| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 28, December 2024, pages 1-23
Role of dietary lipids and gut microbiome-derived lipids in regulation of intestinal homeostasis and modulation of inflammatory diseases
Chi Yan†, Shou-He Huang†, Huafang Ding, Wen-Sen He, Hanyue Zhu, Zhen-Yu Chen*
School of Life Sciences, Faculty of Science, The Chinese University of Hong Kong, Shatin, NT, Hong Kong, China
†These authors contributed equally to this work.
*Corresponding author: Zhen-Yu Chen, School of Life Sciences, Faculty of Science, The Chinese University of Hong Kong, Shatin, NT, Hong Kong, China. E-mail: zhenyuchen@cuhk.edu.hk
DOI: 10.26599/JFB.2024.95028391
Received: December 13, 2024
Revised received & accepted: December 20, 2024
| Abstract | ▴Top |
Gut health is the foundation of overall health. Inflammatory bowel disease (IBD) including Crohn’s disease and ulcerative colitis is a kind of chronic relapsing and idiopathic immune dysbiosis in the intestinal tract. Recent studies have highlighted the effects of various dietary components on the progress of IBD. Mounting evidence has demonstrated that both dietary lipids and gut microbiome-derived lipids play a crucial role in gut health. They can directly or indirectly change the composition of gut microbiota, modulate the metabolism of colonic epithelial cells, influence the integrity of gut barrier and regulate the immune function. This review aims to define the key classes of dietary lipids and microbial-derived lipids, elucidate the interaction of these lipids with gut microbiota, discuss their effects on the intestinal homeostasis, and provide the future perspective in the research of gut health.
Keywords: Inflammatory bowel disease (IBD); Gut microbiota; Dietary lipids; Gut microbiome-derived lipids; Gut dysbiosis
| 1. Introduction | ▴Top |
The gastrointestinal (GI) tract is the largest immune organ in humans, consisting of complicated networks of various immune cells and epithelial cells (Kaur et al., 2023). Intestinal homeostasis is the foundation of overall health in humans. Intestine-related inflammation can trigger a variety of systemic diseases including inflammatory bowel disease (IBD) and colorectal cancer (Jia, et al., 2020b). IBD, such as Crohn’s disease and ulcerative colitis, is a kind of chronic relapsing and idiopathic immune dysbiosis in GI tracts, accompanied by dysregulation of immune responses, causing a large number of destructive inflammatory cell infiltration and excessive production of pro-inflammatory factors (Lloyd-Price et al., 2019). Recent epidemiological studies have clearly demonstrated that the incidence of IBD is substantially increasing (Chen et al., 2023a). The number of patients with IBD has risen from 3.7 to more than 6.8 million from 1990 to 2017 around the world (Alatab et al., 2020), demonstrating a need in management of GI health. Although numerous investigations have been performed to study the etiology of IBD, its underlying mechanisms remain unclear. It is hypothesized that IBD is associated with susceptibility of gene loci, environmental risk factors, and gut microbiota composition (Kobayashi et al., 2020; Wu et al., 2021).
Numerous therapies and medicines are available to treat IBD. Mesalazine (decreasing the expression of pro-inflammatory factors), corticosteroid (suppressing the activation of immune systems), biological therapy (fecal microbiota transplantation), surgical management (removing the damaged intestinal tissue), and immunosuppression (regulating the amount of relevant immune cells) have been widely used to alleviate inflammatory symptoms of IBD, but the treatments outcomes are still challenging with some serious and unexpected side-effects, for example, medicine dependence, liver and kidney toxicity (Chen et al., 2023b; Hartwig et al., 2021). In addition, there are also some other limitations for current IBD treatments. For example, the application of fecal microbiota transfer, which helps to re-establish gut microbial communities of patients, is still controversial due to its transient outcomes, potential pathogenic infections, and unstable reproducibility (Pigneur and Sokol, 2016). With the advances in medical development, some novel concepts and strategies have been proposed to enable physicians to have better approaches for IBD management.
Recent studies have also highlighted the crucial role of various dietary components in the progress of IBD (Ananthakrishnan, 2015). Lipids are one of the three largest nutrients for humans (Hosomi et al., 2020). Intake of lipids can cause both beneficial and harmful effects in humans, for example, dietary fat is required for humans as the major energy income, however, its excessive intake can cause numerous chronic diseases, such as atherosclerosis, obesity, and even cancer. After consumption, dietary lipids are first digested in the small intestine and approximately 95% of lipids will be absorbed in the GI tracts (Armand, 2007). Numerous studies have indicated that dietary lipids have significant effects on intestinal homeostasis and are related to various inflammatory diseases, including the regulation of the colonic physical barrier and immune systems (Okamura et al., 2021; Rohr et al., 2020). Furthermore, dietary lipids can exert various effects on gut health by modulating the composition of gut microbiota (Ye et al., 2021).
Gut microbiota-derived molecules have demonstrated to be one of the important factors that can affect the physiological conditions of the host (Koppel et al., 2017). The effect of lipids derived from gut microbiota on host health has been understudied (Eichelmann et al., 2022). Recent studies have shown that gut microbiota-derived lipids can affect the homeostasis in GI tracts and modulate the inflammatory diseases (Brown et al., 2023). The bacterial-derived lipids can directly stimulate the metabolism of colonic epithelial cells and improve the integrity of gut barrier (Kelly et al., 2015). In addition, gut microbiota can also produce various kinds of lipids that have been proven as potential factors to regulate immune systems, such as short-chain fatty acids (SCFAs) and phospholipids (Cai et al., 2022; Yao et al., 2022).
There are some research gaps about the influence of dietary and gut microbiota-derived lipids on gut health. For example, some functional edible oils have been applied to improve the symptoms of IBD, but the discrete roles of different lipid components in IBD have not been well understood (Prabha et al., 2023). Many researchers have focused on the functionality of gut microbiota in health, but the important roles of lipids from the gut microbiota membrane are ignored. This review aims to highlight and update the biological effects of different dietary lipids on gut microbiota in the regulation of intestinal homeostasis and IBD. The potential underlying mechanisms of lipids in the modulating of gut health via gut microbiome-dependent or gut microbiome-independent manners will be discussed.
| 2. Gut homeostasis and mechanisms of IBD | ▴Top |
The gut homeostasis is a complicated equilibrium maintained by a large number of factors. Beginning from the surface of the GI tract, the crucial factor is the gut bacteria which can educate immune systems and maintain gut barrier functions. A healthy intestinal condition can prevent the infiltration of bacteria across the gut barrier and establish immune tolerance toward gut microbiota (Zheng et al., 2020). The intestinal homeostasis and health mainly depend on complicated interactions among the gut microbiome, intestinal barrier, and host immune systems (Maloy and Powrie, 2011). Although the pathophysiology of IBD is multifaceted and is not fully elucidated, current research allows us to summarize some main components that trigger and contribute to the incidence of intestinal inflammation: gut microbiota, barrier, and immune systems (Figure 1).
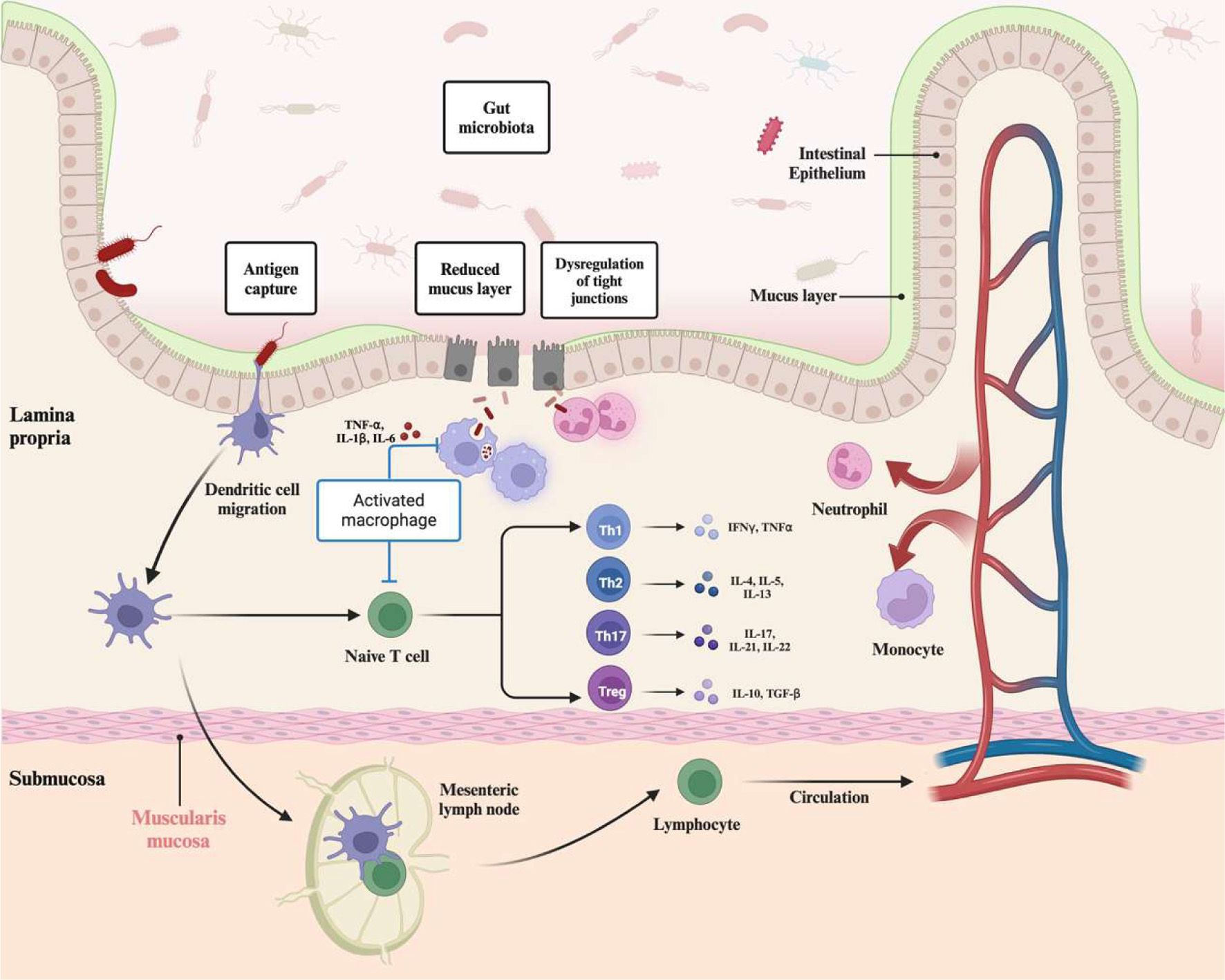 Click for large image | Figure 1. Dysbiosis and pathophysiology of inflammatory bowel disease (IBD). Multiple associated pathways, including gut microbiota, intestinal barrier, and immune systems, contribute to the incidence of IBD. In homeostasis conditions, trillions of commensal bacteria in the gastrointestinal tracts can help to mature the immune systems and produce beneficial metabolites, such as short-chain fatty acids. In IBD conditions, the composition of gut microbiota and metabolic profiles are altered, accompanied by an increase in some harmful bacteria, such as Escherichia coli. Impairment of the mucus layer and tight junction proteins permit the infiltration of microbiota and related antigens in lamina propria, activating innate immune systems and antigen-presenting cells to recruit neutrophils as the first responders in IBD. Neutrophils infiltrate and contribute to the first immune line via constructing neutrophil extracellular traps, excessive antigens can be recognized by macrophages and dendritic cells to further stimulate the activation of the adaptive immune system. With the stimulation and binding with antigen-presenting cells, naïve T cells can polarize and differentiate into various T helper and regulator cells, including Th1, Th2, Th17, and Treg, further contributing to the progress of IBD with the production of numerous cytokines. Figure 1 was created in Biorender.com (Agreement number: QL27CARACU). |
2.1. Gut microbiota
The GI tract is home to trillions of bacteria that perform diverse roles in human health. Studies have declared that numerous gut microbiota could be linked with the gene expression in hosts (Alexander et al., 2022; Venema, 2010). There is adequate evidence that the offspring can obtain gut microbiota from mothers in many ways (Yang et al., 2021). The environmental factors that come after birth, including dietary components, medicines, and even sleeping models can exert crucial effects on modulating the composition of gut microbiota in fetuses, whilst the composition of gut microbiota can be more stable in adults even though it can still be affected by various factors (Dominguez et al., 2011). Extensive studies based on antibiotics-treated mice or germ-free mice have revealed the indispensable roles of gut microbiota in human development and health. In normal conditions-raised mice colonized with the conventional microbial community, some dietary factors especially some complex carbohydrates can also be further digested and broken by bacteria and then provide nutrients that can nourish locally or systemically to the host (Oliphant and Allen-Vercoe, 2019). In contrast to wild-type mice, the cecum of germ-free mice is enlarged by four to eightfold due to the accumulation of undigested fibers and intestinal transit is also prolonged (Manca et al., 2020). In addition to dietary factors digestion as afore-described, the defective gut microbiota in germ-free mice can also induce a decrease of intestinal barrier integrity, a thinner mucus layer in the colon part, through decreasing Muc2 expression and secretion in the proximal colon (Bergstrom et al., 2020). Immune systems are also impacted in germ-free mice, for example, germ-free mice have hypoplastic Peter’s patches and diminished IgA-producing and CD4+ T cells in the intestine (Lin and Zhang, 2017).
