| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 28, December 2024, pages 50-58
Lenthionine, a key flavour compound of shiitake mushrooms, prevents platelet aggregation by inhibiting αIIbβ3 activation
Shoichiro Shimada, Kazuki Kumagai, Soichiro Mochizuki, Ryuji Hirata, Yuki Tanabe, Kyohei Yamada, Yusuke Yamaguchi, Hitomi Kumagai*
Graduate School of Bioresource Sciences, Nihon University, 1866 Kameino, Fujisawa-shi 252-0880, Japan
*Corresponding author: Hitomi Kumagai, Graduate School of Bioresource Sciences, Nihon University, 1866 Kameino, Fujisawa-shi 252-0880, Japan. E-mail: kumagai.hitomi@nihon-u.ac.jp
DOI: 10.26599/JFB.2024.95028395
Received: September 12, 2024
Revised received & accepted: September 20, 2024
| Abstract | ▴Top |
Cardiovascular diseases, which are frequently associated with thrombosis and platelet aggregation, are a significant global health concern. The inhibition of platelet aggregation is a crucial strategy for the prevention of thrombotic disorders. Our previous studies have demonstrated that lenthionine, a cyclic polysulphur compound derived from shiitake mushrooms, possesses platelet anti-aggregatory properties. However, the underlying mechanism of action remains unclear. This study aimed to elucidate the inhibitory effects of lenthionine on platelet aggregation and αIIbβ3 activation. Lenthionine inhibits platelet aggregation induced by various types of agonists. Flow cytometry and scanning electron microscopy revealed that lenthionine suppresses platelet activation and morphological changes. Moreover, lenthionine did not affect the phosphorylation/dephosphorylation of myosin light chain (MLC), cofilin, and talin, but significantly inhibited the dephosphorylation of α-actinin. Our findings suggest that lenthionine may inhibit αIIbβ3 activation by potentially inhibiting the activity of protein disulfide isomerase (PDI), which is crucial for αIIbβ3 activation.
Keywords: Shiitake mushroom; Lenthionine; Cyclic polysulphur compound; Platelet aggregation; αIIbβ3 activation
| 1. Introduction | ▴Top |
According to the WHO Global Health Estimates in 2019, the top global causes of death were cardiovascular diseases such as ischaemic heart disease and stroke, which are often associated with thrombosis triggered by platelet aggregation (Li et al., 2010; Bledzka et al., 2013; Estevez and Du, 2017; Shin et al., 2017; Chen and Ju, 2020; Lordan et al., 2021: Harishkumar et al., 2022). Therefore, inhibition of platelet aggregation has been recognised as an important strategy for the prevention and treatment of atherothrombotic disorders (Jackson, 2007).
Signal transduction for platelet aggregation is summarised in Figure 1. Platelets circulates in the bloodstream in a discoid shape (Shin et al., 2017; Sang et al., 2021). However, when collagen is exposed to the bloodstream, the interaction between the collagen-bound von Willebrand factor (vWF) and glycoprotein (GP) Ib on platelets allows collagen to contact GPVI, triggering Ca2+ signalling (Andrews and Berndt, 2004; Nieswandt et al., 2009). The hydrolysis of phosphatidylinositol 4,5-biphosphate (PIP2) by phospholipase C (PLC) produces inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG) (Kahner et al., 2006). The produced IP3 releases Ca2+ from intracellular stores through IP3 receptors in the endoplasmic reticulum (ER) membrane, whereas DAG activates protein kinase C (PKC) (Kahner et al., 2006; Nieswandt et al., 2009; Ngo et al., 2018).
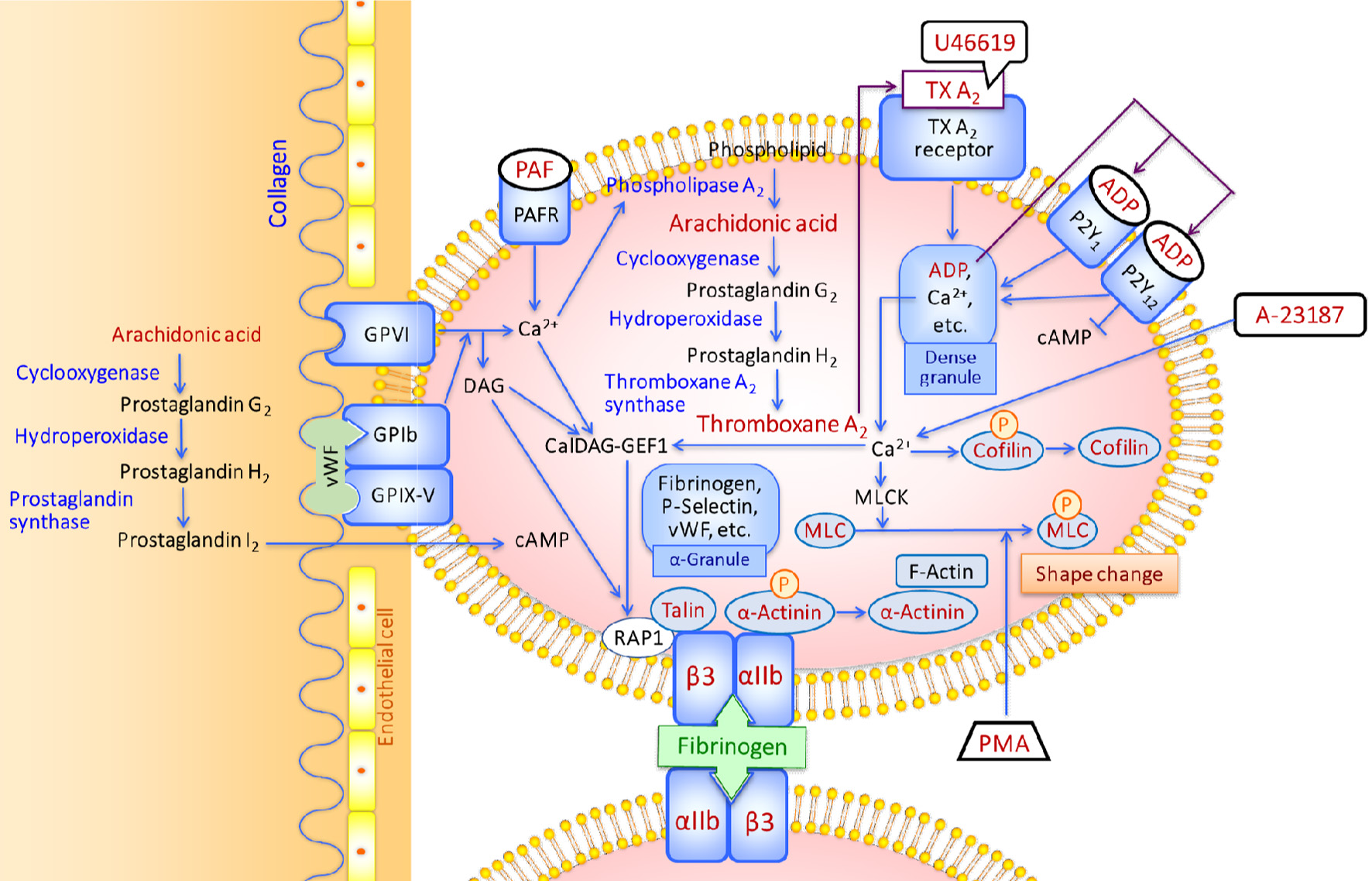 Click for large image | Figure 1. Schematic diagram of platelet activation by various types of agonists. |
An increase in intracellular Ca2+ activates phospholipase A2, which dissociates arachidonic acid (AA) from membrane phospholipids (Lordan et al., 2021; Zhou et al, 2023). AA is converted to prostaglandin H2 by cyclooxygenase-1 (COX-1) and hydroperoxidase and then to thromboxane A2 (TxA2) by thromboxane synthetase (Davì and Patrono, 2007; Lordan et al., 2021; Zhou et al, 2023). Binding of the produced TxA2 with the TxA2 receptor induces the release of the secondary mediators such as adenosine diphosphate (ADP) and Ca2+ from dense granules. ADP binds with P2Y1 and P2Y12 receptors. P2Y1 coupled to G protein regulates Ca2+-depending signalling events, while P2Y12 activates αIIbβ3 by inhibiting cAMP production by adenyl cyclase (Andrews and Berndt, 2004) and induces dense granule release (Manickam et al., 2008; Panova-Noeva et al., 2013).
