FBP (green line). Climbing activity was examined every 5 day until 35 days after eclosion (n = 100 from five repeated assays). The points on the y-axis represent the mean climbing scores, which reflect the locomotor activity of the flies. Two-way ANOVA with Tukey’s multiple comparison test. There are significant differences (p < 0.0001) between the treatments for those fed with or without FBP. There are also significant differences in time (p < 0.0001) and treatment-time interactions (p < 0.0001). |
In Drosophila adults, accumulating oxidative stress in the muscles causes locomotor activity to decline over time (Le et al., 2019; Suzuta et al., 2022; this study). However, this decline was partially suppressed in adults fed 0.5 mg/ml FBP. There are two possibilities for the target tissue: acting on the muscles or the nervous system of the brain. For example, sesamin affects both the tissues (Le et al., 2019; Le and Inoue, 2021). To obtain further evidence concerning the antiaging effects of FBP and to identify its target tissues, we next examined whether the accumulation of ubiquitinated aggregates in adult muscles with age could be suppressed by FBP feeding.
3.4. FBP suppressed the accumulation of ubiquitinated protein aggregates reflecting the age- and/or oxidative stress-dependent impairment of protein homeostasis
As FBP exhibited lifespan extension and antioxidant effects in Drosophila adults, we investigated whether FBP consumption also suppressed the aging phenotype that appeared in the muscle. To add muscle-specific oxidative stress to flies, which makes protein homeostasis easier to observe, flies harboring muscle-specific depletion of Sod1gene were used (Oka, et al., 2015; Le et al., 2019; Suzuta et al., 2022). We fed the flies a diet supplemented with FBP (0.5 mg/ml) and counted the number of ubiquitinated protein aggregates that accumulated in the indirect flight muscles as they aged (Demontis and Perrimon, 2010; Oka et al., 2015). Ubiquitinated aggregates that accumulated in adult muscles at 5 days (B), 20 days (D), and 30 days after eclosion, close to the mean lifespan of the knockdown flies (F), were observed under confocal microscopy. The number of aggregates was counted in the fluorescence images. An average of 700 pixels of ubiquitinated aggregates per confocal microscopic field was observed at 5 days after eclosion, 1,200 pixels at 20 days, and 3,200 pixels at 30 days in adults fed the diet