Dysbiosis of gut microbiota has been related to the occurrence of IBD at the clinical level (Chassaing and Darfeuille–Michaud, 2011; de Souza and Fiocchi, 2016). In patients suffering from Crohn’s disease, the relative abundance of Bacteroidetes and Proteobacteria increased, whereas Firmicutes was reduced compared with healthy individuals (Man et al., 2011). Meanwhile, some studies have observed that gut microbiota abnormalities in Crohn’s disease are more significant than ulcerative colitis (Qiu et al., 2022). A decrease in Roseburia hominis and Faecalibacterium prausnitzii was reported in ulcerative colitis patients (Machiels et al., 2014). The pathogenic roles of some bacteria have also been evaluated in animal models, such as Eggerthella lenta which is enriched in IBD patients and exacerbated dextran sodium sulfate (DSS)-induced colitis in mice via inducing the activation of Th17 cells in antigen-independent mechanisms (Alexander et al., 2022). In addition, the decrease of some crucial metabolisms from gut microbiota was also reported, for example, SCFAs which have been connected with gut barrier functions and immune system regulation based on various murine models (Furusawa et al., 2013; Kelly et al., 2015). Remarkably, some studies showed that improvement of IBD could be achieved in some patients via fecal microbiota transplantation (Moayyedi et al., 2015; Paramsothy et al., 2017). Although this transplantation treatment is not sufficient for some patients to cure IBD and the therapy outcome is still challenging to predict, the important roles of gut microbiota in IBD have been revealed with this evidence.
With the increasing evidence and studies about the influence of gut microbiota on IBD, there are also emerging questions that still limit the application of related therapy in IBD management. Evidence exists that the function and roles of bacteria can be different in IBD progress. A prior study demonstrated that the relative abundance of Akkermansia muciniphila significantly decreased in TLR4−/− mice and then contributed to the exacerbation of DSS-induced colitis, thereby proving the potentially crucial role of Akkermansia muciniphila in gut homeostasis (Liu et al., 2022). Previous studies also suggested that treatment of Akkermansia muciniphila could ameliorate colitis symptoms by enhancing gut barrier integrity, decreasing inflammatory factors, and improving microbiota ecology (Bian et al., 2019). However, adverse effects of Akkermansia muciniphila in IBD have also been reported based on animal models. Intake of dietary sugars (such as glucose) could induce severe colonic inflammation in DSS-treated or IL10−/− mice by modulating gut microbiota composition, in particular, the increase of Akkermansia muciniphila abundance could cause the decrease of the mucus layer and facilitate gut barrier damage during inflammation (Khan et al., 2020). Similar harmful effects of Akkermansia muciniphila on IBD were also reported in IL-10 knockout mice (Seregin et al., 2017). Those conflicting results and effects of gut microbiota on IBD could be explained by various factors, including the different genotypes of animal models and gut microbiota baseline. Another question is whether gut microbiota dysbiosis is a primary or secondary trigger for IBD. Numerous studies have emphasized the important roles of gut microbiota on IBD, however, recent evidence suggested that even inflammation per se could cause gut microbiota dysbiosis (de Souza and Fiocchi, 2016; Lupp et al., 2007). DSS treatment could induce colitis and gut dysbiosis in mice, however, DSS could not induce any significant changes in murine microbiota in vitro, including composition and metabolic pathways (Krause et al., 2024). Individual delivery methods can significantly influence the gut microbiota composition, but it is unlikely to substantially influence the incidence of IBD (Bruce et al., 2014). Those results demonstrated that gut microbiota dysbiosis alone may not be sufficient to trigger IBD. In this regard, more information about the interaction between gut microbiota and the host is desired.
2.2. Intestinal barrier
The first line of defense in human GI tracts is the intestinal barrier which can prevent the infiltration of potentially harmful compounds from intestinal content. In healthy individuals, the presence of an intact gut barrier, including epithelial cells and mucus layer, can prevent most bacteria or antigens from contact with lamina propria. The tolerance mechanism of humans can limit the dysregulation of immune cells and related inflammatory responses in the intestinal part. However, when the breach of the gut barrier happens, severe infiltration of luminal antigens can induce the failure of immune system tolerance and trigger the incidence of IBD (Kobayashi et al., 2020). Therefore, the gut barrier has attracted the attention of the academic community recently, and current evidence also proves that maintaining the gut barrier might be a crucial therapeutic target.
The mucus layer is mainly comprised of gel-forming mucins produced by goblet cells, acting as a crucial physical barrier at the top of epithelial cells. The structure of the mucus layer is different in the small intestine and colon. The small intestinal tract constitutes a single and penetrable layer, but in the colon, there are two layers of mucus, including a penetrable outer layer and an impenetrable inner layer ( Johansson et al., 2013; Johansson and Hansson, 2016). The importance of the mucus layer in gut health has attracted the attention of researchers for many years, and the damage and defect of the mucus layer have been observed with decreased mucus layer thickness due to the decrease of mucin 2 expression (Van Klinken et al., 1999). The conduction of Muc2−/− mice also illustrates the important role of mucus as a physical barrier in preventing luminal components infiltration and IBD (Van der Sluis et al., 2006). The relationship between gut microbiota and mucus is reciprocal, as previous experiment results also proved that the depletion of gut microbiota in germ-free and antibiotics-treated mice could reduce the thickness of the mucus layer in the colon via decreasing the expression of Muc2 in the proximal colon part (Bergstrom et al., 2020). Furthermore, the mucus layer can also provide nutrients and habitat for gut microbiota, a prior study identified a distinctive gut microbiota community in Muc2−/− mice which could further exacerbate DSS-induced colitis in wild-type mice after fecal microbiota transfer (Leon-Coria et al., 2021).
Immediately below the mucus barrier are the epithelial cells which maintain a high renewal rate and also act as a physical barrier to restrict the free passage of antigens from the intestinal lumen. The defect of gut epithelial cells has been linked to the trigger of IBD, for example, in the early stage of ulcerative colitis, the apoptotic foci could be found while the epithelium was normal under endoscopy test (Gitter et al., 2001). The GI tract is the most important digestion and absorption organ, thus, in addition to the crucial barrier functions, intestinal epithelial cells should also selectively allow the permeability of some nutrients and water. Tight junction proteins, including occludin, claudins, and zonula occludens (ZOs), are the most crucial components for intracellular interactions between epithelial cells, equipping epithelial cells with those functions that seal the paracellular space and selectively restrict the entry of components (Chelakkot et al., 2018b). Due to the remarkable turnover rate of epithelial cells, the accurate regulation of tight junction proteins is required to maintain gut homeostasis. Current studies support that abnormal alterations of tight junction proteins can be detrimental to gut health and have been regarded as the crucial causes for the increase of gut permeability (Edelblum and Turner, 2009). For example, the decrease in occludin expression has been reported in active Crohn’s disease (Zeissig et al., 2007), and the deficiency of mdr1a could trigger the development of spontaneous colitis in mice by impairing phosphorylation of tight junction proteins (Martini et al., 2017; Resta-Lenert et al., 2005).
Apart from forming a gut barrier, intestinal epithelial cells also play a crucial role as the interface between the immune regulation of the host and the gut microbiota. Some pattern recognition receptors have been found in intestinal epithelial cells, such as the Toll-like and NOD-like receptors, which can be constantly stimulated by gut microbiota-related molecular patterns and then activate epithelial cells to express antimicrobial peptides (including angiogenin-4) and cytokines (IL-33 and IL-25) (Hooper et al., 2003; Schiering et al., 2014; Zaph et al., 2008). Those factors are required for the forming of tolerogenic immune regulation which helps to preserve a symbiotic environment and regulate the gut microbiota ecology. Previous studies have revealed that the dysregulation of pathogen recognition receptor expression could be widely observed in patients with IBD, for example, the mutation of NOD-2 has been identified in patients suffering from Crohn’s disease (Ogura et al., 2001). NOD-2 expression in intestinal epithelial cells has been linked with gut inflammation and homeostasis (Ferrand et al., 2019). Nod2−/− mice were also conducted to prove the functions of NOD-2 in the intestine, indicating that deficiency of NOD-2 could induce the expansion of Bacteroides vulgatus and exacerbate mucosal inflammation (Ramanan et al., 2014). Another example of the connections between gut microbiota, epithelium, and immune systems is the Toll-like receptor 4 (TLR4), which has also been connected with IBD and expressed in epithelial cells (Dheer et al., 2016). TLR4−/− mice experienced enhanced susceptibility to colon inflammation due to the dysregulation of Akkermansia muciniphila-associated immune response (Liu et al., 2022).
2.3. Immune systems
The intestinal immune systems can be divided into innate and adaptive immunity protecting the gastrointestinal tracts from various diseases. In homeostasis conditions, the immune system maintains a delicate balance between numerous immune cells and contributes to the tolerance of humans to dietary factors and gut microbiota. However, the unbalanced modulation of immune systems has also been regarded as a potential trigger for the incidence of IBD.