Elevated calcium concentration in the cytosol induces the phosphorylation and dephosphorylation of proteins. Cofilin, a regulator of actin filament dynamics, is dephosphorylated (Wang et al., 2005; Pandey et al., 2006), and then binds to actin (Shin et al., 2017). In contrast, the myosin light chain (MLC) is phosphorylated and increases actin-activated ATPase activity reflecting the contractile activity of actomyosin and actin polymerisation (Getz et al., 2010; Aburima et al., 2017).
In addition, an increase in the calcium concentration activates Ca2+- and diacylglycerol-regulated guanine nucleotide exchange factor (CalDAG-GEF1), which functions as a GEF for Ras-related protein 1 (Rap1) (Lagarrigue et al., 2020). Rap1 GTPases are the primary regulators of talin-1-dependent integrin activation that occurs by talin-1 binding to the integrin β3 subunit cytoplasmic domain in αIIbβ3 (Ratnikov et al., 2005; Verhaar et al., 2008; Fang et al., 2019; Lagarrigue et al., 2020). The binding of talin-1 disrupts the salt bridge between the transmembrane regions of the α and β integrin subunits, which results in a conformational change in the extracellular domains and ligand binding (Nieswandt et al., 2009; Tadokoro et al., 2011).
The β3 subunit of αIIbβ3 and αvβ3 integrins contains four epidermal growth factor (EGF)-like domains and each domain harbours four disulfide bonds (Mor-Cohen et al., 2012). The disulfide bonds in the EGF domains of β3 are intact to keep αIIbβ3 in an inactive state (Kamata et al., 2004), and disruption of unique disulfide bonds in EGF domains of β3 activates αIIbβ3 (Chen et al., 2001; Essex et al., 2001; Manickam et al., 2008; Mor-Cohen et al., 2012). Upon platelet activation, α-granule releases fibrinogen, vWF, P-selectin, etc. (Grammel et al., 2016). The activated αIIbβ3 serves as a receptor for ligands such as fibrinogen and vWF to form a bridge to other αIIbβ3 on adjacent platelets (Chen et al., 2001; Mor-Cohen et al., 2012; Bledzka et al., 2013), while P-selectin is translocated to the cell surface (Merten and Thiagarajan, 2000). P-Selection interactions between platelets stabilise the initial αIIbβ3-fibrinogen interactions (Vallés et al., 2002).
α-Actinin plays a potential role in setting integrins to a default low-affinity ligand-binding state in resting platelets and regulating αIIbβ3 activation by inside-out signalling. α-Actinin is bound to the integrin cytoplasmic tail in resting platelets (Bozulic et al., 2007; Tadokoro et al., 2011). However, αIIbβ3 activation signals from protease-activated receptors (PARs) dephosphorylate and dissociate α-actinin from αIIbβ3 (Tadokoro et al., 2011). Dephosphorylation of α-actinin is regulated by the protein tyrosine phosphatase (PTP) SHP-1 (also known as PTP1C, SHPTP-1, SHP, HCP, and PTPN6), which is the main PTP expressed in platelets (Tadokoro et al., 2011). α-Actinin also has an N-terminal actin-binding domain (Hampton et al., 2007), and serves as a cross-linker by binding to F-actin of the polymeric filamentous form that contributes to the shape change of platelets (Bearer et al., 2002; Shin et al., 2017). The final activated platelets are spiny spheres with long, thin filopodia that extend several micrometers from the platelets (Shin et al., 2017). When αIIbβ3 activation signalling dwindles, α-actinin becomes rephosphorylated, which decreases the affinity to F-actin and α-actinin reassociates with αIIbβ3 (Tadokoro et al., 2011; Shin et al., 2017).
Platelet aggregation can be inhibited by inhibiting a certain part of these signalling pathways, leading to the prevention of atherothrombotic disorders. Some of the sulphuric compounds from garlic have been reported to inhibit platelet aggregation such as methyl allyl trisulfide (MATS) (Ariga et al., 1981), diallyl trisulfide (DATS) (Hosono et al., 2020), and (E,Z)-4,5,9-trithiadodeca-1,6,11-triene 9-oxide (Ajoene) (Apitz-Castro et al., 1986). These volatile sulphuric flavour compounds are produced from S-allyl-L-cysteine sulfoxide (ACSO) and S-methyl-L-cysteine sulfoxide (MCSO) by C-S lyase when garlic is cut or crushed (Yamaguchi and Kumagai, 2020). Oral administration of ACSO in rats also inhibited platelet aggregation (Akao et al., 2008). MATS suppresses TXA2 production by inhibiting hydroperoxidase activity, which is the second reaction of the bifunctional COX-1 (Ariga and Seki, 2006). Therefore, platelet aggregation is inhibited when AA is used as an agonist to activate platelets but not inhibited when U46619, an analogue of TXA2, is used (Nishimura and Ariga, 1992). Acetylsalicylic acid (aspirin) is known to inhibit the first reaction of COX-1 (Ariga and Seki, 2006), but it also inhibits cyclooxygenase-2 (COX-2) in vessel walls suppressing the production of anti-aggregatory prostaglandin I2 (PGI2), which results in “aspirin dilemma” (Sakata et al., 2013). Therefore, if COX-2 in the vessel walls is more strongly inhibited than COX-1 in platelets, platelet aggregation may occur. Despite the inhibitory activity of MATS against the production of PGI2, its antithrombotic effect prevents thrombus formation (Ariga and Seki, 2006). Unlike MATS, DATS inhibits platelet aggregation even when U46619 is used as the agonist (Hosono et al., 2020). As DATS has high reactivity with SH groups, its inhibitory activity against platelet aggregation is estimated to be due to the covalent binding to the free thiol in integrin αIIbβ3 (Hosono et al., 2020).