without FBP. The number of aggregates increases with age. In contrast, muscle samples were prepared from flies fed 0.05, 0.5, and 2 mg/ml FBP. Adults were fed a diet containing FBP for 5, 20, and 30 days after eclosion. The results showed that the average number of aggregates was lower in adults fed FBP at every concentration than in adults reared without FBP at the same age (Figure 3B, D, F). The differences in numbers were statistically significant (p < 0.01∼0.0001, one-way ANOVA). Thus, age-dependent accumulation of ubiquitinated protein aggregates in adult muscles was suppressed by FBP consumption.
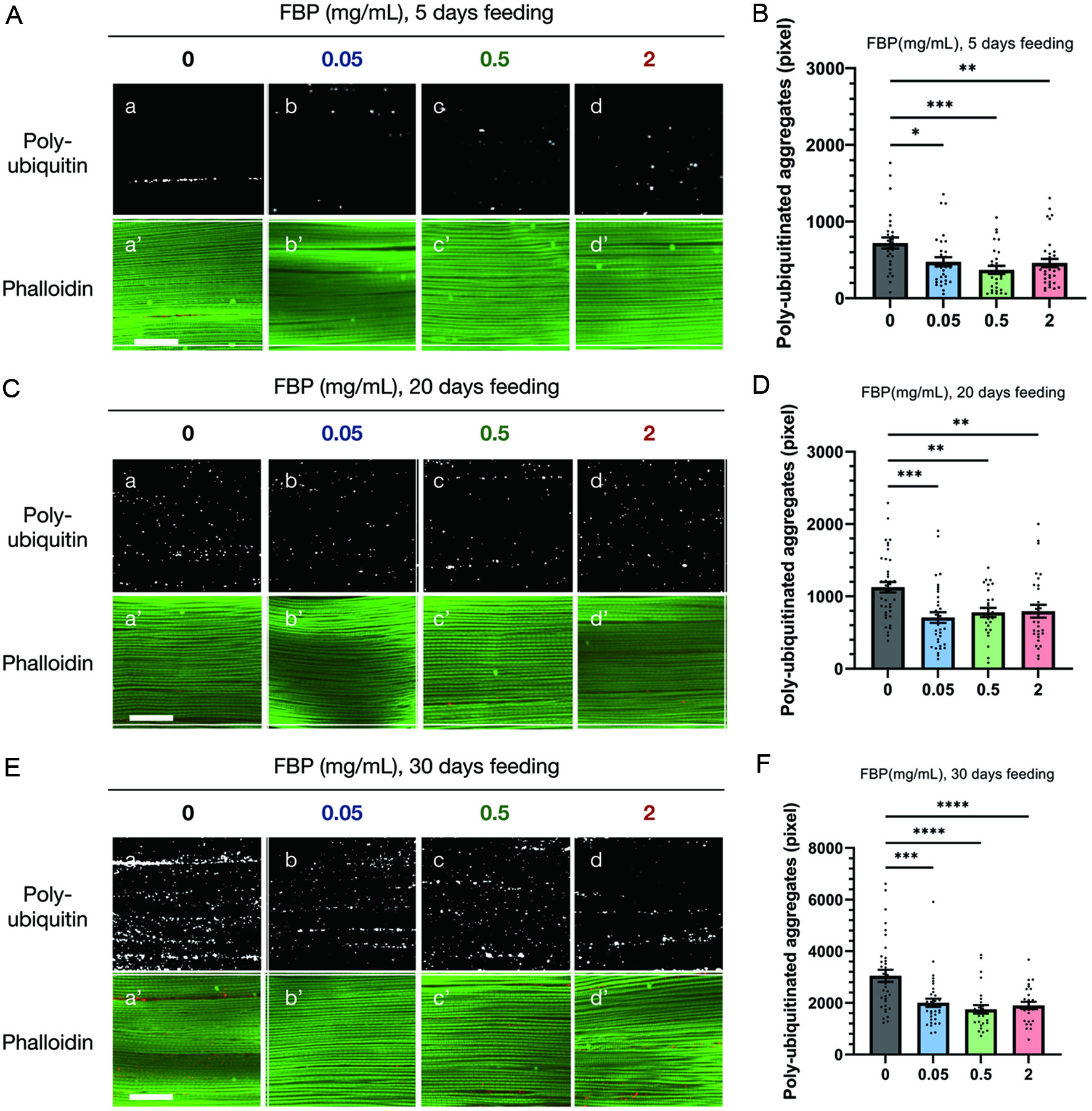 Click for large image | Figure 3. FBP suppresses the accumulation of ubiquitinated protein aggregates in the myofibrils of the indirect flight muscles. (A, C, E) Immunostaining of the indirect flight muscles collected from flies with a muscle-specific depletion of Sod1 (Mef2>Sod1RNAi) using an anti-ubiquitin-conjugated antibody (white) (a-d), and phalloidin staining for F-actin (green) (a′-d′). Young flies, collected within 24 hours after eclosion, were aged for 5 (A, B), 20 (C, D), and 30 (E, F) days on a diet supplemented without FBP (control) (a-a′), or with 0.05 (b-b′), 0.5 (c-c′), and 2 mg/mL FBP (d-d). Scale bar represents 30 μm. (B, D, F) The average pixel total areas of the sum of protein aggregates containing ubiquitinated proteins per single confocal optic fields in control (grey bars), 0.05 mg/mL (blue bars), 0.5 mg/mL (green bars), and 2 mg/mL (red bars) FBP-fed flies are shown on the y-axis. *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001. One-way ANOVA followed by the Bonferroni post-hoc test was used to assess the differences. The error bars represent the standard error of the mean (SEM). |
Accumulation of ubiquitinated aggregates in adult muscles with age indicates a decline in the ability of muscle cells to maintain protein homeostasis. Consequently, the extent of this accumulation has been used to estimate the progression of muscle aging (Oka et al., 2015; Le and Inoue, 2021; Ozaki et al., 2022; Suzuta et al., 2022). These results suggest that FBP has the potential to inhibit this accumulation, thereby preventing muscle aging. If an equivalent system that can quantify the progression of muscle aging by measuring similar biomarkers in mammalian models (Lai et al. 2024) would be available, it is valuable to investigate the effects of FBP in mammals.