The innate immune system, including neutrophils, macrophages, and dendritic cells (DCs), constitutes the first line of defense against any infiltration and exposure. The infiltration of neutrophils in intestinal tissues which can be observed throughout the progress of active IBD is one of the earliest markers for gut-related inflammatory diseases (de Souza and Fiocchi, 2016). Neutrophils contribute to the progress of IBD via numerous mechanisms, such as neutrophil extracellular traps and myeloperoxidase which are also activated and upregulated in patients suffering from ulcerative colitis (Bennike et al., 2015; Dinallo et al., 2019). A prior study has also observed the crucial role of neutrophils in IBD with the conduction of BATF3 deficiency mice, supporting that intestinal epithelium-derived BATF3 in mice could promote colitis and even colonic cancer via increasing the number of neutrophils (Lin et al., 2021). The functions of macrophages in IBD are plastic, and macrophages have been classified as M1 and M2 with different cytokine patterns and various biological effects (Lissner et al., 2015; Wynn et al., 2013). M1-type macrophages perform important roles in promoting inflammation conditions via secreting cytokines, including IL-1β, IL-6, TNF-α, etc., and promoting expression of inducible nitric oxide synthase (iNOS) (Yip et al., 2021). With the expression of those factors, macrophages can further influence the regulation of adaptive immune systems and promote IBD. In contrast, M2 macrophages have been classified as the anti-inflammatory type that can produce immunosuppressant factors, such as IL-10 and TGF-β (Yang et al., 2022). Previous research based on DSS-induced colitis in mice also indicated that colonic inflammation could be promoted via facilitating macrophage M1 polarization (Wei et al., 2020). Moreover, there are also some strategies tried to manipulate the polarization of macrophages to alleviate IBD (Liu, Ren et al., 2022). Besides secreting various cytokines, macrophages can also act as antigen-presenting cells to link innate and adaptive systems, and this similar function can also be observed in DCs, which can monitor the surrounding conditions and present antigens to induce adaptive immune responses. Intestinal DCs normally maintain a “hyporesponsive and tolerogenic state” in humans, however, studies implied an improper conditioning of DCs that were activated with high levels of TLR2 and TLR4 in patients suffering from IBD (Hart et al., 2005). Those dysregulated DCs could migrate to peripheral lymphoid tissues and further activate the antigen-specific response of T cells. The crucial role of DCs in the progress of IBD was also proved by previous studies that the DCs-derived CD134L was involved in the incidence of T cell transfer-induced colitis in murine models (Malmström et al., 2001).
In contrast to the innate immune systems, initiating adaptive immunity requires antigens and activated antigen-presenting cells. The gut homeostasis requires the delicate regulation between T cells, the key players of adaptive immunity. Previously, Crohn’s disease was postulated to be a Th1-driven condition due to the increasing expression of IL-12 and IFN-γ, while ulcerative colitis was closely linked with unconventional regulation of Th2 accompanied by elevated expression of IL-5 and IL-13 (Camoglio et al., 1998; Kobayashi et al., 2020). Th1 cells are crucial for eradicating intracellular pathogens, such as bacteria and viruses (Raphael et al., 2015), these cells can be induced with IL-12 produced by antigen-presenting cells upon antigens recognition (Gomez-Bris et al., 2023). In homeostasis condition, only a few CD4+ T cells could be observed in the gut, however, Th1 cells accumulated in the GI tracts of some patients with IBD and have been linked with the progress of diseases (Imam et al., 2018). IFN-γ has been applied as the defining cytokine expressed by Th1 cells, Nava et al. (Nava et al., 2010) demonstrated that IFN-γ could regulate intestinal epithelial homeostasis and loss of IFN-γ decreased colitis in mice models, indicating the potential pro-inflammatory of IFN-γ in IBD. However, some studies also mentioned the controversial role of IFN-γ in intestinal inflammation, Muzaki et al. (Muzaki et al., 2016) found that intestinal CD103+CD11b− DCs improved IBD via IFN-γ-induced anti-inflammatory responses. Th1 cells have also been defined as a source for the expression of TNF-α which has also been connected with the dysregulation of the intestinal barrier in intestinal inflammation and anti-TNF therapies have been applied for IBD treatment in patients (Kobayashi et al., 2020; Nava et al., 2010). Th2 cells normally contribute to the elimination of the parasite and participate in the regulation of immunity with other cells by producing various cytokines, including IL-4, IL-5, and IL-13 (Zeng, 2013), which can prevent the development of Th1 cells and activate the regulation of macrophages (Gomez-Bris et al., 2023; Raphael et al., 2015).
Besides the participation of Th1 and Th2 cells in IBD, emerging studies also demonstrated that there are some other subsets of CD4+ T cells contributing to the progress of IBD. Th17 cells have been regarded as pathogenic cells concerning the incidence of IBD, and previous clinical results observed an increased number of Th17 cells in patients suffering from IBD compared with healthy individuals (Annunziato et al., 2007; Caprioli et al., 2008; Lu et al., 2022). These cells can contribute to the regulation of immune systems via producing various cytokines, including IL-17, IL-21, and IL-22. Alexander et al. have demonstrated that gut microbiota can directly worsen DSS-induced colitis in mice via inducing the activation of Th17 cells and the expression of IL-17 (Alexander et al., 2022). Treg cells are essential for maintaining immunity tolerance, and the defect in Treg cells underlie autoimmune-related diseases and IBD (Mayne and Williams, 2013). Treg cells (CD4+FoxP3+CD25+) have been proven to be the existence in the mucosal of mice (Mottet et al., 2003). The crucial functions of these cells in IBD have also been identified with adoptive transfer colitis in mice, which induced colonic inflammation via adoptive transferring CD4+ T cells (CD4+CD45RBhigh T cells) into Rag1-/- mice (Kiesler et al., 2015). The initial IBD cause in this model has been linked to the lack of Treg cells in those transferred naïve CD4+ T cells. The anti-inflammatory and negative immunoregulatory roles of Treg in the gastrointestinal tracts can be exerted by numerous mechanisms, such as the expression of IL-10 and TGF-β (Vignali et al., 2008). Previous studies have highlighted the immunomodulatory functions of IL-10 with the conduction of IL10−/− mice as spontaneously developed colitis models, showing the important role of IL-10 in the regulation of immune homeostasis (Gunasekera et al., 2020; Wilson et al., 2011).
Overall, these various and complicated mechanisms for the incidence of IBD present great challenges in understanding the interactions between lipid compounds and gut health. Dietary and gut microbiota-derived lipids may influence intestinal homeostasis and IBD progress through the mentioned one or several aspects.
| 3. Regulation of IBD by dietary lipids | ▴Top |
The GI tract can digest and absorb nearly 95% of dietary lipid substances and those left lipids can therefore interact with gut microbiota. Accumulating results have suggested that dietary oils and fat could contribute to the modulation of gut microbiota composition and regulate gut homeostasis conditions (Murphy et al., 2015; Röytiö et al., 2017). With these studies, it has been shown that the amount of daily intake of different fats with varying fatty acid composition and other fat-soluble components can be linked with gut inflammation and homeostasis regulation.
3.1. High-fat diet
Previous studies have connected high-fat diets (HFD), especially saturated fats, with systemic inflammation and diseases (Murphy et al., 2015). The gut microbiota as another fat consumer in humans can also be affected by HFD. Due to the exposure to dietary factors, the composition of these microbes colonizing in the gut also differentially develops reflecting the fluctuating diet. It has been demonstrated that HFD can induce a decrease in the richness and diversity of gut microbiota composition. A study has reported that HFD could induce a decrease in Bacteroidetes accompanied by an increase in Proteobacteria (Hildebrandt et al., 2009; Wolters et al., 2019). Although the clear mechanisms remain unknown, emerging studies have suggested that HFD-induced gut microbiota dysbiosis could act as a regulator to promote the related symptoms of IBD, demonstrating a promoted bacteria-derived endotoxemia as a direct evidence of increased gut permeability and increased lipopolysaccharide (LPS) in animal models and human (Cani et al., 2008; de la Serre et al., 2010; Serino et al., 2011). Moreover, research on HFD-induced gut dysbiosis has provided information for underlying mechanisms revealing the harmful effects of HFD-modulated gut microbiota on the intestinal barrier. HFD could reduce the abundance of some intestinal barrier-improving bacteria, including Bifidobacterium, Lactobacillus, and Akkermansia muciniphilia, accompanied by increasing bacteria lined with damaged barrier functions, including Oscillibacter and Escherichia coli (Anderson et al., 2010; Cani et al., 2007; Devkota et al., 2012; Lam et al., 2015; Lin et al., 2016; Sun et al., 2016; Sun et al., 2020). Although the clear mechanisms of the interactions between those specific bacteria and the gut barrier are still unclear, bacteria-derived metabolites may play a role in the maintenance of the gut barrier. Extracellular vesicles produced by Akkermansia muciniphilia play a crucial role in controlling gut barrier functions by promoting the expression of tight junction proteins (Chelakkot et al., 2018a). Similarly, Bifidobacterium and Lactobacillus may have beneficial effects on improving gut barrier functions and tight junction proteins via unknown mechanisms (Anderson et al., 2010; Hsieh et al., 2015). In contrast, the HFD-induced increased bacteria, Oscillibacter, has been directly linked to a decrease in the expression of tight junction proteins and an improvement of intestinal barrier (Lam et al., 2012). At this point, an increase in LPS associated with HFD feeding has also been found to modulate tight junction proteins and promote permeability in cell levels by activating the NF-κB pathway (Guo et al., 2013). Moreover, Escherichia coli as the pathogenic microorganism is also able to modulate the barrier functions by degrading the mucins and damaging the mucus layer with the expression of proteases (Paone and Cani, 2020). In addition, previous studies have also emphasized that the gut bacteria from HFD-treated mice was able to activate some pro-inflammatory pathways in germ-free mice, proving the potential interactions between HFD-modulated gut microbiota and immune systems (Ding et al., 2010). The pro-inflammatory effects of HFD on the intestinal tracts are normally linked with the increase of LPS which is a pro-inflammatory factor in immune systems, for example, LPS can induce the polarization of M1 macrophages (Zheng et al., 2013). Meanwhile, the decrease in some specific bacteria may also induce the modulation of immune systems. With the conduction of TLR4−/− mice, Liu et al. reported that the decrease of Akkermansia muciniphilia might induce the dysregulation of RORγt+ Treg cell and further exacerbated DSS-induced colitis in mice (Liu et al., 2022). Interestingly, the influence of HFD on gut microbiota can also modulate the gut homeostasis and development of offspring. Xie et al. (Xie et al., 2018) found that the consumption of HFD during pregnancy could induce gut microbiota dysbiosis and low-grade inflammation in offspring, even further promoting susceptibility to experimental colonic inflammation in adulthood.
In addition to the gut microbiota that has been described, HFD can also modulate gut homeostasis and contribute to the progress of IBD via direct or indirect ways. Recent studies have pointed out that the potential influence of HFD on the composition and functions of the mucus layer is mainly to affect its major protein, mucin, which is a high molecular weight and heavily O-glycosylated protein expressed by goblet cells. For example, HFD feeding for 25 weeks changed the oligosaccharide chain of mucin in mice and further contributed to colon inflammation (Mastrodonato et al., 2014). Although clear mechanisms were not provided in this study, the influence of HFD feeding on mucin was hypothesized to be connected with the glycosylation defect or incomplete maturation of goblet cells. The consumption of HFD can also induce endoplasmic reticulum /oxidative stress in goblet cells, triggering the unfolded protein response and damaging the formation of mucus layer (Gulhane et al., 2016). Moreover, in the small intestine, HFD can modify the PPAR-γ pathway in mice to repress the expression of Cftr gene, leading to a reduction in chloride and loss of mucus barrier integrity, which may contribute to the incidence of IBD (Tomas et al., 2016). Other studies also parsed out the influence of HFD on other components of the intestinal barrier, for example, an HFD pattern reduced the expression of tight junction proteins and thus promoted gut permeability in mice (Cani et al., 2008; Kirpich et al., 2012). Similarly, effects of HFD on tight junction proteins were also observed in obese (Otsuka Long Evans Tokushima Fatty) and lean rat (Long Evans Tokushima Otsuka) strains, indicating that the suppression of tight junction proteins could be mainly attributed to HFD rather than obesity and related modulated metabolism (Suzuki and Hara, 2010). Apart from the influence of HFD-modulated gut microbiota, the direct impact of HFD on tight junction proteins was also observed based on related cell models. Directly treating Caco-2 cells with dietary fatty acids contained in HFD could induce promoted tight junction permeability via protein kinase C (PKC) regulation (Usami et al., 2003). Those results indicated that HFD can also interact with intestinal epithelium and change its functions. With these concepts, other studies also found that the HFD could also induce gut barrier dysfunction by increasing the apoptotic rate of intestinal epithelial cells. The accumulation of long-chain fatty acids in the intestine with HFD treatment has been proven as a potential cause to increase ROS production, mitochondrial dysregulation, and pro-activating apoptotic-related pathways (Li and Li, 2020). Moreover, the consumption of HFD can also modulate the bile acids profile, such as the increase of taurocholic acid and deoxycholic acid, then inducing the damage of intestinal epithelial cells (Barrasa et al., 2011; Wan et al., 2020; Wolf et al., 2020).
