| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 27, September 2024, pages 88-103
Rutin modulates the TGR5/GLP1 pathway and downregulates proinflammatory cytokines genes in streptozotocin-induced diabetic rats
Olusola Olalekan Elekofehintia, Olorunfemi Raphael Molehinb, Moses Orimoloye Akinjiyana, *, Aderonke Elizabeth Fakayodea
aBioinformatics and Molecular Biology Unit, Department of Biochemistry, Federal University of Technology, PMB 704, Akure, Ondo State, Nigeria
bDepartment of Biochemistry, Ekiti State University, PMB 5363, Ado-Ekiti, Ekiti State, Nigeria
*Corresponding author: Moses Orimoloye Akinjiyan, Bioinformatics and Molecular Biology Unit, Department of Biochemistry, Federal University of Technology, PMB 704, Akure, Ondo State, Nigeria. E-mail: moakinjiyan@futa.edu.ng
DOI: 10.26599/JFB.2024.95027390
Received: June 26, 2024
Revised received & accepted: September 18, 2024
| Abstract | ▴Top |
Rutin, a bioactive component of citrus has been used to treat diabetes mellitus, but there is a paucity of information on the mechanism. In this study, rutin’s impact on the expression of genes related to inflammation and insulin secretion were examined. Rats induced with streptozotocin (60 mg/kg) were grouped into six: normal control, diabetes untreated, metformin and rutin-treated groups (25, 50, and 100 mg/kg), for 28 days. The blood glucose were evaluated at 3-day intervals with glucometer. The mRNA expression of PDX-1, TGR-5, GLP-1, DPP4, TNF-α, IL-1β and IL-6 was investigated utilizing RT-PCR. Schrödinger suites was used to dock rutin with TGR-5, GLP-1 and DPP4. After treatment, the groups administered with rutin had decreased blood glucose levels than the diabetes untreated group. Compared with those in the untreated diabetes group, the expression of PDX-1, TGR-5 (25 and 100 mg/kg), and GLP-1 (25 and 100 mg/kg) was significantly upregulated, and the expressions of DPP4, IL-1β, TNF-α, and IL-6 were significantly downregulated in the 25 mg/kg rutin-treated group. The docking results showed that rutin is a potent ligand of the selected proteins investigated. The antidiabetic effects of rutin could be linked to its potential to downregulate proinflammatory cytokines and modulate the TGR5/GLP-1 signally, leading to normoglycemia.
Keywords: Rutin; diabetes mellitus; gene expression; inflammation; molecular docking
| 1. Introduction | ▴Top |
The most common sign of diabetes mellitus (DM), a collection of metabolic diseases, is persistent hyperglycemia (high blood glucose), which can be caused by insufficient insulin production or improper cell response to insulin (Lim et al., 2021; Song et al., 2022; Ojo et al., 2023). Glucose, though present in the blood in diabetic patients, is unable to be fully incorporated and utilized by mitochondria, the major site of energy and ATP generation. DM is a significant global health issue and one of the top six causes of death globally, accounting for around 2 million deaths yearly (Talib et al., 2021). According to estimates, there were 422 million adults worldwide with diabetes in 2014, and 463 million in 2019. This figure is expected to be 593 million by 2035 and 643 million by 2040 (Zimmet et al., 2016)
Due to their high therapeutic potential and the presence of phytochemicals like flavonoids, medicinal and traditional plants have been employed for a long time to manage a variety of conditions, including diabetes mellitus (Ahn, 2017, Ijatuyi et al., 2024). Flavonoids are naturally occurring plant-based products with a wide range of biological and therapeutic uses. This broad class of substances exhibits hepatoprotective, nephroprotective, anticarcinogenic, antihyperglycemic, and antihyperlipidemic properties (Mutha et al., 2021). These are a class of naturally occurring substances with polyphenolic structures that are commonly present in fruits and vegetables and are also added to some drinks as naturally derived colours. The fundamental structure of flavonoids includes fifteen carbon atoms and two phenolic rings carrying one or more hydroxyl groups (Mahmoud et al., 2019). Flavonoids have two aromatic rings attached to a heterocyclic pyrane ring (Mahmoud et al., 2019). These compounds are rich in double bonds and include rutin, hesperidin, hesperetin, and eriodictyol among others. Recently, studies have been carried out on these phytochemicals to determine their mode and mechanism of action. Rutin is a citrus flavonoid that has many therapeutic effects and has been used to manage DM (Mahmoud et al., 2019; Liang et al., 2021).