Shiitake (Lentinula edodes) is a popular edible mushroom often used in Japanese and Chinese cuisine. The characteristic flavour of shiitake is 1,2,3,5,6-pentathiepane (lenthionine), a volatile cyclic polysulphur compound with disulfide and trisulfide bonds, produced from lentinic acid by γ-glutamyl transpeptidase and C-S lyase. We have previously demonstrated that lentinic acid suppresses plasma ethanol elevation (Uto-Kondo et al., 2020) and lenithionine induces phase II detoxification enzymes (Katayama et al., 2012) and inhibits platelet aggregation, both in vitro (Shimada et al., 2004) and in vivo (Shimada et al., 2008). As lenthionine inhibits platelet aggregation even when U-46619 is used as an agonist, the inhibitory site is assumed to be in the downstream of the AA cascade, but this has not yet been confirmed. Therefore, in this study, we measured the inhibitory activity of platelet aggregation, activation of αIIbβ3 by using various agonists, and phosphorylation and dephosphorylation of proteins to activate αIIbβ3.
| 2. Materials and methods | ▴Top |
2.1. Reagents
Lenthionine was obtained from Ogawa & Co., Ltd. (Tokyo, Japan). A23187, ADP and acetylsalicylic acid were obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Platelet-activating factor (PAF) and phorbol 12-myristate 13-acetate(PMA)were obtained from Funakoshi Co., Ltd. (Tokyo, Japan). CD61 PerCP, CD62P PE, and PAC-1 FITC antibodies were obtained from Nippon Becton Dickinson Company, Ltd. (Tokyo, Japan). BD CellFIX was obtained from Thermo Fisher Scientific Inc. (Waltham, USA). Prostaglandin E1 and anti-phosphotyrosine, clone 4G10 were obtained from Sigma-Aldrich (Tokyo, Japan). Anti-phosphorylated MLC antibody (p-MYL9, Thr 18/Ser 19, sc-12896), anti-phosphorylated cofilin antibody (p-Cofilin 1 (hSer 3)-R, sc-12912-R), anti-rabbit secondary antibody (goat anti-rabbit IgG-HRP, sc-2004) and anti-α-actinin antibody (sc-17829) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, USA). Anti-phosphorylated talin antibody (phospho-Talin (Ser 425) Antibody) was obtained from Cell Signaling Technology (Tokyo, Japan). Protein G Sepharose 4 Fast Flow was purchased from GE Healthcare Technologies Inc. (Tokyo, Japan). All the other reagents used were of a reagent special grade or equivalent.
2.2. Blood collection
Approximately 4.5 mL of blood samples were collected from healthy volunteers who had not taken any medication for at least two weeks using a vacuum blood collection tube (Venoject II Vacuum Blood Collection Tube containing 0.5 mL of 3.8% sodium citrate, Terumo Corporation, Tokyo) and a blood collection needle (Venoject II Blood Collection Needle S 22 G x 1 1/2, Terumo Corporation, Tokyo).
2.3. Preparation of platelet-rich plasma (PRP) and platelet-poor plasma (PPP)
As platelets aggregate upon cooling, the following procedure was performed at room temperature. Citrated blood obtained by blood collection was centrifuged at 300×g for 10 min (H-500FR, Kokusan Co., Ltd., Tokyo, Japan), and the upper layer was used as PRP. The remaining bottom layer of the prepared PRP was further centrifuged at 1200×g for 15 min, and the top layer was used as PPP. Platelets were used within approximately 3 h of blood collection to prevent a decline in platelet function.
2.4. Inhibition of platelet aggregation induced by various agonists
The inhibition of platelet aggregation was measured using an aggregometer (NKK HEMA TRACER 1 MODEL PAT-4, SSR Engineering Corporation, Tokyo, Japan) with a CHROMATOPAC recorder (C-R4A, Shimadzu Corporation, Kyoto, Japan). To a silicone-coated cuvette containing a stir bar, 300 μL of PPP or PRP was added and preheated at 37 °C. Transmittance was recorded while stirring at 37 °C and 1,000 rpm. The transmittance of PPP was set to 0%, whereas that of PRP was set to 100%. A span adjustment was performed for each measurement. After span adjustment, 2 μL of lenthionine at a final concentration of 0.316 mM or ethanol used as dilution solvent was added to PRP and stirred for 3 min at 37 °C. Then, 18 μL of agonists to induce platelet aggregation (the final concentrations of PAF, ADP A23187, and PMA, were 1 μM, 10 μM, 50 μM, and 5 μM, respectively) was added and the change in transmittance was recorded for 10 min. Only the diluted solvent of lenthionine (ethanol) was added as a control.
The inhibition rate of platelet aggregation was determined by analysing the changes in the transmittance (T%) of the PRP. The change in transmittance was attributed to a change in the aggregation rate of the platelets (Agg. %), and the highest value was defined as the maximum aggregation rate (Agg. max %). The inhibition rate (inhibition%) was calculated as (1-T′/T)×100, where T is the maximum transmittance of lenthionine-free PRP and T′ is that of lenthionine-added PRP. The IC50 value was determined by plotting each concentration of lenthionine on the horizontal axis and the inhibition percentage of the corresponding concentration on the vertical axis, and identifying the concentration that inhibited aggregation by 50%.
2.5. Status of αIIbβ3 evaluated by flow cytometry
Platelets for flow cytometry were prepared from PRP obtained by the blood collection and diluted 10-fold with PBS(-). 2 µL of lenthionine with a final concentration of 0.316 mM or with diluent (ethanol), was added to the platelets and stirred gently for 3 min. After stirring, 18 µL of each agonist solution (the final concentrations of U-46619, A23187, PMA, and ADP were 1 μM, 50 μM, 5 μM, and 10 μM, respectively) was added. For non-activated platelet samples, PBS (-) was used instead of the agonist solution. After stirring for 2 min, CD61 PerCP, CD62P PE, and PAC-1 FITC antibodies were added to the platelets. The platelets were then stirred for 20 min and subjected to an antigen-antibody reaction. After the reaction, platelets were fixed with adding BD CellFIX and left to stand on ice for 30 min before analysis using a flow cytometer (BD FACSCanto, Becton, Dickinson and Company).