HFD has also been connected with the disordered regulation of immune systems in IBD. In the DSS-induced colitis model, the treatment of HFD exacerbated the symptoms of inflammation accompanied by decreased Treg cells in the colon (Ma et al., 2007). Apart from the influence of HFD-changed gut microbiota and bile salts on the intestine (Zhao et al., 2020), there are emerging studies that have reported the potential effects of HFD-induced obesity and adipose tissue on immune dysregulation in IBD and related diseases. HFD-induced obesity could accelerate experimental colitis-associated colorectal cancer by exacerbating inflammation and dysregulation of immune systems (Wunderlich et al., 2018). The obesity-exacerbated colonic cancer has been mainly attributed to the shifted macrophage polarization by obesity-induced IL-6, which further expresses CCL-20 and recruits CCR-6-expressing B cells and γδ T cells. Similarly, Wei et al. also revealed that HFD could modulate the miRNA profiles of visceral adipose exosomes, changing these exosomes from anti-inflammation to pro-inflammation type. Interestingly, those pro-inflammatory exosomes can then circulate to the GI tracts and exacerbate DSS-induced colitis in mice by enhancing M1 macrophage differentiation (Wei et al., 2020) (Figure 2).
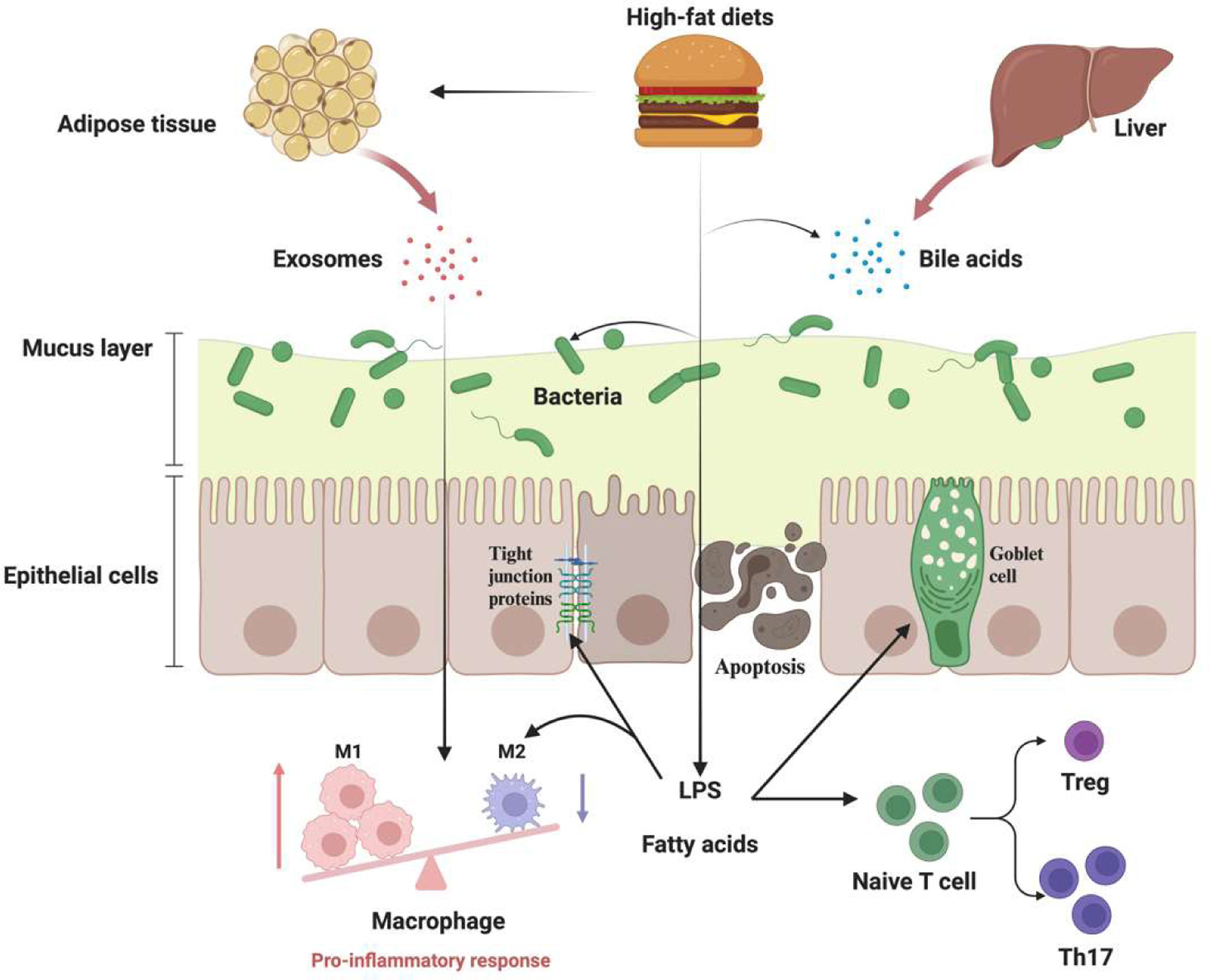 Click for large image | Figure 2. The potential effects of high fat diets (HFD) on intestinal homeostasis and inflammatory bowel disease (IBD). The HFD-induced dysbiosis of gut microbiota, including the decrease in richness and imbalance of the Firmicutes/Bacteroidetes ratio, has been widely linked with bacteria-derived lipopolysaccharide (LPS) that can further trigger low-grade inflammation and immune dysregulations. The mucus layer, a crucial physical barrier in the intestinal, can also be damaged with HFD due to the dysfunction of goblet cells and alteration of peroxisome proliferator-activated receptor gamma (PPAR-γ) pathway. HFD can also induce gut permeability by modulating tight junction proteins and epithelial cell apoptosis. HFD-induced obesity is also associated with the progress of IBD via promoting M1 macrophage differentiation and recruiting lymphocytes. Figure 2 was created in Biorender.com (Agreement number: MC27CARFOD). |
3.2. Ketogenic diet
A ketogenic diet with about 90% calories from fat has been practiced by many people for weight loss and blood sugar control (Hall and Chung, 2018). Ketogenic diets are effective in achieving ketosis which is an anti-inflammatory state in humans with an increase in blood acetoacetate and β-hydroxybutyrate. Even though it is controversial, some studies also found that ketogenic diets could affect the gut microbiota composition in humans with a promotion abundance of some SCFAs-producing bacteria (such as Lactobacillus and Bacteroides) (Alsharairi, 2022). Due to the nonnegligible roles of SCFAs in IBD, it was also hypothesized that the consumption of the ketogenic diet might exert beneficial effects on IBD patients. The significant impact of ketogenic diets on gut microbiota composition was also detected in another inpatient crossover study (Ang et al., 2020).
The effects of ketogenic diets on IBD and the potential underlying mechanisms have been examined based on animal models. The modulation effects of ketogenic diets on gut microbiota were also observed in mice, accompanied by a significant decrease in bifidobacteria (Ang et al., 2020). Furthermore, this study showed that the adjusted gut microbiota composition in ketogenic diet-fed mice could be mainly attributed to the increased level of ketone bodies. As a consequence of ketogenic diet-induced gut microbiota alteration, this dietary pattern could reduce the levels of Th17 cells in the intestinal tissues, emphasizing the potential anti-inflammatory effects of ketogenic diets in IBD. Analogical effects of ketogenic diets on gut microbiota and immune systems were also observed in mice with DSS-induced colitis, Kong et al. suggested that ketogenic diets could alleviate colonic inflammation by decreasing the production of RORγt+CD3− group 3 innate lymphoid cells (Kong et al., 2021). With the conduction of germ-free mice and FMT experiments, the beneficial effects of ketogenic diets on colitis and immune system regulation have been connected with the regulation of gut microbiota composition. Conversely, another study also demonstrated that ketogenic diets could aggravate DSS-induced colitis in mice with promoted inflammatory symptoms and gut microbiota dysbiosis when compared with control diet-fed groups (Li et al., 2021). Some human studies and investigations also mention the potential negative influence of ketogenic diets on gut health and microbiota, highlighting the necessity to further examine the safety and clear mechanisms of ketogenic diets and gut ecology (Gentile and Weir, 2018).
3.3. Fatty acids
In addition to the amount of total dietary fat, the composition of fatty acids could also influence the gut microbiota and even induce harmful or beneficial effects (Table 1). Previous studies have suggested that the composition of gut microbiota is significantly different between murine models treated with polyunsaturated fatty acids enriched fish oil and saturated fatty acids enriched with lard (Li et al., 2017). Meanwhile, Haskey et al. (2022) compared the influence of different dietary oils on the progress of IBD by conducting Muc2−/− mice as a model, implying that the fatty acid composition of fat might also impact colitis in mice.
 Click to view | Table 1. The main effects of dietary fatty acids on the gut microbiota |
3.3.1. Saturated fatty acids (SFAs)
Numerous studies have demonstrated that SFAs are potential pro-inflammatory dietary factors for human health, especially in gastrointestinal tracts. The intake of lard oil could significantly dysregulate the composition of gut microbiota with a decrease in diversity and richness (Liu et al., 2019). The previous results also reported the potential connections between SFAs-induced gut microbiota dysbiosis and IBD, Devkota et al. (2012) showed that the treatment of SFAs (milk-derived) could promote the expansion of Bilophila wadsworthia which is a kind of pathobiont and exacerbate colitis in IL10−/− mice with Th1 immune response. Some clinical studies also emphasized the potentially harmful influence of dietary SFAs on gut microbiota in humans, for example, the increase in SFA consumption has been linked to the modulation of some pro-inflammatory bacteria (including Proteobacteria) during pregnancy (Mandal et al., 2016). These studies clearly revealed that the intake of SFAs could modulate the composition of gut microbiota, and this disturbed bacterial ecology may further aggravate intestinal inflammation.
Although the mechanisms remain unclear, the direct pro-inflammatory effects of SFAs in the gastrointestinal tract have also been reported in previous studies. It has been revealed that SFAs might increase intestinal permeability and serum LPS amount by damaging the gut barrier. Ghezzal et al. (2020) showed that palmitic acid treatment could decrease the expression of tight junction proteins and cause their mislocalization between cells, leading to a decrease in the mucosal bar. This study also demonstrated that the SFA treatment could promote the expression of pro-inflammatory factors in mice, including IL-1β. The similar pro-inflammatory effects of SFAs-contained diets have been widely proven, the administration of myristate and stearate could induce proinflammation activation via activating IL-1β and TNF-α in human monocytes, whereas these effects could not be induced with the unsaturated fatty acids (Pillon et al., 2016). The pro-inflammatory pathway, NF-κB, could also be modulated with the treatment of SFAs (Lee et al., 2003). In summary, these studies clearly showed that SFAs and related diet patterns may negatively influence gut permeability and these dietary factors can also promote the production of inflammatory-related factors, which may further contribute to the progress of IBD.