3.5. Suppression effect of FBP on the muscle aging phenotype in aged Drosophila adults equivalent to middle age in human
As described above, the continuous consumption of FBP from the young adult stage suppressed the muscle aging phenotype. Next, we investigated whether FBP exhibits a suppressive effect in Drosophila adults aged to a certain extent. Young control flies (Mef2>+) were collected within 24 hours after eclosion and reared for 15 days on a standard fly diet without FBP. Then, we gave the flies with the diet supplemented with 0.5 mg/mL of FBP for 5 days or 15 days. After FBP consumption, ubiquitinated protein aggregates in the adult muscle were observed using confocal microscopy (Figure 4A). Ubiquitinated aggregates accumulated in adult muscles at 20 days (15 + 5 days) after eclosion (Figure 4Aa ) and at 30 days (15 + 15 days) (Figure 4Ac). An average of 100 pixels of ubiquitinated aggregates per confocal microscopic field was observed at 20 days and 1,300 pixels at 30 days in normal adults fed a diet without FBP. These numbers increased with age (Figure 4B). In contrast, 40 pixels of the aggregates on average were counted in the 15-day-old flies fed with 0.5 mg/ml FBP for 5 days and 460 pixels for 15 days. The 15-day-old adults were fed a diet containing FBP for 5 and 15 days (Figure 4Ab, Ad, B). The differences in aggregate size between normal flies fed a diet without FBP and those fed a diet supplemented with FBP were statistically significant (p < 0.0001, one-way ANOVA and Bonferroni post-hoc test). These data suggested that 0.5 mg/ml FBP suppressed the accumulation of ubiquitinated protein aggregates, reflecting reduced protein homeostasis.
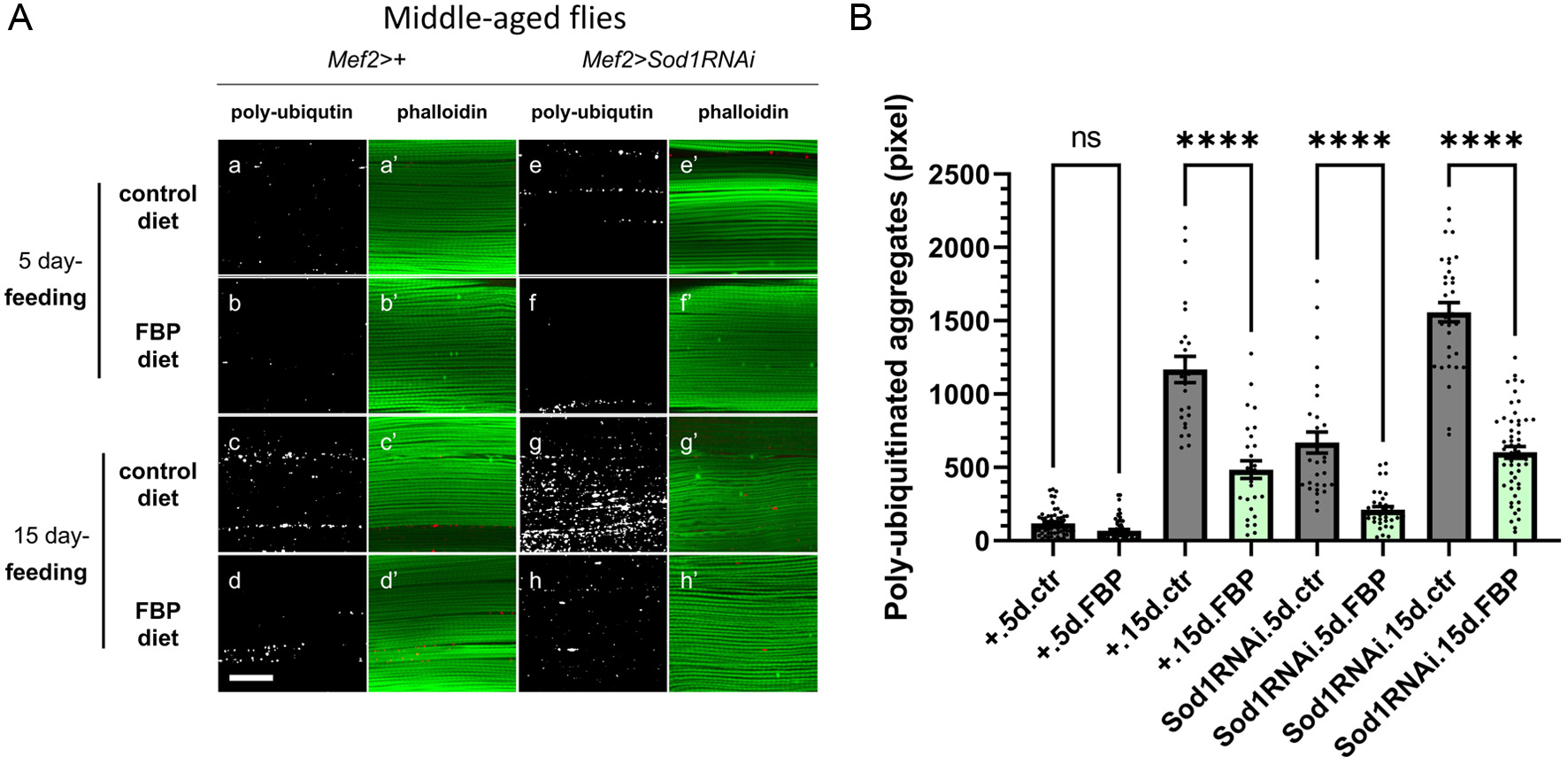 Click for large image | Figure 4. FBP suppresses the accumulation of ubiquitinated protein aggregates in the myofibrils of the indirect flight muscles in middle adult age. (A) Immunostaining of the indirect flight muscles dissected from normal flies (Mef2>+) (Aa-Ad′) or flies with additional oxidative stress accumulation in muscle by the Sod1 depletion (Mef2>Sod1RNAi) (Ae-Ah′) using an anti-ubiquitin-conjugated antibody (white) (Aa-Ah), and phalloidin staining for F-actin (green) (Aa′-Ah′). Young flies, collected within 24 hours after eclosion, were aged on a standard fly food without FBP for 15 days, and then aged on a diet supplemented with or without FBP for 5 (Aa-Ab′, Ae-Af′) or 15 (Ac-Ad′, Ag-Ah′) days. Flies were raised