Mitochondria are the site of cellular respiration and this organelle is also often referred to as the powerhouse of a cell because the energy it generates powers other cell activities (Kim et al., 2008; Anderson et al., 2019). This energy is generated by the breakdown of glucose, a process known as glycolysis (Navale and Paranjape, 2016). Because of its continuous activity, it is the site of free radical and pro-oxidant generation, which is further increased by hyperglycemic conditions in DM. This results in oxidative stress and inflammation.
Pancreatic and duodenal home box 1 (PDX-1) is a gene associated with mitochondrial regeneration in the pancreas (Wang and Farhana, 2023). The antidiabetic mechanism of rutin action was investigated in this study on genes associated with mitochondrial biogenesis, insulin secretion, and inflammation in the pancreas and liver of STZ-induced rats. The pancreas is a key organ involved in glucose regulation since its beta-cells secrete insulin via Takeda G-protein-coupled receptor 5 (TGR 5) and glucagon-like peptide 1 (GLP-1) signally, insulin hormone is crucial in glucose metabolism (Edgerton et al., 2021).The liver helps in the distribution of glucose throughout the body, and it also stores excess glucose in the form of glycogen (Nakrani et al., 2023). The genes associated with insulin sensitivity and glucose metabolism examined in this study included TGR 5, GLP-1, and dipeptidyl peptidase-4 (DPP4) (Cai, et al., 2021). Genes linked to inflammation in pancreatic and hepatic cells, such as interleukin-6 (IL-6), interleukin-1beta (IL-1β), and tumour necrosis factor-alpha (TNF-α), were also investigated in this study.
| 2. Materials and methods | ▴Top |
2.1. Materials
Analytical grade solvents and reagents were employed in the investigation. The sources of streptozotocin and rutin were Sigma-Aldrich (St. Louis, MO, USA). From Inqaba Biotech, Africa Genomics Company (South Africa), we acquired TRIzol, reverse transcriptase, specific gene primers, loading buffer, DNA ladder, nuclease-free water, agarose gel powder, EZ Vision, ethidium bromide, and gel loading dye. We bought regular rat feed and metformin from local suppliers.
2.2. Animals
Male Wistar rats used in the experiment were obtained from the University of Ibadan in Nigeria, weighing an average of 189.33 ± 5 g. The rats were acclimated for two weeks at room temperature in galvanized cages at the animal house of the Department of Biochemistry, Federal University of Technology, Akure. The rats were kept on a 12-hour light/12-hour dark cycle, with free access to water and standard rat chow (Oloyede et al., 2022). The National Institutes of Health’s (NIH) Guidelines for the Care and Use of Laboratory Animals were adhered to when working with the animals.
2.3. Experimental design
The experimental rats were then intraperitoneally induced with streptozotocin (60 mg/kg) in 0.1 molar citrate buffer (pH 4.5) with the aid of a needle and syringe (Ghasemi and Jeddi, 2023) Streptozotocin (60 mg/kg) is widely reported to reduce beta-cell levels, and this destructive ability is believed to be caused by a continuous cycle of oxidative stress (Nahdi et al., 2017). The rats’ blood sugar was devaluated with a glucometer (ACCU-CHEK) and glucose strip after 72 hrs (3 days) of induction, with readings of rats equal to or greater than 200 mg/dl considered diabetic. They were then put into six groups viz:
- Group 1 = the normal control group;
- Group 2 = diabetic control group;
- Group 3 = 100 mg/kg metformin (standard drug);
- Group 4 = 25 mg/kg rutin treatment;
- Group 5 = 50 mg/kg rutin treatment;
- Group 6 = 100 mg/kg rutin treatment.
The rats were treated daily orally with the aid of gavage as described above for 28 days (31 days after induction), and the rats’ blood sugar and body weight mass were evaluated at 3-day intervals. After that, the mice were sacrificed, and the tissues of interest were harvested as described previously by (Elekofehinti and Akinjiyan, 2020).