3.3.2. Medium-chain fatty acids (MCFAs)
MCFAs are normally characterized as fatty acids with shorter carbon chains (C6-C12) that can be directly absorbed by humans and quickly supply energy (Jia et al., 2020a). Nowadays, the potential influence of MCFAs on IBD has been elucidated with some clinical and epidemiological results. With the analysis of fecal samples with the GC-mass method, significantly decreased MCFAs (including pentanoate, hexanoate, heptanoate, and octanoate) have been reported in IBD patients, and MCFAs have been regarded as a potential metabolic biomarker (De Preter et al., 2015). Similar results were also shown in adolescent patients with ulcerative colitis, the amount of lauric acid in the blood was higher during the active phase compared with quiescent conditions (Kikut et al., 2022). These results supported that MCFAs might play a crucial role in the treatment and prediction of IBD. However, the actual application of MCFAs as the medical therapeutic for IBD is still challenged, while the supplementation of MCFAs may cause the deficiency of other functional fatty acids and fat-soluble nutrients (Łoś-Rycharska et al., 2016).
MCFAs can improve the progression of IBD by modulating inflammatory factors and the gut barrier. The treatment of lauric acid could decrease the promoted level of some inflammatory factors via modulating TLR4/MyD88 pathway in rats with LPS-induced inflammations (Khan et al., 2021). Moreover, glycerol monolaurate, the mono-ester formed from glycerol and lauric acid, was reported that can attenuate DSS-induced colitis by promoting colonic Foxp3+ Tregs cells and ratio of serum anti-inflammatory/proinflammatory cytokines, as well as reconstructing microbial communities(Mo et al., 2021). The Black Soldier Fly Larvae’s oil which contains a high amount of MCFAs could ameliorate colitis in mice (Richter et al., 2023). Further results from transcriptome analysis provided potential mechanisms that these functional oil components could regulate mTOR signaling and promote the expression of PPAR-related genes. The beneficial effects of MCFAs were also observed in TNBS-induced colitis in mice, while the treatment of dietary medium-chain triglycerides could alleviate colitis with the inhibition of IL-1β, TNF-α, and MPO expression (Kono et al., 2010). The impact of MCFAs on the gut barrier has also been examined in epithelial cell lines, caprylic and nonanoic acid could reduce bacterial translocation and enhance barrier functions by attenuating the activity of the histone deacetylase pathway (Wang et al., 2018). Based on the samples from IBD patients and healthy individuals, the influence of sodium caprate on paracellular permeability and tight junction proteins has also been confirmed. Although clear information is still limited in IBD, the gut microbiota regulation roles of MCFAs have also been reported. MCFAs could prevent body weight gain in high-fat diet-treated mice and modulate the gut microbiota composition, including decreased Firmicutes/Bacteroidetes ratio and Proteobacteria (Zhou et al., 2017). Moreover, an increase in SCFAs was also induced with the treatment of MCFAs, emphasizing the potential beneficial role of MCFAs on gut ecology.
3.3.3. Monounsaturated fatty acids (MUFAs)
MUFAs that contain a carbon double-bond and mainly include palmitoleic and oleic acid can be found in the human daily diets, such as olive oil and nuts (Las Heras et al., 2022). Numerous studies have linked MUFAs with some beneficial effects on gut microbiota and inflammation-related diseases (Statovci et al., 2017). Among MUFAs, the effective anti-inflammatory activities of palmitoleic acid in IBD have been reported. When cultured colonic tissue of IBD patients was treated with palmitoleic acid, the expression of inflammation-related factors, such as IL-6 and TNF-α, was significantly reduced (Chen et al., 2023b). This study also observed that the oral intake of palmitoleic acid could alleviate experimental colitis in mice models and reshape gut microbiota composition with an increase in the abundance of Akkermansia muciniphila. Macrophage is another potential target, chronic palmitoleic acid supplementation can reduce the systemic level of IL-1β by evoking the lipidomic remodeling of the endoplasmic reticulum in macrophages (Çimen et al., 2016). Souza et al. also reported that palmitoleic acid could decrease LPS-induced pro-inflammatory responses in macrophages and alter the ratio of M1/M2 in a PPARα-independent manner (Souza et al., 2017).
The effects of another MUFA (oleic acid) on gut health and related mechanisms are unclear. With the application of 7-day food diaries in prospective cohort investigation, oleic acid was recognized as a beneficial dietary factor in decreasing the incidence of ulcerative colitis (de Silva et al., 2014). The animal studies based on rats treated with acorn-fed ham which contains high levels of oleic acid supported the potential intervention role of oleic acid in the management of IBD (Fernández et al., 2020). In this study, the consumption of oleic acid could alleviate the symptoms of IBD and also reduce the amount of some proinflammatory factors (including IL-17 and IFN-γ). The reconstruction of gut microbiota composition was also mentioned in this model, showing that the treatment of oleic acid-enriched diets was sufficient to promote the abundance of some anti-inflammatory bacteria and the concentration of SCFAs. Similarly, supplementation of oleic acid could significantly counteract the HFD-perturbed gut microbiota dysregulation, accompanied by increased total bacteria density (Mujico et al., 2013). However, some contradictory results were also reported, a clinical study previously compared the influence of two dietary patterns with different lipid compositions (79% and 28% oleic acid) on patients suffering from active Crohn’s disease, reporting that the remission rate of the higher oleic acid treated group was lower (Gassull et al., 2002). Furthermore, some studies also supported that the intake of oleic acid might promote the risk of the incidence of IBD (Sugihara et al., 2018; Ye et al., 2021). Therefore, more studies and clinical data are still required to further clarify the influence of oleic acid on gut health and diseases based on various models.
3.3.4. Polyunsaturated fatty acids (PUFAs)
PUFAs, including ω-3 and ω-6 fatty acids that contain multi-double bonds, are also abundant fatty acids in human diets, The supplement of PUFAs is crucial for many biological activities in humans because they cannot be directly produced by humans and daily diets are the major exogenous resource. Numerous studies have been conducted to declare the potential beneficial effects of ω-3 fatty acids including α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). However, there are still some debates about the influence of ω-6 fatty acids including linoleic acid (LA) and arachidonic acid (AA) as many studies conflict with others and generate the mixed results of the potential pro-inflammatory or anti-inflammatory activity.
Accumulating evidence based on clinical trials suggested the crucial roles of PUFAs in the progression of IBD. A decreased level of serum ω-3 fatty acids has been found in patients suffering from ulcerative colitis and Crohn’s disease compared with normal individuals (Jiang et al., 2023). A previous 170,805 women included prospective study suggested that cumulative energy-adjusted consumption of PUFAs could not promote the incidence and risk of IBD, and the excessive dietary ω-3 fatty acids could be linked with a lower risk of ulcerative colitis (Ananthakrishnan et al., 2014). Similar anti-inflammatory effects of ω-3 fatty acids were also supported by a previous Mendelian randomization analysis, demonstrating the beneficial role of ω-3 fatty acids in IBD (Astore et al., 2022). Different from the desired influence of dietary ω-3 fatty acids in IBD, numerous studies have concluded that ω-6 fatty acids could be associated with increasing IBD pathogenesis. Dietary LA is metabolized to AA and then causes a direct influence on health. In patients with active IBD, the amount of AA was significantly increased compared with healthy individuals, and these results have been partially attributed to the promoted infiltration of inflammatory cells (Nishida et al., 1987). A prospective cohort study that involved 203,193 individuals was also conducted further to uncover the role of LA in IBD and demonstrated that increased LA consumption could contribute to the promoted risk of IBD (Tjonneland et al., 2009). Conversed with other ω-6 fatty acids, conjugated linoleic acid (CLA) could exert an anti-inflammatory activity in patients with Crohn’s disease by suppressing the expression of IL-17, IFN-γ, and TNF-α from CD4+ and CD8+ T cells (Bassaganya-Riera et al., 2012).
Dietary PUFAs can influence the incidence and pathogenesis of IBD by directly modulating inflammatory-related factors and pathways. The clear role of ω-3 fatty acids in IBD was discovered based on transgenic fat-1 mice that could produce ω-3 fatty acids without any diet supplement. Those endogenous ω-3 fatty acids could relieve DSS-induced colitis and the anti-inflammatory effects have been partially connected with the suppressed NF-κB activity (Hudert et al., 2006). Similar results were also observed in rats with experimental-induced Crohn’s disease, intake of ω-3 fatty acids could improve the symptoms of IBD accompanied by an increase in the activation of PPAR-γ (Yao et al., 2017). Although underlying mechanisms are still unclear, various studies have been performed to investigate the influence of some individual ω-3 fatty acids on IBD. For example, previous studies have shown that oral intake of ALA could improve DSS-induced colitis in mice (Kim et al., 2020). The anti-inflammatory effects of ALA were further revealed in Caco-2 cells, where the treatment of ALA could inhibit the expression of IL-8, COX2, and iNOS (Reifen et al., 2015a). The administration of EPA or DHA has also been supported as a potential measurement for IBD management based on animal results, showing that intake of EPA and DHA could attenuate DSS-induced colitis. This study also emphasized that EPA was more effective compared with DHA, and the underlying mechanisms might involve the NLRP3/IL-1β and IL-6/STAT3 inflammation-related pathways (Zhang et al., 2021). The exacerbation effects and potential mechanisms of ω-6 fatty acids have also been discussed based on in vivo and vitro models. A diet rich in LA could accelerate and exacerbate colitis in IL10−/− and wild-type mice (Deol et al., 2023). The metabolomic analysis showed that these adverse effects of LA could be linked to the increase of oxylipins and the decrease in endocannabinoid system metabolites. In patients with Crohn’s disease, the decreased activity of glutathione peroxidase 4 (GPX4) was observed in the small intestinal epithelial cells. The previous studies indicated that an AA diet could induce intestinal inflammation in Gpx4+/−IEC mice by triggering cytokine production similar to ferroptosis (Mayr et al., 2020). Different from LA and AA, intake of CLA could ameliorate chemically induced colitis in a dose-dependent manner, accompanied by decreasing inflammatory factors and improving gut barrier (Chen et al., 2019). Another study further provided evidence based on in vivo experiments and declared that the protective effects of CLA on IBD could be partly due to the activation of PPAR γ and δ (Bassaganya-Riera et al., 2004).
The dysbiosis of gut microbiota plays a crucial role in the progression of IBD, and PUFAs may impact IBD via modulating gut bacterial ecology. The associations between dietary ω-3 fatty acids and gut microbiota have been observed based on the data from 876 twins, emphasizing that ω-3 fatty acids supplementation could improve microbiome composition, especially for Lachnospiraceae (Menni et al., 2017). In the colitis mice model, ω-3 fatty acids could also improve the inflammation symptoms and modulate the composition of gut microbiota, including some differential bacterial Akkermansia, Alistipes, and Lactobacillus (Dong et al., 2022). Moreover, the beneficial effects of CLA on IBD have also been partly attributed to its modulation functions on gut microbiota. The administration of 40 mg CLA per day could rebalance the DSS-induced dysbiosis in gut bacteria, while a decrease in Bacteroides and an increase in Bifidobacterium have been reported in the CLA-treated group (Chen et al., 2019). Promoting effects of LA on gut dysbiosis have also been revealed in previous study, which reported an increased abundance of Escherichia coli in LA-fed mice and this bacteria might contribute to the exacerbation of IBD (Deol et al., 2023). Conflicts in the interactions between PUFAs and gut microbiota should also be mentioned, the intake of high-dose omega-3 PUFAs could not directly induce significant modulation effects on gut microbiota in healthy humans (Watson et al., 2018). These results clarified that the influence of PUFAs on gut microbiota and IBD is still unclear, and the potential effects of PUFAs on bacterial ecology may also be affected by other molecules or the health conditions of individuals. Due to the complexity and importance of the gut microbiome, more detailed and well-designed experiments (such as germ-free mice and FMT) are desired to be conducted to provide more information.