on a diet supplemented without FBP (control) (Aa-Aa′, Ae-Ae, ′ Ac-Ac′, Ag-Ag′), or with 0.5 mg/mL FBP (Ab-Ab′, Af-Af′, Ad-Ad′, Ah-Ah′). Scale bar represents 30 μm. (B) The average pixel total areas of the sum of protein aggregates containing ubiquitinated proteins per single confocal optic field in control (grey bars), 0.5 mg/mL FBP (green bars)-fed flies are shown on the y-axis. One-way ANOVA followed by the Bonferroni post-hoc test was used to assess differences, ns; not significant, ****p < 0.0001. The error bars represent the SEM. |
Additionally, we investigated the effect of FBP on the accumulation of ubiquitinated protein aggregates in adults with excessive muscle oxidative stress caused by Sod1 depletion (Mef2>Sod1RNAi). On average, 520 pixels of the aggregates were counted in the 15-day-old flies fed without FBP for 5-day-old (Figure 4Ae). 1,530 pixels for 15-day-old (Figure 4Ag). Ubiquitinated aggregates in the muscle of 15-day-old adults fed 0.5 mg/ml FBP for 5 days and 15 days decreased in size (from 500 pixels to 180 pixels for 5-day consumption, from 1,500 pixels to 600 pixels for 15-day consumption) (Figure 4Af, Ah, B). The differences in aggregate size between the knockdown flies fed the diet without FBP and those fed with FBP were statistically significant (p < 0.0001, one-way ANOVA and Bonferroni post-hoc test) (Figure 4B). Drosophila adults harboring muscle-specific accumulation of oxidative stress also showed significant inhibition of the muscle-aging phenotype after FBP consumption.
These data indicate that FBP has a suppression effect on the muscle aging phenotype even in adults aged to a certain extent. To date, only a few substances and foods that exhibit antiaging capacity, even in aged animals, have been reported. Recently, Singh and colleagues reported that when middle-aged mice were continuously administered taurine, a substance similar to the amino acids contained in seafood, their lifespan extended. Further, age-dependent impairment of their health indicators, such as bone mass, also improved (Singh et al., 2023). Similarly, the index of muscle aging was reduced by the continuous administration of FBP from middle age. In the future, if the antiaging substances contained in FBP could be identified, it may be possible to prevent the decline in locomotor activity in Drosophila whose aging has already begun, along with taurine. However, this should be further examined in mammalian models.