2.4. Gene expression
Using TRIzol reagent (Thermo Fisher Scientific), RNA was extracted from the tissues and then converted to cDNA using a Proto Script First Strand cDNA Synthesis Kit (NEB). Using the primer sets listed in Table 1, OneTaq®2X Master Mix (NEB) was used to create PCR amplicons. The amplification of selected genes of interest was performed using a thermocycler, followed by agarose gel electrophoresis of the sample amplicons.
 Click to view | Table 1. Gene expression primer sets |
The pancreas was examined for the expression of the genes for dipeptidyl peptidase-4 (DPP4), glucagon-like peptide-1 receptor (GLP-1), Takeda-G-protein receptor-5 (TGR-5), pancreatic and duodenal home box 1 (PDX-1), and interleukin 6 (IL-6). Both the pancreas and the liver were used to study the expression of interleukin-1 beta (IL-1β) and tumour necrosis factor α (TNF-α).
2.5. Agarose gel electrophoresis
One per cent agarose gel electrophoresis was applied to polymerase chain reaction amplicons. A camera was used to take pictures of the gels under an ultraviolet (UV) transilluminator, revealing their band intensities.
2.6. Molecular docking
2.6.1. Ligand Preparation
The rutin compound’s structure was obtained from https://pubchem.ncbi.nlm.nih.gov/.and ready for docking in Maestro, and Schrödinger suites using the LigPrep module. Appropriately chiral low-energy three-dimensional structures were produced. At a physiological pH of 7.2 ± 0.2, the likely ionization energy for the rutin ligand structure was generated and reduced with the OPLS3 force field (Iwaloye et al., 2021).
2.6.2. Molecular docking study
Using the “receptor grid generation” option in the glide-v7.5 program of Maestro-v11.5, receptor grids were created around the binding sites of Takeda-G-protein-receptor-5 (PDB ID: 7BWO), glucagon-like peptide-1 receptor (PDB ID: 6XLA), and dipeptidyl peptidase-4 (DPP4) (PDB ID: 2OQV). Next, using the Schrödinger suite workflow module’s extra precision (XP) workflow module with default parameters, the prepared rutin ligands were docked into the receptor grids. Maestro’s virtual screening workflow was utilized to score and dock rutin with specific receptors linked to insulin regulation (Arowosegbe et al., 2020; Elekofehinti et al., 2024).
2.7. Data analysis
Densitometric quantification of the band intensities were performed using ImageJ software (http://imagej.en.softonic.com/). The mean ± standard error of the mean (SEM) was used to represent the results. PRISM 5.01 (GraphPad Software, Inc.) was utilized for these statistical analyses, which involved one-way analysis of variance (ANOVA) and post-hoc Dunnett’s multiple range test, with a statistically significant variance at p < 0.05.
| 3. Results | ▴Top |
3.1. Effect of rutin administration on the fasting blood glucose of streptozotocin-induced diabetic rats
The effects of rutin on blood glucose levels in type 1 diabetic rats are displayed in Figure 1. Three days after induction, blood glucose levels in the induced group were significantly higher than in the nondiabetic control group due to diabetes induced by STZ (Figure 1, p < 0.05). When rutin (25 and 50 mg/kg) and metformin were administered for 28 days (31 days after induction), the blood glucose concentrations of the treated diabetic groups were significantly lower than those of the group receiving no treatment for diabetes.