2.6. Preparation of washed platelets
Acetylsalicylic acid was added to PRP at a final concentration of 2 µM. The mixture was then incubated at room temperature for 30 min. Prostaglandin E1 was added to PRP at a final concentration of 1 µM. The mixture was then incubated at room temperature for 3 min before centrifugation at 800×g for 10 min to obtain platelet pellets. The platelet pellets were washed by resuspending them in wash buffer (129 mM NaCl, 8.9 mM NaHCO3, 0.8 mM KH2PO4, 0.8 mM MgCl2, 5.6 mM dextrose, 10 mM HEPES, pH 7.4) and centrifuging at 800×g for 10 min. The process was repeated twice, and the washed pellets were then resuspended in a resuspension solution (129 mM NaCl, 8.9 mM NaHCO3, 0.8 mM KH2PO4, 0.8 mM MgCl2, 1 mM CaCl2, 5.6 mM dextrose, 10 mM HEPES, pH 7.4) at 37 °C to obtain washed platelets.
2.7. Observation of platelet morphology by scanning electron microscopy
Lenthionine, at a final concentration of 0.316 mM, or a diluent (ethanol), was added to the washed platelets and allowed to react with stirring for 2 min. The platelets were then incubated on a rotator for 1 h at 4 °C in the dark. Subsequently, the platelets were washed thrice with PBS, suspended in a cold 2% osmium tetroxide solution, and incubated for another hour at 4 °C in the dark. After incubation, the platelets were washed thrice with PBS. Subsequently, the platelets were dehydrated, subjected to critical point drying, and ion sputtered. Finally, scanning electron microscopy was performed to observe and capture images of the platelets using a scanning electron Microscope S-3500N (Hitachi, Ltd., Japan).
2.8. Phosphorylation/dephosphorylation of skeletal proteins
The washed platelets were divided into three portions of 300 µL each. One portion was used as a control, while the remaining two portions were treated with 2 µL of lenthionine at a final concentration of 0.316 mM or with diluent (ethanol) and agitated for 2 min. Then, 50 µM of A23187 was added to the washed platelets, excluding the control, and allowed to react for an arbitrary time. After the reaction, equal volumes of Laemmli’s sample buffer were added in equal volume to each portion, including the control, to terminate the reaction and lyse the samples. The samples were run on SDS-PAGE and transferred to PVDF membranes. Membranes were blotted with an anti-phosphorylated MLC antibody (1:500), anti-phosphorylated cofilin antibody (1:1,000), and anti-phosphorylated talin antibody (1:1,000) before incubation with an anti-rabbit secondary antibody (1:2,000).
Phosphorylated α-actinin was detected using anti-α-actinin antibodies following immunoprecipitation with anti-phosphotyrosine antibodies to recover phosphoproteins. For each sample that reacted with A23187, 2× neutral detergent lysis buffer (15 mM HEPES, 150 mM NaCl, 2% (v/v) TritonX-100, 10 mM EGTA, 1 mM Na3VO4, and 1% protease inhibitor cocktail with a pH of 7.4) was added instead of Laemmli’s sample buffer to stop the reaction and the cell lysate was obtained by centrifugation (MX-160, TOMY Corporation) at 4 °C, 22600×g for 10 min. Protein G Sepharose was added to the cell lysate and stirred on a rotator for 1 h. The mixture was then centrifuged at 2500×g for 1 min, and the supernatant was collected to remove IgG from the cell lysate. Next, anti-phosphotyrosine (clone 4G10) was added to the supernatant, and the mixture was stirred on a rotator for 1 h. Finally, protein G Sepharose was