3.4. Cholesterol
Cholesterol is present in our daily diets and humans intake around 200 to 600 mg per day (Liu et al., 2023). It performs numerous crucial roles in humans, including maintaining the fluidity and structure of the cell membrane and serving as the precursor for the production of sex hormones and vitamin D (Olkkonen et al., 2017). However, the excessive intake of dietary cholesterol can be associated with the high risk of cardiovascular diseases (Zhong et al., 2019) and even cancer (Hu et al., 2012). The daily diet is one of the major sources of cholesterol for humans, and this exogenous cholesterol can enter the small intestine as micelle forms. It has been reported that only 50% of cholesterol is absorbed in the small intestine, indicating that the direct interaction between dietary cholesterol and colon cannot be negligible (van der Wulp et al., 2013). Meanwhile, the absorbed cholesterol could be applied to synthesize bile acids in the liver and further released to the gastrointestinal tracts which may also have some impacts on the homeostasis conditions of the gut (Collins et al., 2023). With those direct and indirect interactions between dietary cholesterol and the gut, the potential influence of high-cholesterol diets on IBD has received some attention.
Prolonged consumption of high-cholesterol diets has been connected with various chronic and inflammatory diseases in intestine tracts. Previous studies have also demonstrated that intake of high-cholesterol diets could induce the acute inflammation response in the intestine with an IL-1β-dependent increase of myeloid cells in both murine and zebrafish models (Progatzky et al., 2014). These inflammatory responses during the treatment of high-cholesterol diets have been mainly attributed to the direct influence of ingested dietary cholesterol on the inflammasome activation in epithelial cells. Concerning the potential influence of high-cholesterol diets on inflammasome, some studies have investigated the interactions between high-cholesterol diets and inflammasome-related diseases. Du et al. (Du et al., 2016) reported that the intake of high-cholesterol diets could promote inflammatory responses in the colon by the activation of NLRP3 inflammasome. Gao et al. (Gao et al., 2010) found that cholesterol diets could weaken the colon unfolded protein responses and then exacerbate colitis in mice, causing gut barrier damage.
Dietary cholesterol may also modulate the gut microbiota composition. Previous studies have reported that the long-term intake of high-cholesterol diets could drive liver cancer by altering the composition and metabolites of gut microbiota in mice (Zhang et al., 2021). Hao et al. (Hao et al., 2019; Hao et al., 2020) have also reported that the consumption of high-cholesterol diets could modulate the composition of gut microbiota and cause a decrease in short-chain fatty acids (SCFAs), including acetic, propionic, and butyric acids. As a favorable modulator of the immune system, gut barrier, and gut microbiota, the decrease in SCFAs has been regarded as a potentially harmful factor for the incidence of IBD (Caetano and Castelucci, 2022). Therefore, the potentially harmful effects of high-cholesterol diets on IBD may also be partially mediated by gut microbiota.
3.5. Oxidized cholesterol (OXC)
Dietary cholesterol is susceptible to oxidation to produce oxidized cholesterol (OXC) during various food processing stages (Xu et al., 2011). In diets, the oxidation of cholesterol mainly occurs with reactive oxygen species, and numerous kinds of OXC have been found in human diets, especially 7α-hydroxycholesterol, 7β-hydroxycholesterol, 5,6β-epoxycholesterol, 5,6α-epoxycholesterol, and 7-ketocholesterol. OXC has been widely regarded as a potentially harmful substance for human health (Deng et al., 2023). Furthermore, the amount of OXC in Western diets that contain high concentrations of cholesterol cannot be ignored. Previous studies have shown that the intake of OXC might reach up to 10% of cholesterol (Xu et al., 2009; Xu et al., 2011). OXC may be more toxic and harmful compared with naïve cholesterol in diets (Maldonado-Pereira et al., 2018).
OXC may induce oxidative stress and exert pro-inflammatory effects in humans. OXC has also been shown to be a potential trigger for IBD. The amount of serum OXC was significantly higher than cholesterol in patients suffering from IBD, suggesting potential associations between OXC and IBD (Akerlund et al., 1994). In this regard, Bai et al. (Bai et al., 2005) reported that 25-hydroxycholesterol significantly promoted the expression of IL-8 with the treatment of IL-1β in Caco-2 cell models. The pro-oxidant and pro-apoptosis effects of OXC mixture (including 7α-hydroxycholesterol, 7β-hydroxycholesterol, 7-ketocholesterol, 5α,6α-epoxycholesterol, 5β,6β-epoxycholesterol) were also observed at the cell level (Biasi et al., 2009). A previous proteomic study based on intestinal epithelial cells illustrated that the treatment of 7-ketocholesterol could affect the expression of proteins related to inflammation and mitochondrial functions (Laparra et al., 2015). Other studies also observed that OXC could stimulate the activation of caspase-3, enhance the expression of various inflammation-related factors, including TLR2, TLR9, and IL-6 (Biasi et al., 2009; Guina et al., 2015; Mascia et al., 2010). Exogenous OXC may also impair the function of the gut barrier and promote intestinal permeability. Chalubinski et al. (Chalubinski et al., 2014) found that the treatment of 7-ketocholesterol could damage the barrier function of the human intestinal epithelium, accompanied by a decrease in mRNA expression of ZO-1. OXC-induced fall of IL-10 expression was also observed in these studies, indicating that OXC might induce inappropriate inflammatory regulation. Similar results about the effects of OXC on the permeability of Caco-2 cells were also reported in other studies, finding that OXC could decrease levels of ZO-1, occludin, and junctional adhesion molecule-A (JAM-A) in OXC-treated cells (Deiana et al., 2017). Interestingly, the effects of OXC on intestinal barrier function were also associated with the induction of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) which had been shown to cause gut barrier damage via the degradation of junctional proteins (Meijer et al., 2007; Nighot et al., 2015).
The underlying mechanisms of the adverse effects of dietary OXC on IBD have been hypothesized to be mediated by gut microbiota based on recent animal-related research. The administration of 27-hydroxycholesterol via subcutaneous injection could induce gut microbiota dysbiosis in mice with a decrease in Roseburia and SCFAs, indicating the potential influence of OXC on gut microbiota (Wang et al., 2020). Similar aggravated gut microbiota dysbiosis was also observed in mice models with DSS-induced colitis, showing that exposure to OXC could exacerbate chemically induced inflammation in the intestine tracts with significantly increased body weight loss, colon length decrease, and bleeding (Yan et al., 2022). In this study, the gut microbiota composition was regulated by OXC with an increase in some harmful bacteria, such as Escherichia−Shigella and Bacteroides. With the further conduction of antibiotic treatment and FMT experiments, the exact mechanisms of OXC-promoted colitis in mice have been further revealed, and the crucial role of OXC-modulated gut microbiota was also uncovered (Yan et al., 2023). However, due to the difference between the gut microbiota in humans and mice, the influence of dietary OXC on patients with IBD is still limited, and some clinical or epidemiological investigations are still desired.
3.6. Fat soluble vitamin
Some fat-soluble components in dietary fat also play some roles in metabolic regulation and physiological functions. Recently, the potential influence of dietary fat-soluble vitamins (including vitamins A, D, and E) on gut ecology and inflammation has been examined. It had been shown that fat-soluble vitamins were quantitatively lower in patients with IBD compared with healthy individuals, emphasizing that their appropriate supplementation may improve these inflammatory diseases (Fabisiak et al., 2017).
3.6.1. Vitamin A
The deficiency of vitamin A is a worldwide health issue, especially in some developing countries. A previous study has implied that the deficiency of vitamin A in children could increase the risks of respiratory and diarrheal infections, and these harmful effects could be simply reversed with the intake of adequate vitamin A (Villamor and Fawzi, 2000). Moreover, the composition and dysbiosis of gut microbiota in humans may also be influenced by vitamin A. Human subjects with persistent diarrhea were selected to evaluate the effects of vitamin A deficiency on gut microbiome, and results showed a decrease in bacterial diversity in the vitamin A-deficient group with an increase in Enterococcus(Lv et al., 2016). Considering the potential connections between vitamin A and the incidence of IBD, the efficiency of vitamin A treatment on IBD has also been carried out. A daily intake of vitamin A (25,000 IU) for two months could significantly improve the clinical symptoms of ulcerative colitis, emphasizing the potential positive effects of vitamin A in IBD (Masnadi Shirazi et al., 2018). Various experiments have also been conducted in animal models to further support the underlying mechanisms for vitamin A supplementation. The influence of vitamin A deficiency on gut microbiota composition and ecology was also observed in mice. Results showed a decrease in butyrate in the vitamin A deficient group (Cha et al., 2010; Tian et al., 2018). The analogical influence of vitamin A treatment on the regulation of gut microbiota was also shown in DSS-induced mice. The oral intake of vitamin A could ameliorate colonic inflammation in mice with an increase in SCFAs-producing bacteria, and further FMT experiments also confirmed the positive effects of gut microbiome in the vitamin A-treated group (Pang et al., 2021). The supplementation of vitamin A may also directly exert anti-inflammatory functions in the progression of IBD. It has been demonstrated that the intake of vitamin A could increase the levels of NFR-1 and TFAM in both healthy and inflammatory colonic tissues, which supported the potential effects of vitamin A on the preservation of mitochondrial activity (Reifen et al., 2015b). The metabolite of vitamin A, retinoic acid, could also attenuate colonic inflammation and these anti-inflammatory effects have been partially associated with an increase in the expression of IL-22 by γδ T cells and innate lymphoid cells (Mielke et al., 2013). In addition, the beneficial effects of retinoic acid on IBD have also been connected with the regulation of Treg/Th17 profiles, while the balance and activity of related T cell populations could be regulated by retinoic acid treatment (Bai et al., 2009; Elias et al., 2008).
3.6.2. Vitamin D
Low vitamin D status has been linked with the incidence of various immune systems-related diseases, including IBD (Cantorna and Mahon, 2004). Since there are few natural vitamin D-rich diets, inadequate vitamin D consumption is also problematic. This insufficiency may be more severe in patients with IBD. Vitamin D deficiency has also been regarded as a potential risk involved in the incidence of intestinal inflammation (Levin et al., 2011; Siffledeen et al., 2003; Tajika et al., 2004). In vitamin D receptor-deficient mice, the symptoms of DSS-induced colitis were more severe and further supported the potential crucial roles of vitamin D in the progression of IBD (Kong et al., 2008). Not surprisingly, the supplement of vitamin D in patients with IBD has also been proven as an effective method to improve the disease course. A double-blind clinical study showed that the intake of vitamin D3 (1,200 IU per day) for 12 months could increase the serum levels of vitamin D and decrease the relapse rate of Crohn’s disease from 29% to 13% (Jørgensen et al., 2010). Resemble connections between dietary vitamin D and IBD were also revealed by 72,719 women enrolled prospective cohort study, reporting that higher vitamin D status might reduce the risk of IBD (Ananthakrishnan et al., 2012). Numerous studies have been conducted to further examine the direct role of vitamin D in IBD and underlying mechanisms were explored. In IL-10 knockout mice, the deficiency of vitamin D receptor could exacerbate the spontaneous colitis accompanied by a decrease in the number of lymphocytes, highlighting the potential regulation effects of vitamin D in the regulation of immune systems (Froicu et al., 2006). Another study also reported that the treatment of vitamin D3 could improve experimental colitis in mice by regulating the vitamin D receptor-NLRP6 signaling pathway (Gao et al., 2023). Moreover, the positive effects of vitamin D in IBD may also be mediated by gut microbiota. It has been shown that the gut microbiota dysbiosis occurred in vitamin D receptor knockout mice with an increase in Bacteroidetes and Proteobacteria. The antibiotic treatment further revealed that the aggravated effects of vitamin D deficiency on colitis were mediated by gut microbiota dysbiosis (Ooi et al., 2013).