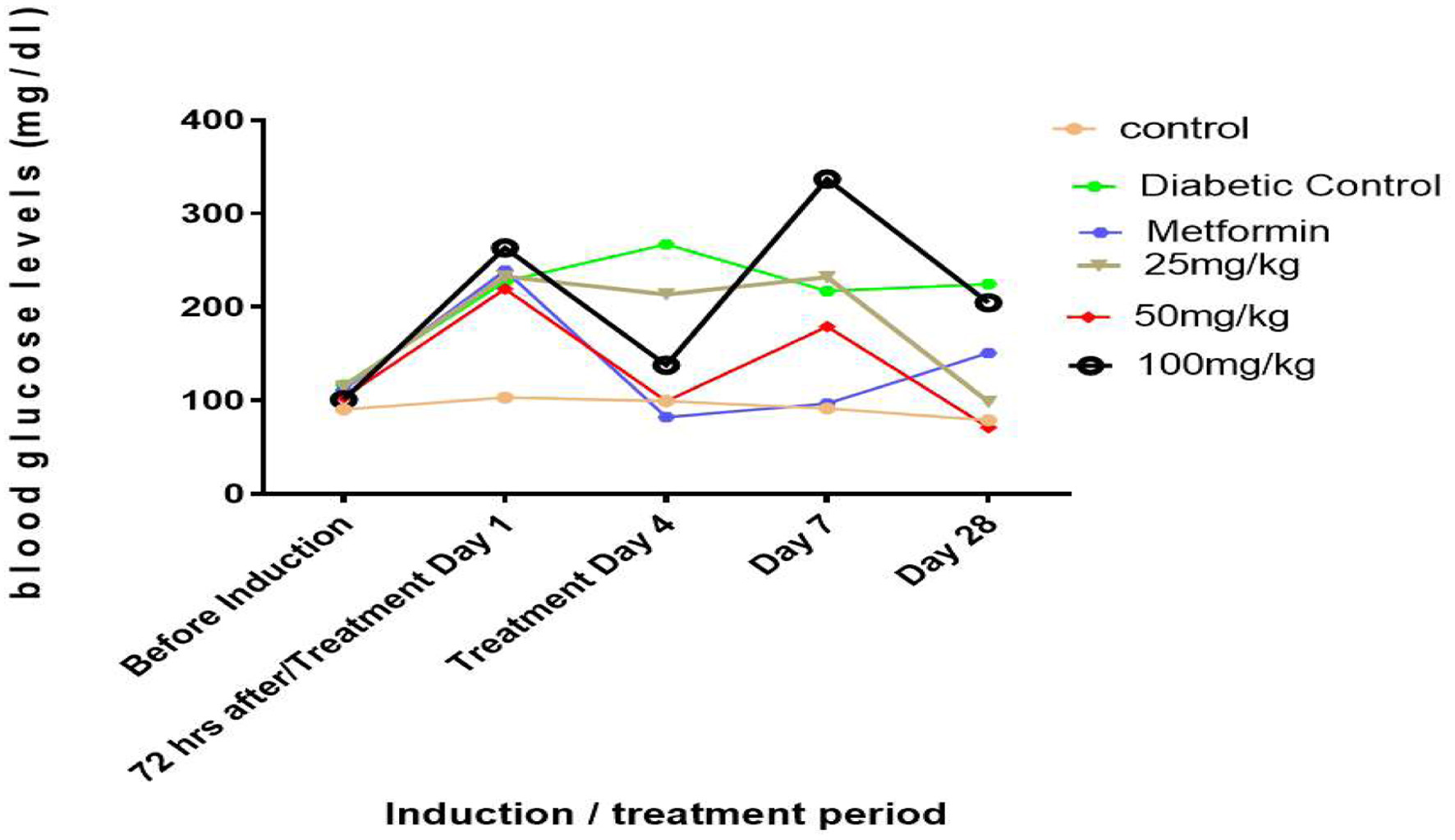 Click for large image | Figure 1. Line graphs showing the hypoglycemic effect of rutin on blood glucose levels in STZ-induced diabetic rats. |
3.2. Rutin treatment and weight patterns of streptozotocin-induced diabetic rats
After 28 days of treatment, diabetic control rats lost weight in comparison to non-diabetic control rats (Figure 2). After 28 days of treatment, the rutin-treated group lost weight in comparison to the non-diabetic control group.