3.6. Suppression effect of FBP on the oxidative damage phenotype appeared in adult midgut treated with paraquat
FBPs effectively suppressed age-related decline in locomotor activity and muscle aging of flies. Some fermented foods contain multiple antioxidants and functional substances that alleviate oxidative damage. Thus, we examined its effects on oxidative damage in the digestive tissues (Figure 5). As flies age, epithelial cells in the intestinal gut are frequently damaged by reactive oxygen species used to eliminate microbial infections and oxidants in foods. Intestinal stem cells proliferate to replace the damaged cells. As the number of damaged cells increases in the digestive gut, or with age, the number of intestinal stem cells increases (Le et al., 2019). Epithelial cells in the intestinal tract were observed by confocal microscopy after DAPI staining. Intestinal stem cells were labelled with specific expression of GFP in the stem cells of the midgut (esg>GFP) (Figure 5). To add oxidative stress to the intestinal tract of flies, we administrated 10mM paraquat for 24 hours to normal Drosophila adults instead of genetically manipulating Sod1 gene. In the 30-day-old flies not subjected to oxidative stress by paraquat, FBP had no significant effect on the proliferation of intestinal stem cells during the same period (Figure 5Aa, Ab, B). However, same-aged flies treated with paraquat before observation exhibited a greater increase in intestinal stem cells than those that were not treated with paraquat. The flies that had continuously been fed FBP for 30 days and treated with paraquat possessed fewer stem cells than those only subjected to paraquat treatment (Figure 5Ac, Ad, B). The increase in the stem cells, which reflects the extent of epithelial damage, was suppressed in Drosophila adults fed with FBP. FBP consumption suppresses oxidative damage to the epithelium of the intestinal tract in Drosophila adults.
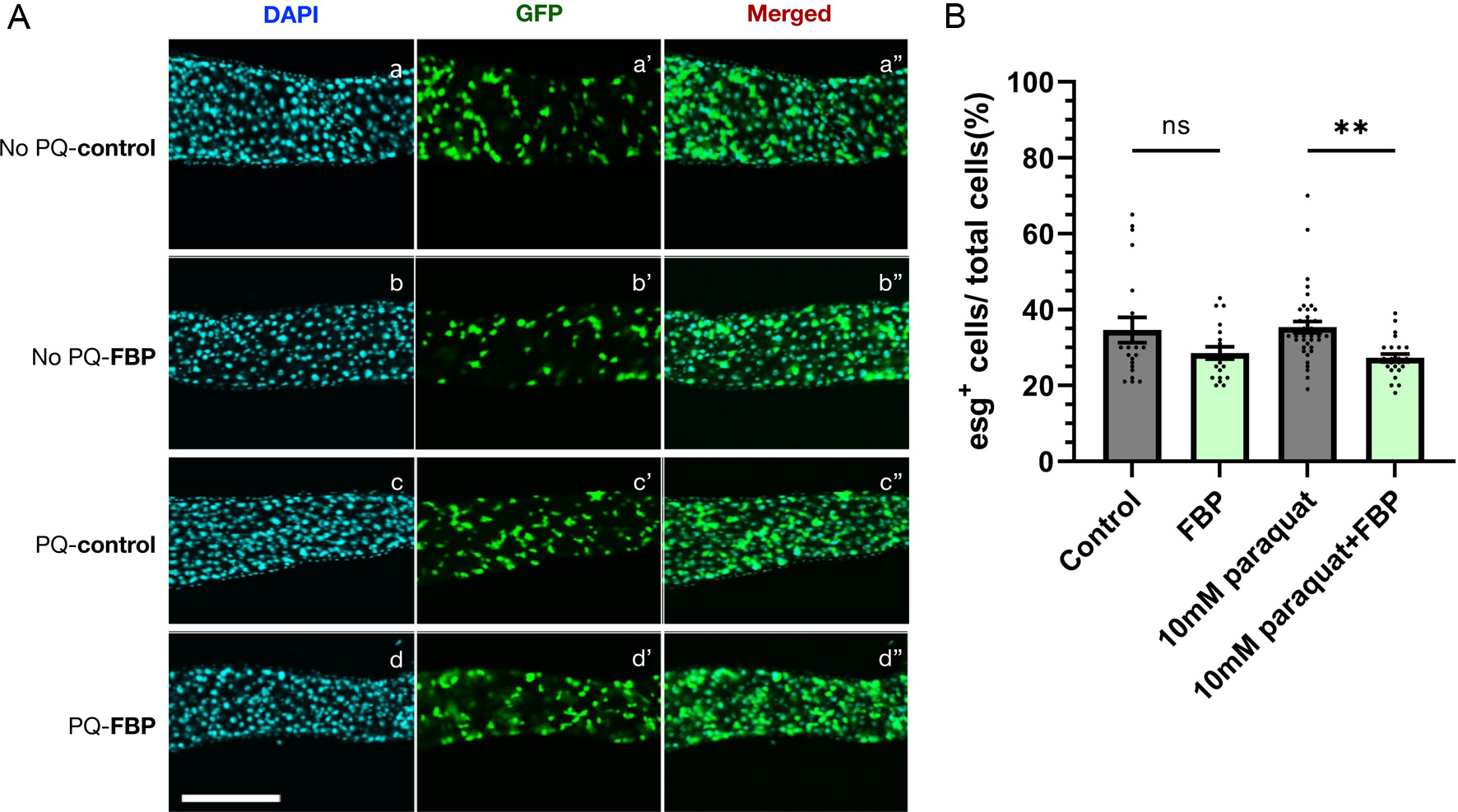 Click for large image | Figure 5. FBP inhibits hyperproliferation of intestine stem cells (ISCs) in intestinal epithelia in aged flies fed with paraquat. (A) Green fluorescence protein (GFP) fluorescence of ISCs expressing GFP in the mid-gut epithelia of esg>GFP flies. Newly eclosed young flies were aged on a standard fly food for 20 days at 28°C, divided into two groups and fed instant medium supplemented with 0.5 mg/mL FBP (Ab-Ab″), or without FBP (Aa-Aa″) for 10 days. For the paraquat-feeding, the 30-day-old flies were incubated in 10 mM paraquat (Ad-Ad″) or without paraquat (Ac-Ac″) for 24 hours. (B) For a quantitative analysis of cell numbers in the adult midgut, the number of 4′,6-diamidino-2-phenylindole (DAPI)-positive (total cells), esg-positive cells (corresponding to intestinal stem cells (ISC)s) in an area of the posterior midgut were counted. The results are presented as the percentage of esg-positive cells among total cells. ISCs are visualized using esg>GFP reporter (green) and DAPI (blue). Scale bar;100 μm. One-way ANOVA followed by the Bonferroni post-hoc test was applied to assess differences; ns not significant, ** p < 0.01. The error bars represent the SEM. |
In Drosophila adults that were not exposed to paraquat, there had no significant effect on the suppression effect of FBP feeding. However, in flies exposed to oxidative stress, FBP inhibited oxidative damage to the midgut epithelium. We concluded that FBP alleviates oxidative damage in the digestive tract in Drosophila adults. This was consistent with the increased tolerance to paraquat (Figure S1). A previous study reported that sesamin, a lignan abundant in sesame seeds, exhibited a similar effect in suppressing oxidative damage in the Drosophila adult midgut (Le et al., 2019). A similar disorganization of the epithelia occurs during Drosophila aging (Choi et al., 2008). FBP consumption elevates IgA and mucin levels, responsible for intestinal immune and barrier functions, in feces of the high-fat-diet fed rats (Yang et al., 2015). It also suppresses the increased deoxyholate level, which cause oxidative stress and DNA damage in the rats. Consistently, FBP exhibited a similar effect on the gut of Drosophila adults under oxidative stress. It would be interesting to investigate the mechanism of how FBP exhibits a suppressive effect on the tissue damage that occurs in the epithelia of the digestive tract.
3.7. FBP consumption did not activate the transcription factor Nrf2, which is essential for the expression of antioxidant genes
Other antiaging substances identified in natural food products activate the transcription factor Nrf2, which is required for the transcription of antioxidant genes (Sykiotis and Bohmann, 2008; Le and Inoue, 2021). Therefore, we investigated whether FBP contains substances with a similar effect using an ARE-GFP reporter harboring a GFP reporter gene downstream of the ARE sequences to which Nrf2 binds. A weak GFP fluorescence was similarly detected in the head regions of both control and FBP-fed flies, while significant expression was not observed in other body parts (Figure S2A). The expression level of the ARE-GFP reporter was quantified by the intensity of GFP fluorescence and the GFP mRNA levels. The fluorescence levels in whole flies did not change after consumption of the diet containing 0.5 mg/ml FBP for 10 days (Figure S2B). Furthermore, the mRNA levels of Gcml and Nqo1, which are the target genes of Nrf2, were also unchanged after feeding FBP for 10 days (Figure S2C).