added, stirred on a rotator for 1 h, and centrifuged at 2500×g for 1 min to collect the precipitate. The precipitate was washed thrice with 2× neutral detergent lysis buffer to obtain a precipitate containing phosphoproteins. Laemmli’s sample buffer was added to the precipitate, which was then incubated at 100 °C for 5 min. After centrifugation at 22600×g for 5 min, the supernatant was subjected to SDS-PAGE and Western blotting with an anti-α-actinin antibody (1:500). The expression of each phosphoprotein was normalised to that found in the control-washed platelets. Subsequently, the differences between groups with and without lenthionine were compared.
2.9. Statistical analysis
The results are presented as the mean ± SE of three experiments. Statistical analysis was conducted using repeated-measures two-way ANOVA.
| 3. Results | ▴Top |
3.1. Inhibitory effect of lenthionine on platelet aggregation induced by various agonists
Four agonists with distinct mechanisms of action (PAF, ADP, A23187, and PMA) were used to investigate the differential inhibitory effects of lenthionine on platelet aggregation. Lenthionine demonstrated inhibitory effects on platelet aggregation against all agonists, with IC50 values of 0.77×10−4 M against PAF, 1.99×10−4 M against ADP, 1.94×10−4 M against A23187, and 1.75×10−4 M against PMA (Figure 2).
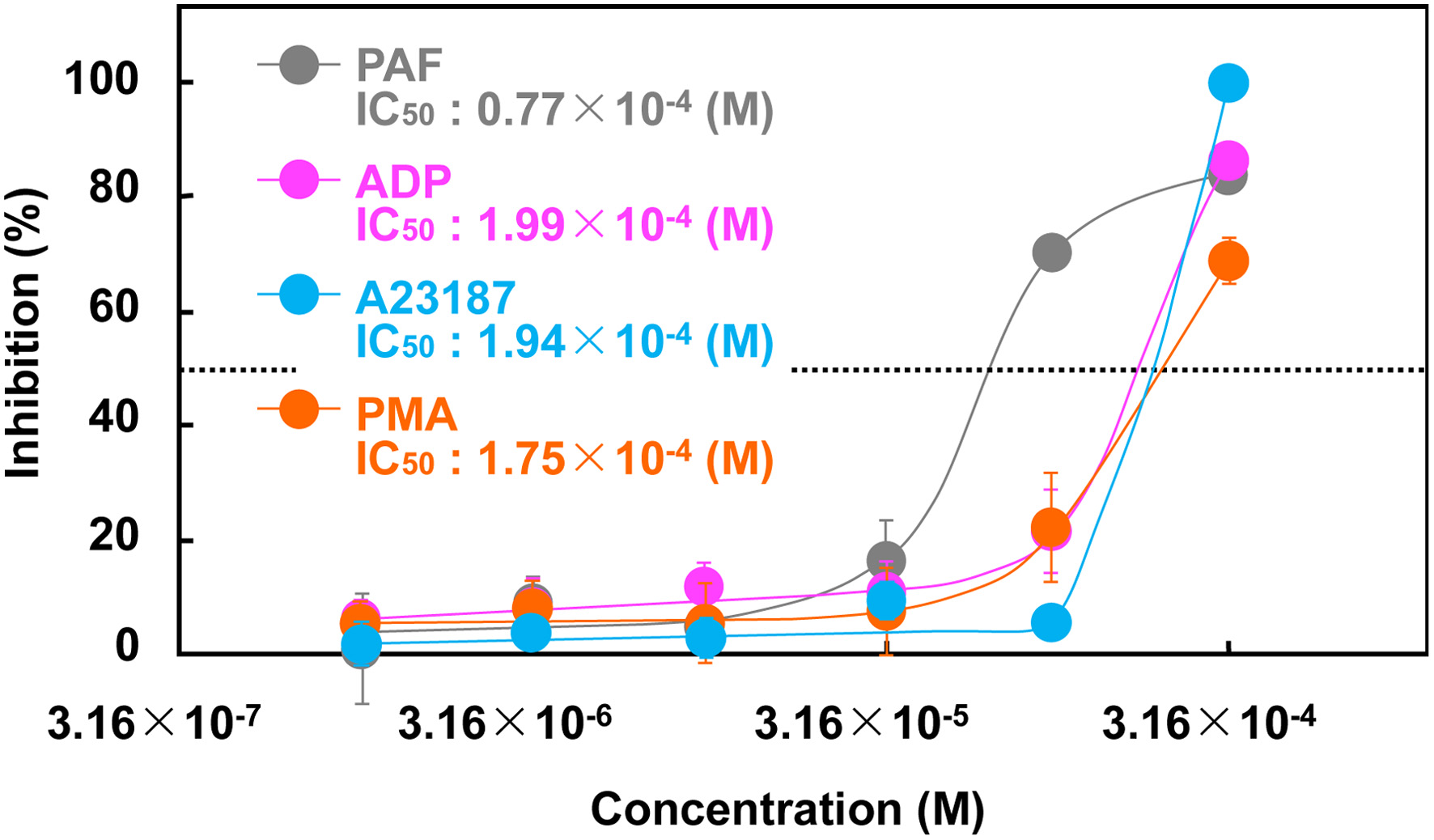 Click for large image | Figure 2. Inhibitory effect of lenthionine on the platelet aggregation induced by A23187. Values are means ± SE of 3 different experiments. |
3.2. Inhibitory effect of lenthionine on platelet activation and morphological change
Fluorescence intensities of CD62P and PAC-1 in platelets were measured by flow cytometry (Figure 3). CD62P is a membrane glycoprotein that migrates from the α-granule membrane to the platelet membrane surface upon activation. PAC-1 is a monoclonal antibody with specificity for the fibrinogen binding site of αIIbβ3. Measurements were performed on platelets activated by four agonists (U-46619, ADP, A23187, and PMA) in the presence and absence of lenthionine. When platelets were exposed to each agonist, the fluorescence intensities of both CD62P and PAC-1 increased compared to those of non-activated platelets without an agonist. In contrast, when the agonists were added to the platelets after lenthionine addition, the fluorescence intensities of CD62P and PAC-1 barely changed. The proportions of platelets in the Q3 region, where the platelets were not activated, were 12.2% for U-46619, 22.7% for ADP, 0.6% for A23187, and 0.2% for PMA without the addition of lenthionine, and 97.7% for U-46619, 98.2% for ADP, 67.1% for A23187, and 66.8% for PMA with the addition of lenthionine.
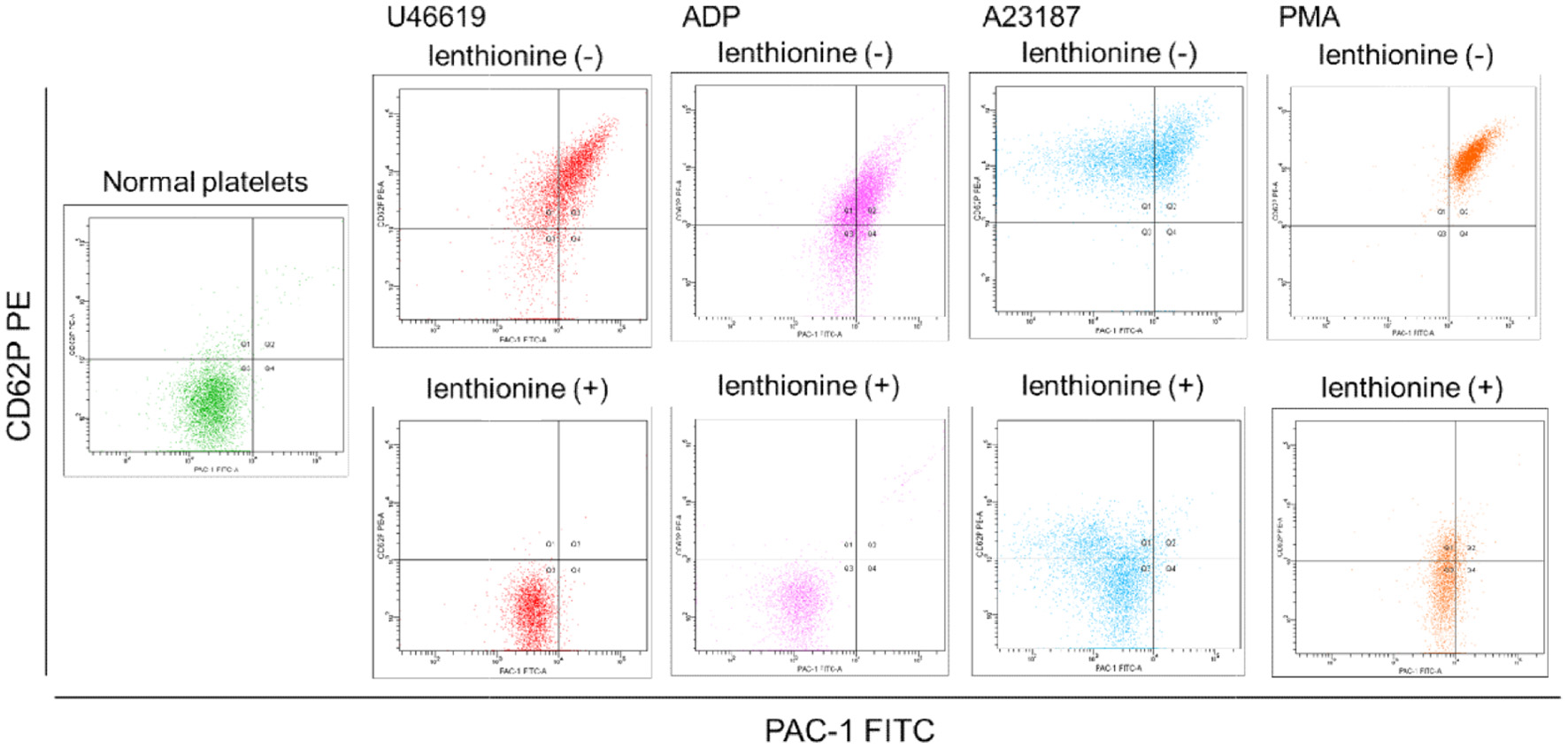 Click for large image | Figure 3. Flow cytometry of CD62P-phycoerythrin (PE) and PAC-1-fluorescein isothiocyanate (FITC) binding to platelets with and without lenthionine, before and after stimulation with various agonists. |
Morphological changes were observed using scanning electron microscopy. Unstimulated platelets were observed as spheres with a diameter of approximately 2 μm, whereas agonists-stimulated platelets (A23187) were observed to change to a flattened, amoeboid pseudopodal shape. However, the addition of lenthionine suppressed the agonist-stimulated morphological changes and the morphology of platelets was similar to that of unstimulated cells (Figure 4).