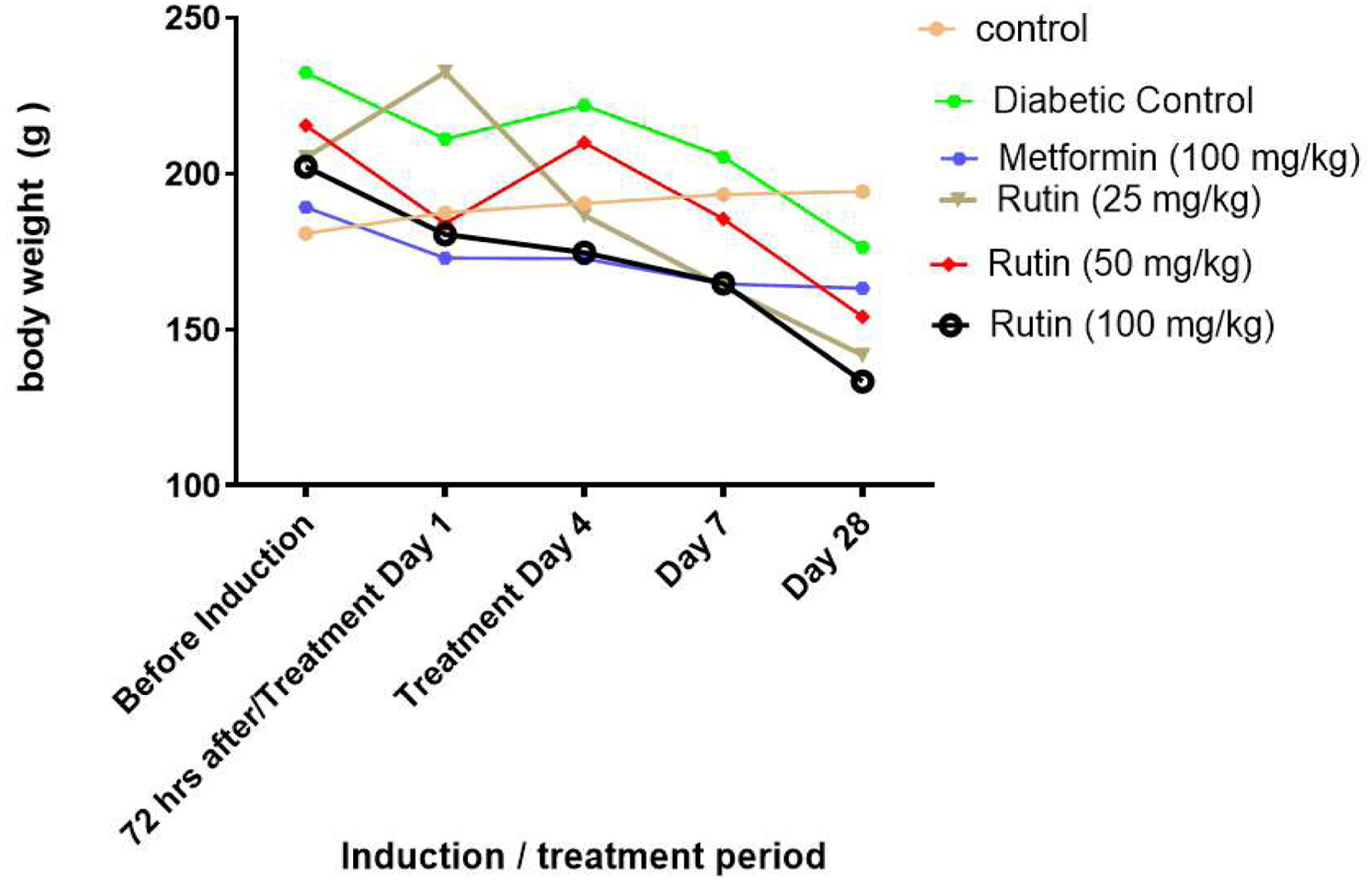 Click for large image | Figure 2. Line charts showing the effect of rutin on the weight of rats with streptozotocin-induced diabetes. |
3.3. Effect of rutin on genes associated with mitochondrial biogenesis in the pancreas of STZ-induced diabetic rats
Pancreatic and duodenal home box 1 (PDX-1) was significantly upregulated (Figure 3, p < 0.05) in the Rutin-treated group compared with the STZ-induced diabetic nontreated and metformin-treated groups at all tested concentrations. This finding suggested the regenerative ability of rutin on pancreatic mitochondria and function.