3.6.3. Vitamin E
The deficiency of vitamin E has also been associated with IBD. Due to the various symptoms of IBD, including abdominal pain and damage in the intestinal tracts, the intake and absorption of some fat-soluble nutrients can be insufficient and inefficient, especially vitamin E (Fabisiak et al., 2017; Wu et al., 2024). Previous studies indicated that the nutritional risk was significantly higher in adolescents suffering from Crohn’s disease with a decrease in vitamin E intake and serum levels (Costa et al., 2015). An increased rate (42.8%) of low serum vitamin E was also shown in severe ulcerative colitis patients, which emphasized the potential connections between vitamin E and IBD (Bousvaros et al., 1998). Some investigations based on humans also supported the positive effects of vitamin E supplementation on IBD. The treatment of d-alpha tocopherol, a vitamin E isomer, could alleviate ulcerative colitis with a decrease in the disease activity index for 64% of patients (Mirbagheri et al., 2008). The anti-inflammatory effects of vitamin E have also been further revealed in animal models. Daily intake of vitamin E (30 U/kg) could suppress the progression of acetic acid-induced colitis in rats with prevented levels of IL-6, MPO, and malondialdehyde (Tahan et al., 2011). Another study also demonstrated that vitamin E could attenuate DSS-induced colitis in mice and further revealed the potential positive effects of vitamin E on DSS-induced barrier damage and gut microbiota dysbiosis (Liu et al., 2021). Although the intake of vitamin E might change the gut microbiota composition of DSS-treated mice with an increase in Roseburia, similar effects could not be detected in healthy mice. These studies highlighted the potential roles of gut microbiota in the treatment of vitamin E and some more detailed mechanisms are also required. As a powerful antioxidant, some studies also supported the positive functions of vitamin E in reducing levels of some pro-inflammatory factors in IBD. The administration of tocotrienol could mitigate DSS-induced colitis in mice with a significant decrease in nitric oxide, malondialdehyde, and COX-2, emphasizing the potential anti-oxidative stress effects of vitamin E in IBD (Saw et al., 2019).
3.7. Curcumin
It has been shown that curcumin possesses the extraordinary anti-inflammatory activity (Pituch-Zdanowska et al., 2022). One study based on healthy humans has found that the intake of curcumin might change the gut microbiota composition (Peterson et al., 2018). To further uncover the outcome of curcumin supplements in IBD, some clinical trials have been conducted. In patients with mild-to-moderate ulcerative colitis, the enema treatment of curcumin with the intake of 5-ASA could induce greater improvements compared with patients who received a placebo and 5-ASA (Singla et al., 2014). The comparable effects of curcumin on IBD were also observed during oral treatments. Intake of 3 g of curcumin per day with mesalamine could significantly improve the symptoms of patients with colitis, while 65.3% of patients achieved remission in clinical responses and only 12.5 % in the control group (Lang et al., 2015). Although the underlying mechanisms are still unclear, the intake of curcumin has been regarded as a potential dietary compound to partly relieve IBD without apparent side effects. Based on experimental IBD animal models, the positive effects of curcumin on gut health are further evaluated. Sugimoto et al. (2002) reported that the administration of a diet with curcumin could ameliorate TNBS-induced colitis in mice with the prevention of CD4+ T cell infiltration and inflammatory pathways activation. Similar results were also observed in DSS-induced colitis, while the treatment of curcumin could decrease the expression of IL-1β and suppress NLRP3 inflammasome activation. In this study, the application of the specific NLRP3 inhibitor could abrogate the effects of curcumin, indicating the potential influence of curcumin on IBD could be associated with the activation of NLRP3 inflammasome (Gong et al., 2018). The interactions between curcumin and gut microbiome have also been declared in animal models. The oral administration of curcumin could improve DSS-induced colitis in mice and change gut microbiota composition with an increase in Coprococcus, Roseburia, and Akkermansia (Guo et al., 2022). However, the exact roles of curcumin-altered gut microbiota are still unknown, and some clinical experiments or FMT are still desired.
3.8. Resveratrol
Resveratrol is a kind of polyphenol dietary component that has attracted much interest with its anti-inflammatory effects and positive influence on the gut microbiome. The potential beneficial impact of resveratrol on IBD has been revealed in previous human clinical trials, the intake of 500 mg of resveratrol for 6 weeks could significantly reduce the plasma levels of TNF-α and activity of NF-κB in peripheral blood mononuclear cells. The life quality of those patients with active mild-to-moderate ulcerative colitis could be improved, and the clinical disease activity index score was lower in the resveratrol-treated group (Samsami-kor et al., 2015). Moreover, the anti-oxidative effects of resveratrol were also observed in another study, demonstrating that resveratrol treatment in patients could increase the activity of superoxide dismutase and decrease the levels of malondialdehyde in serum (Samsamikor et al., 2016). The positive influence of resveratrol on IBD has also been partially linked with gut microbiome. In DSS-induced colitis mice, resveratrol could improve inflammatory symptoms and repress the expression of some pro-inflammatory cytokines, including GM-CSF, IL-1β, and IL-6 (Li et al., 2020). Those anti-inflammatory effects have been correlated with the regulation of gut microbiota composition, such as an increase in Bifidobacterium. Furthermore, fecal microbiota transplantation has also been performed in another study to reveal the crucial role of resveratrol-altered gut microbiome. Alrafas et al. (2019) found that resveratrol could attenuate TNBS-induced colitis and restore the gut microbiota homeostasis with an increase in i-butyric acid. More specifically, this study demostrated that the resveratrol-altered gut microbiota could promote the amount of CD4+FOXP3+ T cells and decrease Th17 cells including CD4+IFN-γ+ and CD4+IL-17+ T cells. Another study also highlighted a potential mechanism of resveratrol treatment in colitis, it suggested that resveratrol might regulate the gut microbiome- macrophage-arginine metabolism axis to improve IBD (Xu et al., 2023). The direct anti-inflammatory effects of resveratrol on IBD have also been evaluated in other animal studies, demonstrating that resveratrol could alleviate DSS-induced colitis in mice by regulating the PI3K/Akt/VEGFA pathway (Zhu et al., 2021). Although mechanisms are poorly understood, these animal and human studies have supported that resveratrol could exert beneficial effects on IBD.
| 4. Gut microbiome-derived lipids in IBD | ▴Top |
Lipid metabolites produced by gut microbiota can affect gut homeostasis and influence intestinal inflammation diseases. The lipid content in bacteria accounts for nearly 10% of the dry weight (Brown et al., 2023). Bacteria-derived lipids have long been investigated as structural components, especially in those bacterial membranes. Recent studies revealed that these substances could also be sensed by the immune system cells and regulate various pathways of the host (Chandler and Ernst, 2017). In this section, the possible influence of bacterial lipids on gut health will be discussed (Figure 3).
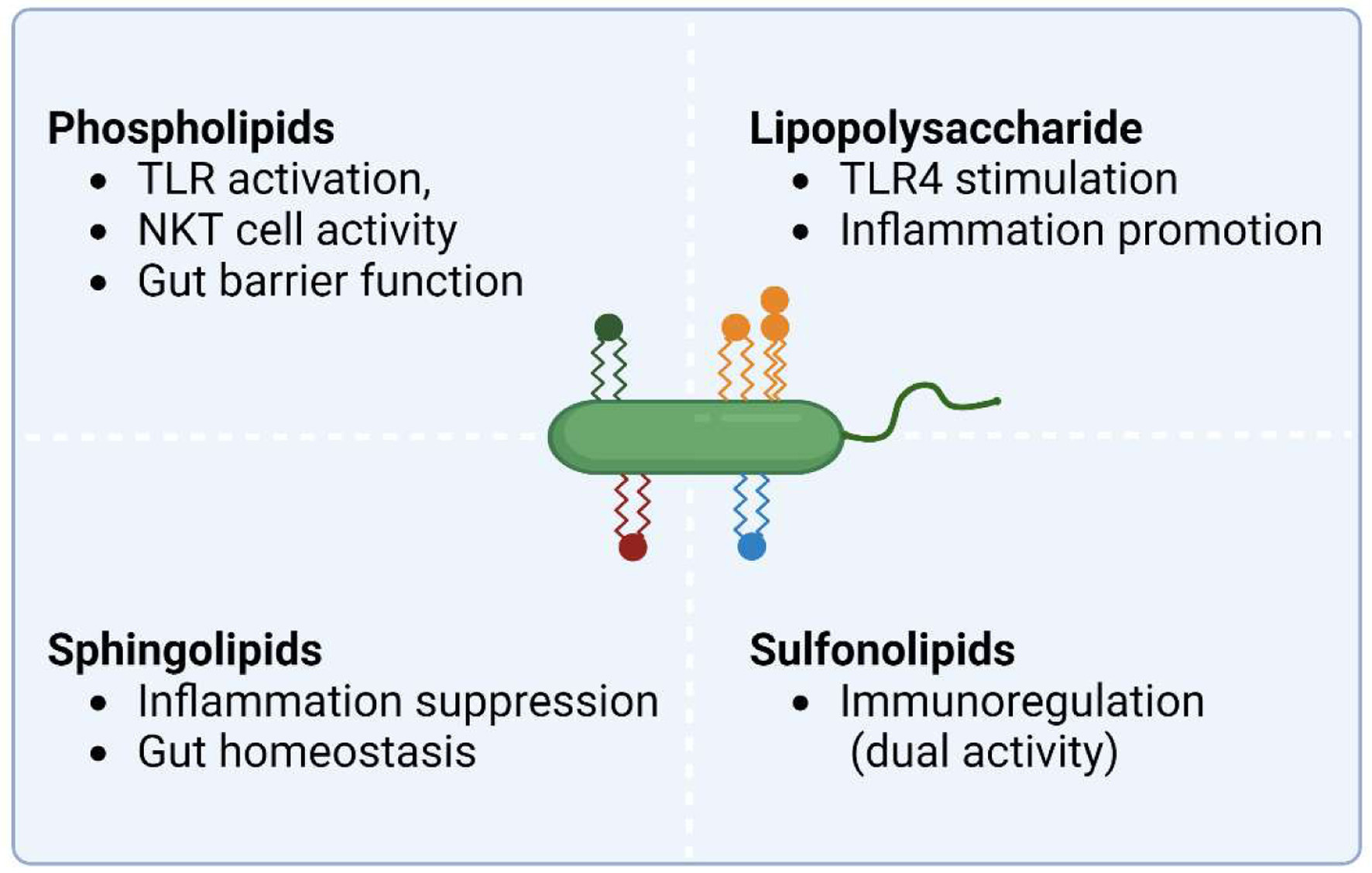 Click for large image | Figure 3. Gut microbiome-derived lipids and their known function in gut homeostasis. Phospholipids are the major components in bacterial membrane, lipopolysaccharide is a component found in Gram-negative bacteria which can activate the immune system through toll-like receptor 4 (TLR4), sphingolipids are produced mostly by commensal Bacteroidetes strains, sulfonolipids is present in some gut bacteria like Bacteroides, Alistipes, and Flavobacterium strains. Emerging studies have shown that these gut microbiota-derived lipids play some roles in gut barrier integrity and immunological regulation. Figure 3 was created in Biorender.com (Agreement number: GF27CARL9T). |
4.1. Phospholipids
Phospholipids have been known to be the most abundant in bacteria membrane. With the conduction of Escherichia coli models, the production and related pathways of phospholipids in bacteria have been uncovered (Zhang and Rock, 2008). It has been suggested that the structure and properties of phospholipids in the membrane could be altered by bacteria to overcome and adapt to a wide range of conditions (Zhang and Rock, 2008). Although the precise mechanisms and connections between gut environment-phospholipids-IBD are still unclear, some studies have already recognized some bacteria-derived phospholipids that could impact the gut health of the host. It has been shown that Akkermansia muciniphila could improve experimental colitis in mice by regulating the balance of T cells (Liu et al., 2022). However, some conflict results were also observed in IL-10 knockout mice, showing that Akkermansia muciniphila could promote intestinal inflammation (Seregin et al., 2017). Another study recently recognized an immunoregulation-related molecule from Akkermansia muciniphila, a diacyl phosphatidylethanolamine with two branched chains (a15:0-i15:0 PE), which could activate TLR2-TLR1 and induce the expression of some inflammatory cytokines, including TNF-α (Bae et al., 2022). Although the data were obtained from in vitro models, the potential immunoregulatory effects of bacteria-derived phospholipids on the progression of IBD cannot be ignored. Meanwhile, the regulation role of bacteria-derived phospholipids in gut health and inflammation was also revealed on some pathogens. Compared with mammalian phosphatidylglycerol, Listeria-derived phosphatidylglycerol contains shorter and fully-saturated anteiso fatty acid lipid tails, and it can bind to CD1b and be efficiently recognized by natural killer T (NKT) cells to regulate the immunity of the host (Wolf et al., 2015). NKT cells are a subset of T cells that have also been involved in the progression of IBD based on both clinical trials and murine models (Liao et al., 2013). In addition, previous studies also suggested the potential role of microbiome-derived phospholipids in the gut barrier possibly by changing the lipid profiles of host cells (Brown et al., 2023).