These results indicate that FBP consumption does not activate ARE-dependent transcription, which corresponds to Nrf2-dependent transcription. Although the Nrf2-Keap1 system is known to be a master regulator that removes cellular oxidative stress (Vomund et al., 2017), it is also possible that FBP may influence other genes, proteins or biochemical pathways in which this transcription factor is not involved. Alternatively, a fluorescence of the ARE-GFP reporter was observed in a limited area, more posterior part, in the brain of FBP-fed fly (Figure S2), although there was no significant increase in the GFP mRNA levels in adult whole bodies. RNA used in this experiment was extracted from whole bodies. Thus, it is possible that this difference was not readily apparent. To detect tissue-specific expression, quantitative PCR should be performed using RNA prepared from each body part. It is also important to confirm in the next study whether long-term FBP consumption affects Nrf2 activation. If FBP activates Nrf2 in specific tissues, it may act as an antioxidant and antiaging health food via a known mechanism. This issue needs to be investigated in future studies using mammalian models.
| 4. Conclusions | ▴Top |
We investigated whether fermented botanical product (FBP) exerts lifespan extension and antiaging effects in living animals using Drosophila aging-accelerated models. Continuous consumption of diluted FBP extends the lifespan of flies. It provides a partial tolerance to oxidants. Furthermore, it suppressed age-dependent impairment of locomotor activity in flies. The aging phenotype of the muscle was suppressed when FBP was administrated. This suppression was observed even in flies that were aged to a certain extent, equivalent to middle aged in humans. FBP has antioxidant and antiaging effects in Drosophila models. Nrf2 hyperactivation is not involved in the antioxidant effects of FBP.
| Supplementary material | ▴Top |
Figure S1. Survival curves of the Drosophila adults fed diets containing the diluted FBP and the paraquat, which gives oxidative stress. (A) Survival rates of the assay flies fed the diet without FBP (control, black lines), supplemented with FBP at 0.05 (blue), 0.5 (green), 2 mg/mL FBP (red) under a mild oxidative stress (0.1 mM paraquat), n ≥ 100 for each. (B) Survival rates of the assay flies fed the diet without FBP (control, black lines), supplemented with FBP at 0.05 (blue), 0.5 (green), 2 mg/mL FBP (red) under a more severe oxidative stress condition (1 mM paraquat), n ≥ 100 for each. Twenty flies were reared in a single culture vial containing the instant medium. Dead adults in each vial were scored every 12 hour. The results represent the average of five or more than five repeated experiments. Curves were plotted using Kaplan-Meier survival analysis. A log-rank test was performed for each pair of adults fed with the control diet and adults fed with the treatment diet. ns not significant, *p < 0.05, **p < 0.01, ***p < 0.001, log-rank test.
Figure S2. Absence of ARE-dependent Nrf2 activation in Drosophila adults fed FBP by observation of fluorescence intensity of the ARE-GFP reporter and quantification by qRT-PCR. (A) Stereo fluorescence micrographs of ARE-GFP reporter flies. ARE-GFP reporter flies, collected within 2 days after eclosion, were raised for 10 days on a diet supplemented without FBP (control) or with 0.5 mg/mL FBP. (B, C) Quantification of total RNA in whole bodies of male flies fed a diet with or without FBP by qRT-PCR. Each analysis (n > 35 flies) was repeated three times to quantify the mRNA levels of each target gene. Statistical analysis was performed using the Student’s t-test (p = 0.44). (C) One-way ANOVA followed by the Bonferroni post-hoc test was applied to assess the differences between more than two groups (p = 0.7255 for Gclm, p > 0.9999 for Nqo1). ns, not significant. The error bars represent the standard error of the mean (SEM).
Acknowledgments
We thank Y. Yonezu, A. Watanabe, and M. Platt for their technical assistance. We acknowledge the Bloomington Stock Center and Drosophila Genetic Resource Center for providing fly stocks. YHI received a research grant from Manda Fermentation Co., Ltd. and the Joint Research Program of the Biomedical Research Centre at the Kyoto Institute of Technology (2022008).
Conflict of interest
The authors declare that they have no conflict of interest.
| References | ▴Top |