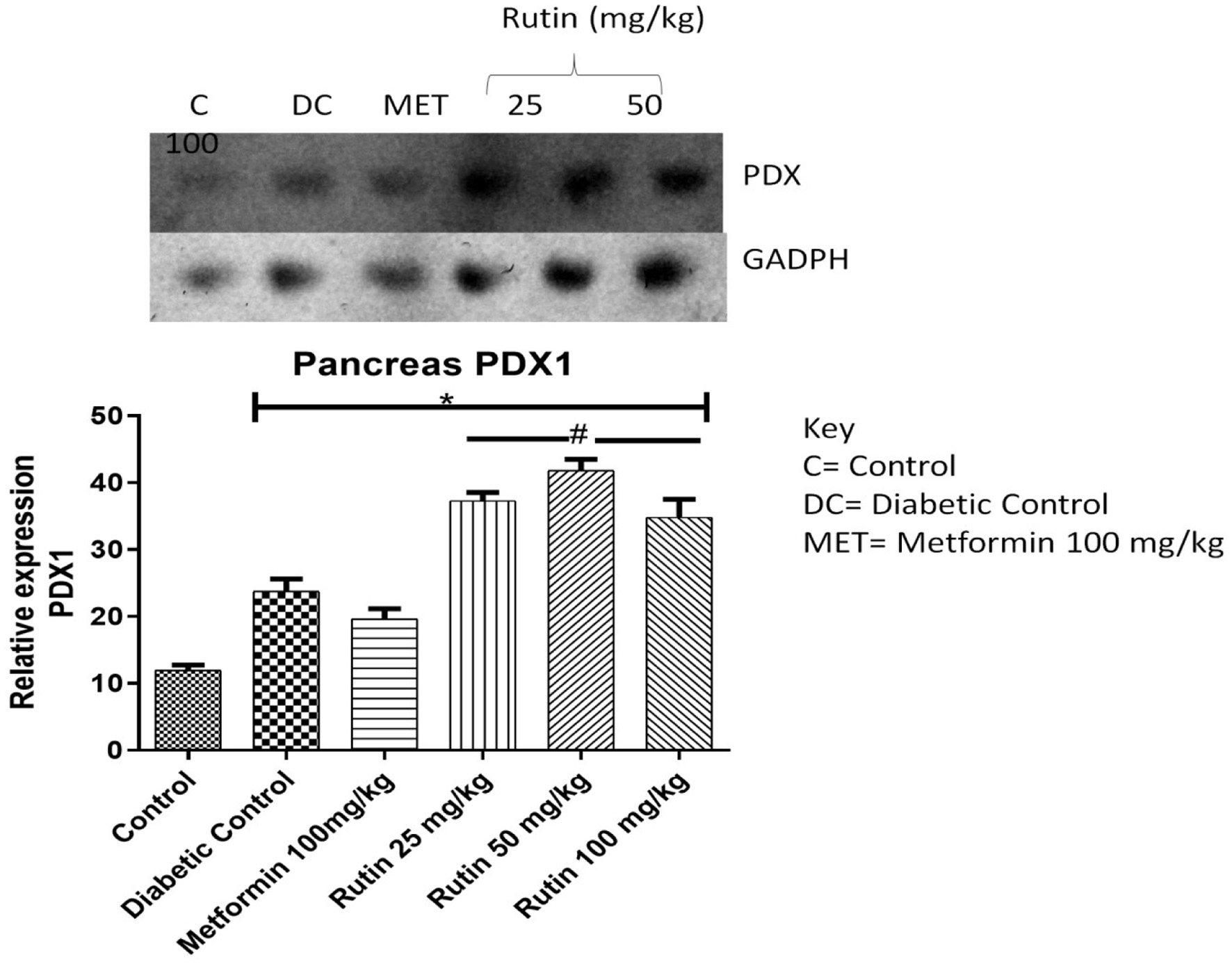 Click for large image | Figure 3. Pictorial view of RT-PCR agarose gel electrophoresis (AGE) for pancreatic and duodenal home box 1 (PDX-1) in rats with STZ-induced diabetes treated with rutin (n = 3) followed by densitometric analysis. * and # represent a significant variation between the control group and the diabetic control group respectively at p < 0.05. |
3.4. Effect of the oral administration of rutin on genes linked to inflammation in the pancreas of streptozotocin-induced diabetic rats
Tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) are proinflammatory cytokines. The expression of these genes in the pancreas was investigated in the present study. The expression of these genes was significantly (p < 0.05) greater in diabetic control rats than in control rats (Figures 4 and 5), which indicates toxicity. Treatment with 25 or 100 mg/kg rutin significantly downregulated IL-1β compared to the diabetic control group. Metformin and rutin (25 and 50 mg/kg) significantly repressed TNF-α relative to the diabetic control class after 28 days of rutin administration.