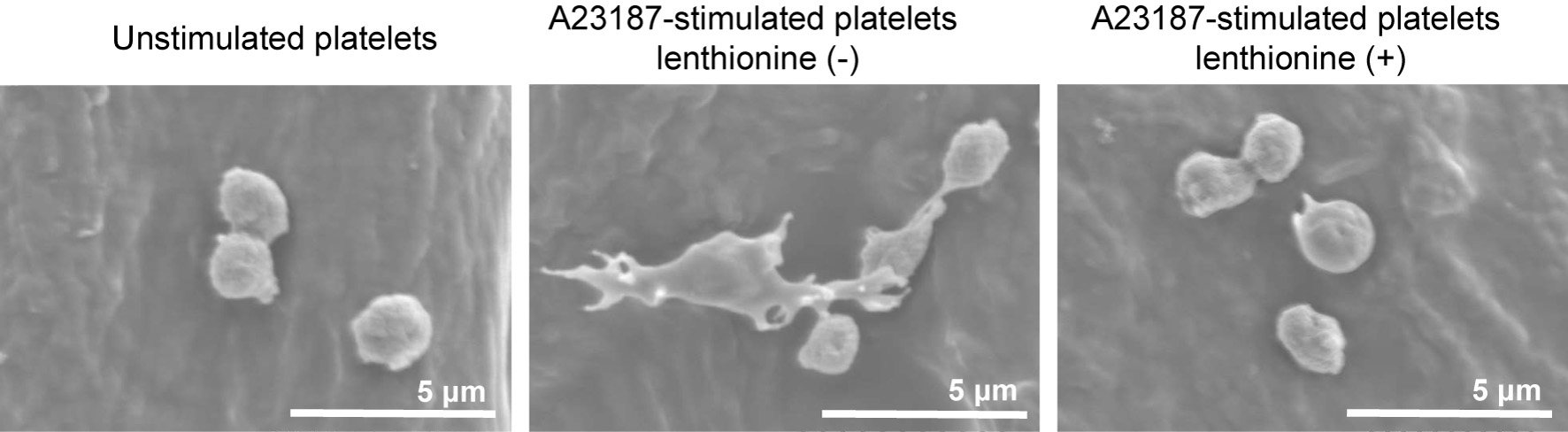 Click for large image | Figure 4. Scanning electron microscopy of platelets with and without lenthionine, before and after stimulation with A23187. |
3.3. Effect of lenthionine on phosphorylation/dephosphorylation of cytoskeletal proteins
The effects of lenthionine on the phosphorylation and dephosphorylation of proteins affecting platelet activation and morphological changes were examined. MLC was phosphorylated approximately 1.5-fold more than in unstimulated platelets with the addition of an agonist (A23187). The addition of lenthionine did not significantly change the degree of phosphorylation (lenthionine treatment, p = 0.505; time, p = 0.207; interaction effects, p = 0.982) (Figure 5a). Cofilin was significantly dephosphorylated by the addition of an agonist (A23187) by approximately 0.2-fold over time compared to that in unstimulated platelets, and it was also dephosphorylated with the addition of lenthionine (lenthionine treatment, p = 0.191; time, p = 0.000; interaction effects, p = 0.685) (Figure 5b). Talin was phosphorylated approximately 2.5-fold with the addition of an agonist (A23187) compared to that in unstimulated platelets, and the addition of lenthionine did not significantly affect the level of phosphorylation (lenthionine treatment, p = 0.900; time, p = 0.818; interaction effects, p = 0.769) (Figure 5c). α-Actinin was dephosphorylated with the addition of an agonist (A23187) to approximately 0.5-fold compared to that of unstimulated platelets, but the addition of lenthionine significantly inhibited the dephosphorylation (lenthionine treatment, p = 0.005; time, p = 0.213; interaction effect, p = 0.274) (Figure 5d).
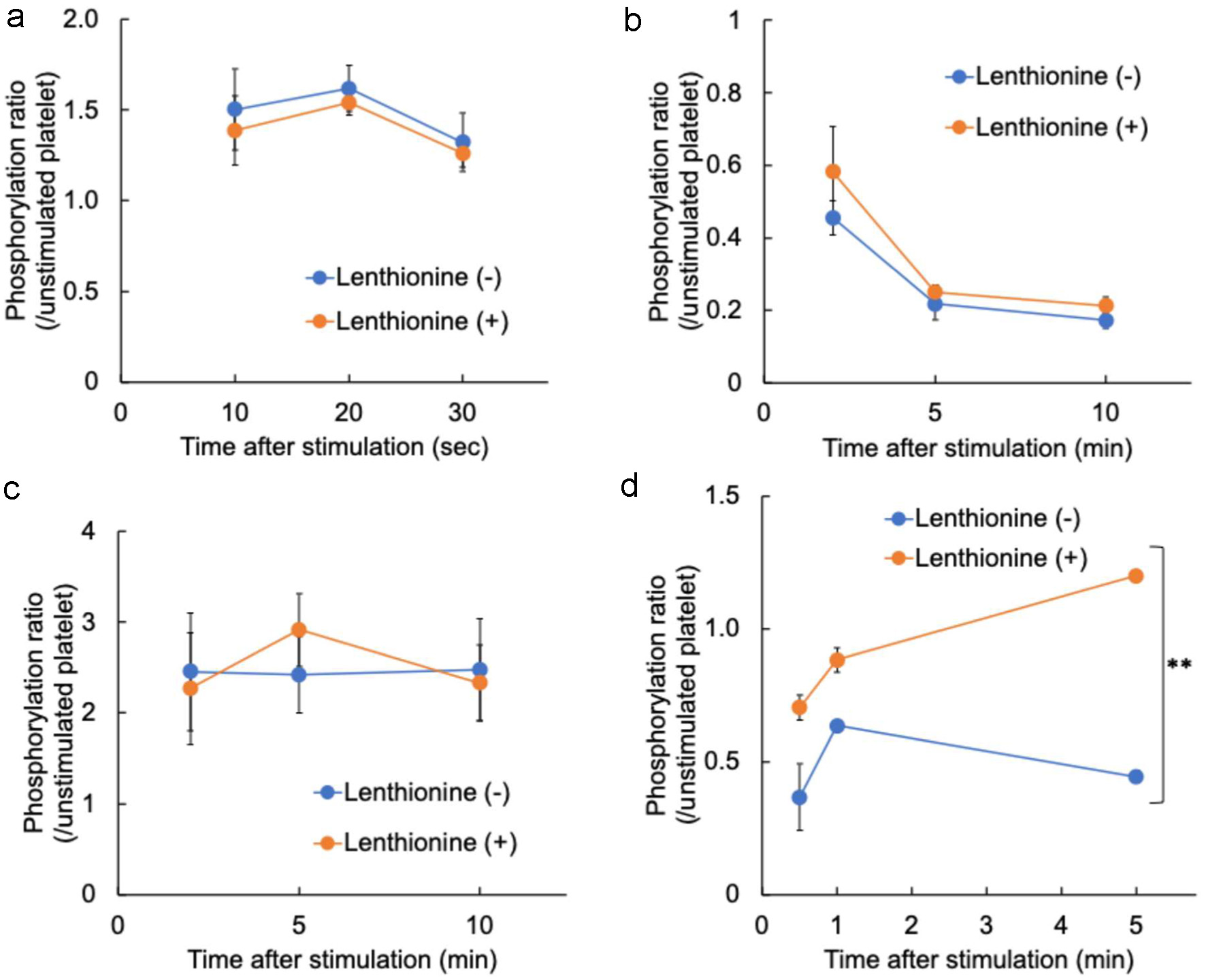 Click for large image | Figure 5. Changes in the phosphorylation levels of (a) myosin light chain, (b) cofilin, (c) talin, and (d) α-actinin induced by A23187 with and without lenthionine. Values are means ± SE of 3 different experiments. |
| 4. Discussion | ▴Top |
Thrombosis is typically performed to achieve haemostasis. When a vessel is injured and vascular endothelial cells detach to expose the subendothelial tissue, platelets first adhere to it and become activated. This results in platelet aggregation and the formation of a primary haemostatic thrombus. Concurrently, the blood coagulation cascade is initiated within the platelet thrombus, whereby fibrinogen is converted to fibrin, forming a robust secondary haemostatic clot that repairs the damaged blood vessels. In normal blood vessels, vascular endothelial cells cover the subendothelial tissue, inhibit platelet aggregation and coagulation, and enhance fibrinolysis to suppress thrombus formation. However, when the vascular endothelium becomes abnormal due to a worsening lifestyle or diseases such as atherosclerosis, the thrombus-suppressing function of vascular endothelial cells is lost, and platelets aggregate even outside the site of vascular injury, causing thrombosis. It is widely acknowledged that the inhibition of platelet aggregation represents a crucial strategy for the prevention and management of thrombotic disorders (Jackson, 2007). We have already reported that lenthionine inhibits platelet aggregation, both in vitro (Shimada et al., 2004) and in vivo (Shimada et al., 2008). The objective of this study aimed to elucidate the mechanism through which lenthionine inhibits platelet aggregation.