4.2. Sphingolipids
Some bacteria possess the ability to produce sphingolipids, mainly including Bacteroidetes. Deficiency of sphingolipids could induce the alteration of membrane structure, promote susceptibility to oxidative stress, and reduce the survival rate in stressful conditions (An et al., 2011; Heaver et al., 2022). The potential effects of bacteria-derived sphingolipids on the host and intestinal inflammation diseases have also been reported. In patients with IBD, the abundance of Bacteroidetes and microbiome-derived sphingolipids were reduced (Brown et al., 2019). In this study, the conduction of germ-free mice and the sphingolipid-deficient bacterial strain showed that the mono-treatment of sphingolipid-deficient bacteria could directly result in intestinal inflammation and gut barrier damage. Combined with the data from in vitro studies, microbiome-derived sphingolipids have been recognized as potential components in immune system regulation. Another study has also reported the immune system regulation effects of bacteria-derived sphingolipids. During neonatal development, sphingolipids produced by Bacteroides fragilis could impede invariant NKT cell proliferation and then reduce its number in adulthood (An et al., 2014). Moreover, the treatment of bacteria-derived sphingolipids could exert beneficial effects on oxazolone-induced colitis. Although the influence of sphingolipids on immune systems was only observed in the colonic tissues in this study, the potential connections between sphingolipids and the liver functions of the host have also been confirmed. Previous studies also demonstrated that the presence of microbiome-derived sphingolipids could improve fatty liver diseases in animal models, indicating that sphingolipids from bacteria may also have a systemic effect on the host (Le et al., 2022).
4.3. Lipopolysaccharide (LPS)
LPS is a type of bacteria-derived glycolipids that is a crucial component of the membrane bilayers in Gram-negative bacteria. As a barrier element for bacteria, LPS can help to maintain the structure of the membrane and protect them from some detrimental agents, such as antibiotics. In a classic view, LPS is not produced by mammals and thus can activate the innate immune systems, for example, the bacteria-derived LPS can stimulate TLR4 on the host and promote inflammation (Brown et al., 2023). In this regard, LPS has also been regarded as an endotoxin and is associated with numerous inflammatory diseases, including IBD (Candelli et al., 2021). Previous clinical results have shown that the serum levels of LPS and lipopolysaccharide-binding protein were promoted in patients who suffered from IBD compared with healthy individuals, emphasizing the potential pro-inflammatory effects of LPS (Rojo et al., 2006; Tulkens et al., 2020). In mice models, the direct effects of LPS on intestinal inflammation have been examined. It has been suggested that the treatment of LPS could elicit inflammation in the small intestine with body weight loss and increased pro-inflammatory factor expression (Im et al., 2012). Structurally, LPS consists of three domains: lipid A, core oligosaccharide, and O antigen. The immune regulation effects of LPS are mainly dependent on the lipid A component, and previous studies have found that the structure of lipid A in LPS could be modulated by various bacteria compared to E. coli (Okahashi et al., 2021; Park et al., 2009). In this regard, the effects of LPS derived from different bacteria could also be different, and previous in vitro studies have supported this speculation. LPS from five species of bacteria could exert different influences on the inflammatory factors’ expression and permeability in Caco-2 cells (Stephens and von der Weid, 2020). This species-specific manner of LPS was also observed in mice models. The symptoms of colitis induced by the transfer of CD4+CD62L+ T cells in Rag1−/− mice were more severe with the presence of higher endotoxic gut microbiota composition (high levels of Enterobacteriaceae and low levels of Bacteroidetes) compared with another group with lower endotoxic gut microbiota composition (low levels of Enterobacteriaceae and high levels of Bacteroidetes) (Gronbach et al., 2014). Similarly, it has been suggested that LPS from Bacteroides species and E. coli could exert different effects on the immune systems of infants (Vatanen et al., 2016), while the LPS from B. dorei could inhibit immune activation and inflammatory factors responses to LPS from E.coli. These results declared that bacteria-derived LPS could educate and regulate the activation of the immune system, which might also further influence the progression of IBD.
4.4. Sulfonolipids
Sulfonolipids are structurally similar to sphingolipids which is known as a potential immunoregulator factor for the hosts. In the gut microbiota of humans and other animal models, some members of Bacteroidetes, including Alistipes and Odoribacter, can produce sulfonolipids (Walker et al., 2017). Although the mechanisms and connections between bacteria-derived sulfonolipids and IBD are still unclear, some emerging evidence has highlighted the potential immunoregulatory effects of sulfonolipids. In patients with IBD, the abundance of sulfonolipids-produced bacteria was significantly reduced, such as Alistipes (Brown et al., 2023; Franzosa et al., 2019). The negative correlation between the abundance of gut microbiota-derived sulfonolipids and IBD has also been directly proven in humans and Il10-deficient mice models, which further emphasizes the potential influence of sulfonolipids on IBD (Older et al., 2024). Interestingly, previous in vitro studies showed that sulfonolipids could exert dual immunomodulatory activity (Hou et al., 2022; Older et al., 2024). In these studies, the treatment of sulfonolipids could exert mild to moderate pro-inflammatory effects on mouse macrophages, however, sulfonolipids could also suppress the severe inflammatory reaction induced by LPS through TLR signaling. These results clearly show that bacteria-derived sulfonolipid is a potential immunoregulatory factor and may mediate beneficial effects on IBD under specific conditions.
4.5. Short-chain fatty acids (SCFAs)
SCFAs are comprised mostly of acetate (C2), propionate (C3), and butyrate (C4) in an approximate molar ratio of 60:20:20, respectively (Martin-Gallausiaux et al., 2021). The production of SCFAs involves anaerobic fermentation by gut microbiota in the colon. In this process, gut microbiota converts indigestible dietary fibers and other complex polysaccharides into simple sugars, which are further metabolized into SCFAs. Acetate is a net fermentation product for most gut bacteria while propionate and butyrate are produced by more specific bacterial species. Specifically, propionate is limited mainly to the phyla Bacteroidetes, some Firmicutes (Clostridium clusters IX and XI), and Actinobacteria (Propionibacterium), and butyrate-producing bacteria belong mainly to the phylum Firmicutes (Clostridium clusters IV and XIVa) (Louis and Flint, 2017; van der Hee and Wells, 2021). Following their production, SCFAs are absorbed by the colonocytes via specific transporters, and a minor fraction of SCFAs that are not metabolized in the colonocytes are transported to the liver, and circulated throughout the body (Koh et al., 2016).
Previous studies have shown that fecal SCFAs levels are reduced in active IBD (Huda-Faujan et al., 2010). A large body of research also demonstrated that SCFAs play multiple roles in maintaining gut homeostasis. Firstly, butyrate constitutes the major energy source of colonocytes (Ardawi and Newsholme, 1985). In addition, SCFAs play an important role in maintaining gut barrier function by maintaining an environment favorable for commensal bacteria and controlling pathogens’ growth. SCFAs, especially butyrate, can regulate the expression of tight junction proteins, which connect adjacent cells in the gut lining, forming a tight seal that prevents the entry of harmful substances. Previous study has shown that butyrate decreases epithelial permeability by regulating the expression of occludin, zonulin and claudins, strengthening the tight junctions and the trans-epithelial resistance in vitro (Zheng et al., 2017). Animal-based experiments found that an orally administered butyrate-releasing derivative can improved intestinal epithelial integrity, preventing tight-junction impairment (ZO-1 and occludin) in murine colitis (Simeoli et al., 2017). Moreover, SCFAs, particularly butyrate, modulate the mucus layer thickness and protect the mucosa. In the colon, MUC2 is the predominant mucin glycoprotein produced by the goblet cells. Acetate and butyrate have been shown to stimulate and increase mucin secretion and production in rat colon by up-regulating MUC2 gene expression (Barcelo et al., 2000). Additionally, SCFAs exert anti-inflammatory effects in intestinal mucosa. Propionate and butyrate could suppress NF-κB reporter activity, immune-related gene expression and cytokine release in vitro (Pedersen et al., 2022; Tedelind et al., 2007). Similarly, in vivo studies revealed that butyrate and propionate treatment could improve the clinical and inflammatory parameters in murine colitis (Simeoli et al., 2017; Tong et al., 2016). In all, these evidences suggest that supplementation of SCFAs may serve as a promising therapeutic strategy in management of IBD.
| 5. Future perspective | ▴Top |
Dietary and gut microbiome-derived lipids are closely associated with the gut health of humans and can also affect the progression of intestinal inflammatory diseases. Recent studies have arrived at various models and conclusions, and some results were even conflicting and unclear. Therefore, further investigations are still desired to understand the underlying mechanisms for the biological functions of lipids on the GI tracks of host. First, the future effort should further investigate the interactions of various lipid components with gut microbiota in humans. Current studies have studied numerous lipids and examined their influence on inflammatory pathways, mucus layer, and oxidative stress. However, the regulatory mechanism of these lipids on some specific bacterial strains and their clear functions are still understudied. Bacteria-derived lipids, and dietary lipids may also induce the production of bacterial metabolites which may further influence the health of the host. Second, the interactions between lipids and other dietary components should also be subjected to further investigation. Lipid substances have the potential to interact with other dietary components and these interactions may also affect the bioactive activities of dietary lipids. In conclusion, it is evident that dietary lipids and bacteria-derived lipids can significantly affect the gut microbiota and GI health in the host. The further research is in need to uncover the underlying mechanism by which lipids either from diet or bacteria metabolites modulate the gut health.
Acknowledgments
This work was supported by the Hong Kong Research Grants Council General Research Fund [Project Number CUHK 14104923 and 14102321]
| References | ▴Top |