To ascertain the mechanism of action of lenthionine in inhibiting platelet aggregation, an initial examination was conducted to assess the inhibitory effects of lenthionine on platelet aggregation against four different agonists, PAF, ADP, A23187, and PMA. PAF increases intracellular Ca2+ level and induces the activation of phospholipase A2,leading to the formation of TxA2 from AA. Lenthionine demonstrated a concentration-dependent inhibitory effect on platelet aggregation induced by PAF, with an IC50 of 0.77×10−4 M (Figure 2). ADP binds to P2Y1 and P2Y12 receptors on platelet membranes, resulting in an elevation of intraplatelet Ca2+, the release of dense granules (Manickam et al., 2008; Panova-Noeva et al., 2013) and the activation of αIIbβ3 (Andrews and Berndt, 2004). Lenthionine exhibited a concentration-dependent inhibition of ADP-induced platelet aggregation, with an IC50 being 1.99×10−4 M (Figure 2). Our previous study, which employed U-46619, a stable analogue of TxA2, as an agonist, yielded comparable results, with an IC50 value of 1.83×10−4 M (Shimada et al., 2008). These findings suggest that lenthionine inhibits platelet aggregation induced by receptor-binding type agonists and the inhibitory site of action is assumed to be the signalling pathway associated with the increase in Ca2+ concentration in platelets, phosphorylation/dephosphorylation of proteins, or αIIbβ3 activation. Consequently, A23187, a calcium ionophore, was used in order to ascertain the effect of lenthionine on platelet aggregation in the context of elevated Ca2+ concentrations in platelets. Lenthionine inhibited platelet aggregation in a concentration-dependent manner, with an IC50 value of 1.94×10−4 M (Figure 2). As elevated Ca2+ concentrations in platelets amplify the activation of various signalling pathways, ultimately resulting in the activation of αIIbβ3 and platelet morphology changes, this result suggests that lenthionine would inhibit the activation of skeletal proteins and/or αIIbβ3, which are involved in platelet morphological changes. Therefore, PMA was used to induce platelet aggregation by directly activating protein kinase C in platelets. Lenthionine was inhibited PMA-induced platelet aggregation in a concentration-dependent manner, with an IC50 value of 1.75×10−4 M (Figure 2).
Subsequently, the effects of lenthionine were examined from the perspective of changes in platelet morphology and αIIbβ3 structure associated with platelet aggregation. This was accomplished by examining the proteins expressed on platelet membranes during platelet activation and the activation state of αIIbβ3 by flow cytometry. CD62P is a membrane glycoprotein present in the α-granule membrane of platelets. Upon platelet activation, it migrates to the platelet membrane surface, where it becomes visible as its fluorescence intensity increases (Johnston et al., 1989; Merten and Thiagarajan, 2000). PAC-1 is a monoclonal antibody that exhibits specificity for the fibrinogen-binding site of αIIbβ3. In an inactive state, PAC-1 exhibits a low affinity for αIIbβ3. However, upon activation, it binds to αIIbβ3 with high affinity and the elevated fluorescence intensity is indicative of an activated state of αIIbβ3. Consequently, in unstimulated platelets, the fluorescence intensities of both remained low, whereas in activated platelets, which are responsible for platelet aggregation, both are high. Indeed, in our experiments, the majority of non-activated platelets were located in the Q3 region, where both fluorescence intensities were minimal (Figure 3). However, upon activation of platelets with various agonists, both CD62P and PAC-1 fluorescence intensities increased and most platelets migrated to the Q2 region (Figure 3), thereby undergoing platelet morphological transformation. Conversely, the elevation in CD62P and PAC-1 fluorescence intensities was not observed when lenthionine was administered prior to agonist addition (Figure 3), indicating the suppression of αIIbβ3 activation and morphological changes. Scanning electron microscopy demonstrated that the activated platelets, which had been induced to aggregate by the agonist, exhibited a spinous spheroid shape with elongated filopodia extending several micrometers from the platelets (Figure 4). In contrast, platelets treated with lenthionine retained a discoidal, smooth cell surface morphology comparable to that observed in inactivated platelets (Figure 4). These results indicate that the mechanism of action of lenthionine is the inhibition of phosphorylation/dephosphorylation of platelet cytoskeletal proteins and/or activation of αIIbβ3.
The actin cytoskeleton plays a pivotal role in platelet elongation and morphological changes, and the actin polymerisation associated with platelet activation is closely related to platelet aggregation. MLC and cofilin, which are activated during platelet aggregation signalling, starting with platelet adhesion to collagen (via vWF), are involved in increasing actomyosin contractility and accelerating actin filament cleavage and depolymerisation. Additionally, they alter the phosphorylation state during platelet aggregation and change platelet morphology (Wang et al., 2005; Pandey et al., 2006; Getz et al., 2010; Aburima et al., 2017; Shin et al., 2017). Moreover, αIIbβ3 forms a robust thrombus by cross-linking with αIIbβ3 of other platelets via fibrinogen. The activation of αIIbβ3 is accompanied by alterations in the phosphorylation state of proteins involved in αIIbβ3 activation, including talin and α-actinin (Ratnikov et al., 2005; Verhaar et al., 2008; Nieswandt et al., 2009; Tadokoro et al., 2011; Tadokoro et al., 2011; Lagarrigue et al., 2020). Additionally, α-actinin possesses an actin-binding domain at its N-terminus, functioning as a cross-linker between actins through its binding to the high molecular filament type F-actin. This contributes to changes in the platelet shape (Bearer et al., 2002; Shin et al., 2017). Therefore, we investigated the effects of lenthionine on these proteins during platelet aggregation. MLC and cofilin, which are related to the actin cytoskeleton, showed 1.5- and 0.2-fold changes in their phosphorylation states, respectively, in response to platelet aggregation induced by A23187. However, the addition of lenthionine did not alter their phosphorylation levels (Figure 5a and b). This indicates that lenthionine is not involved in the contraction of actomyosin, a function of MLC, or in the cleavage and depolymerisation of actin filaments, a function of cofilin, caused by increased Ca2+ concentrations within platelets. If lenthionine does not affect these functions, it may cause changes in the platelet morphology. However, as shown by flow cytometry and scanning electron microscopy images, no morphological changes were observed (Figures 3 and 4). This indicated the potential involvement of an additional molecule in actin polymerisation through its action on lenthionine. Subsequently, we confirmed the phosphorylation status of talin, which is strongly involved in αIIbβ3 activation, and that of α-actinin, which is related to αIIbβ3 activation and F-actin binding. Talin showed a 2.5-fold change in its phosphorylation state upon the induction of platelet aggregation, whereas lenthionine was not involved (Figure 5c). In contrast, α-actinin was dephosphorylated 0.5-fold upon platelet aggregation induced by A23187, but this dephosphorylation was inhibited by the addition of lenthionine (Figure 5d). The observation that lenthionine did not affect the phosphorylation of talin, which is responsible for the activation of αIIbβ3, but inhibited the dephosphorylation of α-actinin, which is dephosphorylated following αIIbβ3 activation, indicates that lenthionine inhibits the activation of αIIbβ3 itself.
The disulfide bonds in the EGF domains of the β3 subunit of αIIbβ3 are typically intact (Kamata et al., 2004), however, when these bonds are cleaved, αIIbβ3 is activated (Chen et al., 2001; Essex et al., 2001; Manickam et al. 2008; Mor-Cohen et al., 2012). The cleavage of disulfide bonds in the EGF domain of β3 is mediated by protein disulfide isomerase (PDI) (Essex and Wu, 2018). PDI is a member of the thiol isomerase family, which promotes oxidative protein folding. The cysteine residue within the redox-sensitive cysteine-glycine-histidine-cysteine (CGHC) motif of the active site undergoes various redox modifications that regulate platelet aggregation. It is postulated that the thiol group of cysteine becomes sulfinylated and activated by oxidative stress associated with platelet aggregation, thereby cleaving the disulfide bond of αIIbβ3 and placing αIIbβ3 in an activated state (Yang et al., 2023). Lenthionine is a volatile cyclic polysulphur compound with a seven-membered ring containing both disulfide and trisulfide structures, rendering it extremely hydrophobic. Our previous study demonstrated that lenthionine inhibits platelet aggregation when ingested orally (Shimada et al., 2008). Therefore, this compound is assumed to be readily incorporated onto the surface of platelet membranes suspended in the blood after oral ingestion. These findings suggest that lenthionine inhibits αIIbβ3 activation by either reverting the sulfenic acid of PDI, which is activated at the platelet membrane surface, to a thiol group or by inhibiting the activity of PDI to cleave αIIbβ3 disulfide bonds.
As lenthionine, which is abundant in shiitake mushrooms, is a natural bioactive compound with a long history of dietary use and exerts the inhibitory effect on platelet aggregation even through oral intake (Shimada et al., 2008). it is a promising candidate for the prevention and treatment of thrombosis. In addition, lenthionine induces phase II detoxification enzymes, namely glutathione-S-transferase and quinone reductase (Katayama et al., 2012). These phase II detoxification enzymes are considered to be crucial for protection of the body from carcinogens, as they facilitate rapid conjugation and detoxification of carcinogens and other foreign substances that enter the body inhibiting DNA mutations. Due to its hydrophobic properties, lenthionine may also be integrated into cell membranes and affect various cellular processes. Further research is required to fully elucidate the biological activities of lenthionine and its potential to provide a range of previously unconfirmed health benefits. Furthermore, the development of lenthionine-fortified dietary supplements and functional foods will facilitate convenient access to the health benefits.
| 5. Conclusion | ▴Top |
In conclusion, lenthionine, a characteristic flavour compound of shiitake mushrooms with cyclic polysulphuric structure, inhibits platelet aggregation by preventing the cleavage of αIIbβ3 disulfide bonds and inhibiting αIIbβ3 activation. This mechanism of action is different from MATS, a flavour compound of garlic, which inhibits hydroperoxidase activity in the AA cascade, and DATS, another flavour compound of garlic, which is estimated to be bound to the free thiol in αIIbβ3. As lenthionine is the major flavour component of shiitake mushrooms, it can be a promising compound to prevent platelet aggregation and thrombosis. Previous studies in rats have demonstrated that lenthionine exhibits the inhibitory effect on platelet aggregation when administered at doses of 1 mg/kg or higher (Shimada et al., 2008). Subsequent studies will seek to ascertain the efficacious dose in human trials and to assess the safety and bioavailability of lenthionine when consumed as part of a diet or supplement. Furthermore, the advanced extraction methods and processing techniques, such as encapsulation and functional food production, will enhance the practicality of lenthionine. The further studies on health benefits of lenthionine will contribute to the development of novel strategies for prevention and management of thrombotic disorders and other health issues.
| References | ▴Top |