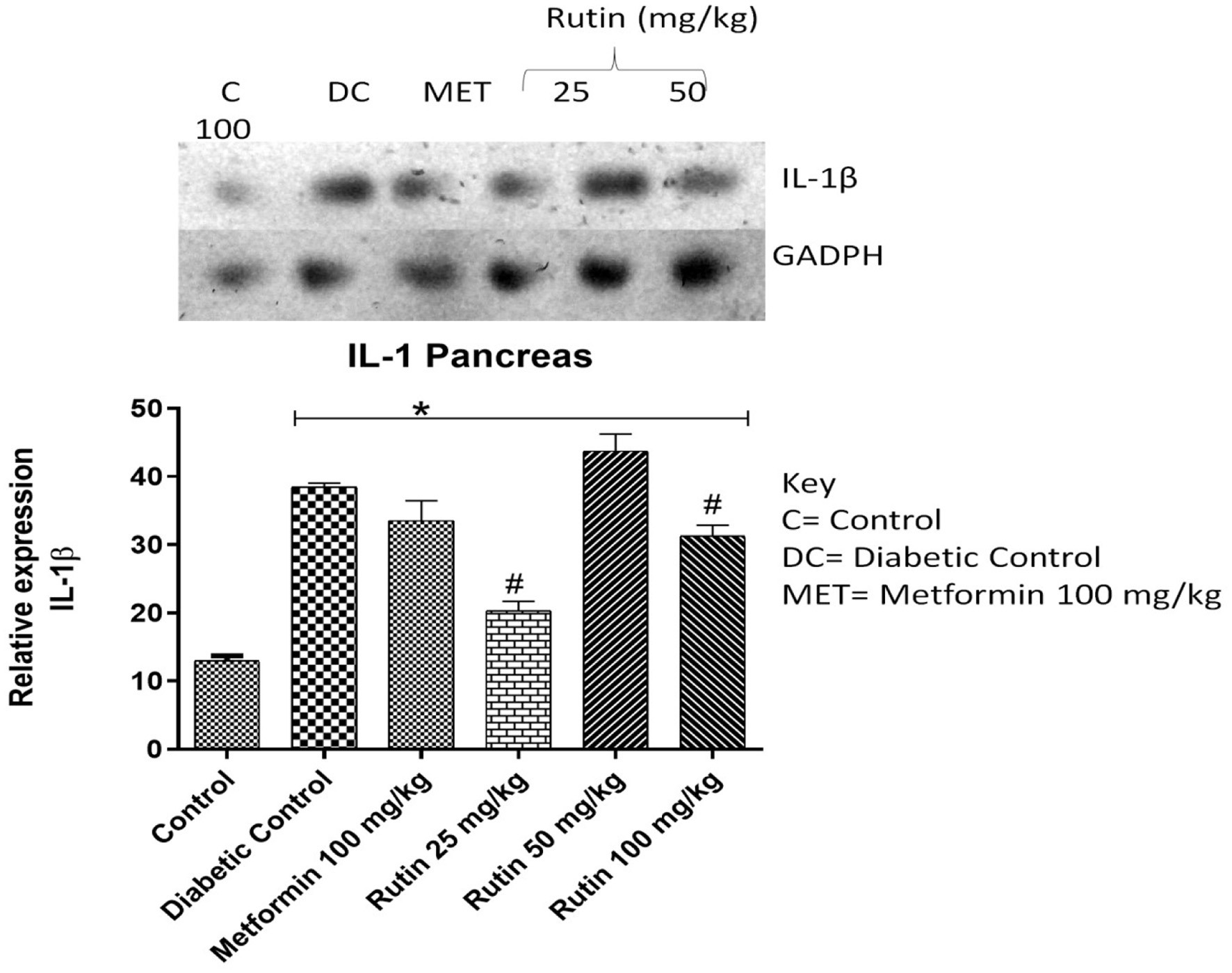 Click for large image | Figure 4. Pictorial view of RT-PCR AGE analysis of pancreatic interleukin-1 beta expression in rats with STZ-induced diabetes treated with rutin (n = 3) followed by densitometric analysis. * and # represent a significant variation between the control group and the diabetic control group respectively at p < 0.05. |
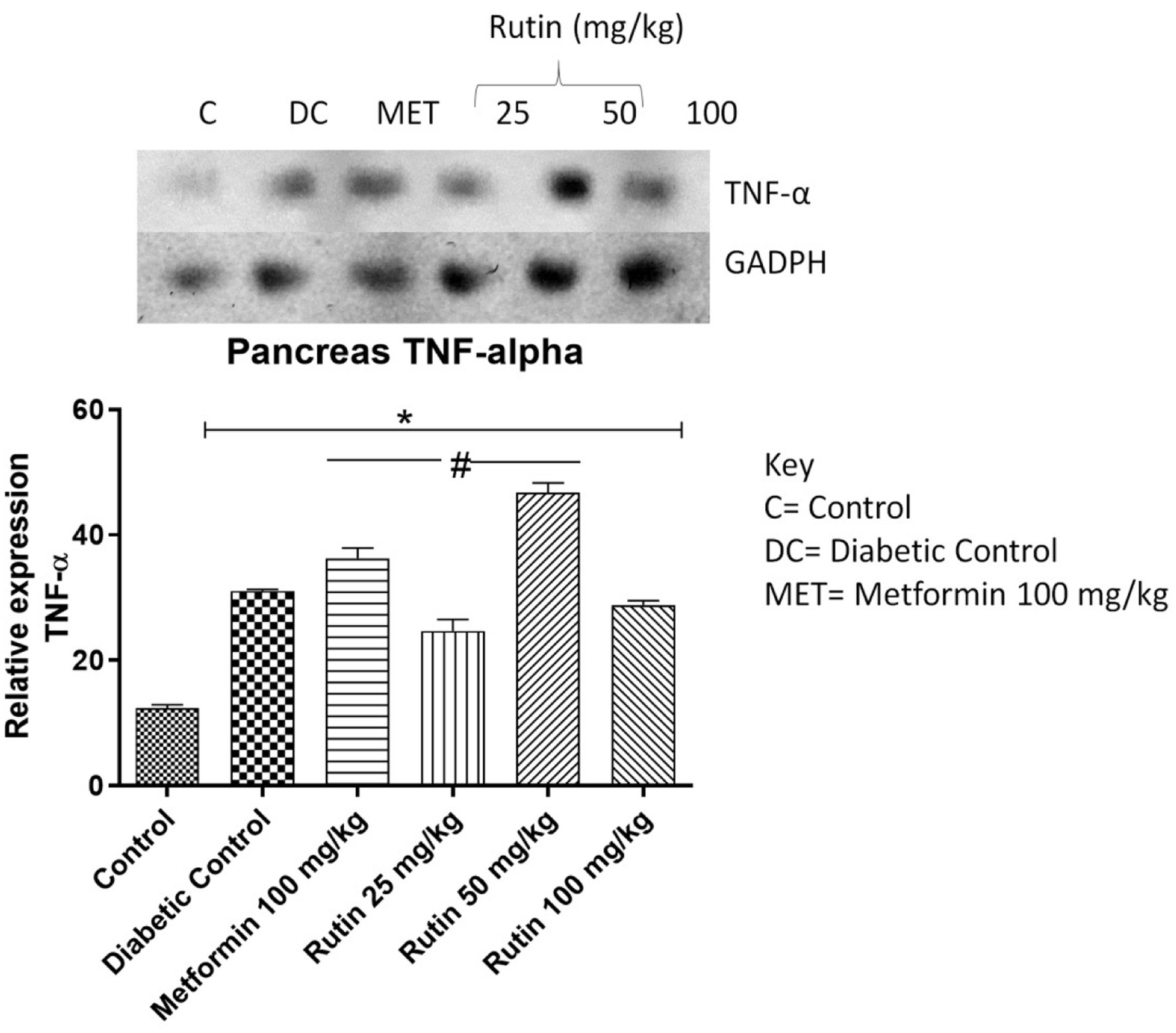 Click for large image | Figure 5. Pictorial view of RT-PCR AGE analysis of pancreatic TNF-α expression in STZ-induced diabetic rats orally administered rutin (n = 3) followed by densitometric analysis. * and # represent a significant variation between the control group and the diabetic control group respectively at p < 0.05. |
3.5. Effect of oral administration of rutin on genes associated with inflammation in the liver of rats with streptozotocin (STZ)-induced diabetes
IL-1β, interleukin-6 (IL-6) and TNF-α expressions were significantly upregulated upon STZ induction compared to that in the noninduced control group. Rutin at 25 mg/kg notably downregulated the expression of these three cytokines in the liver (Figures 6–8). This suggests rutin’s potential to reduce inflammation brought on by diabetes.
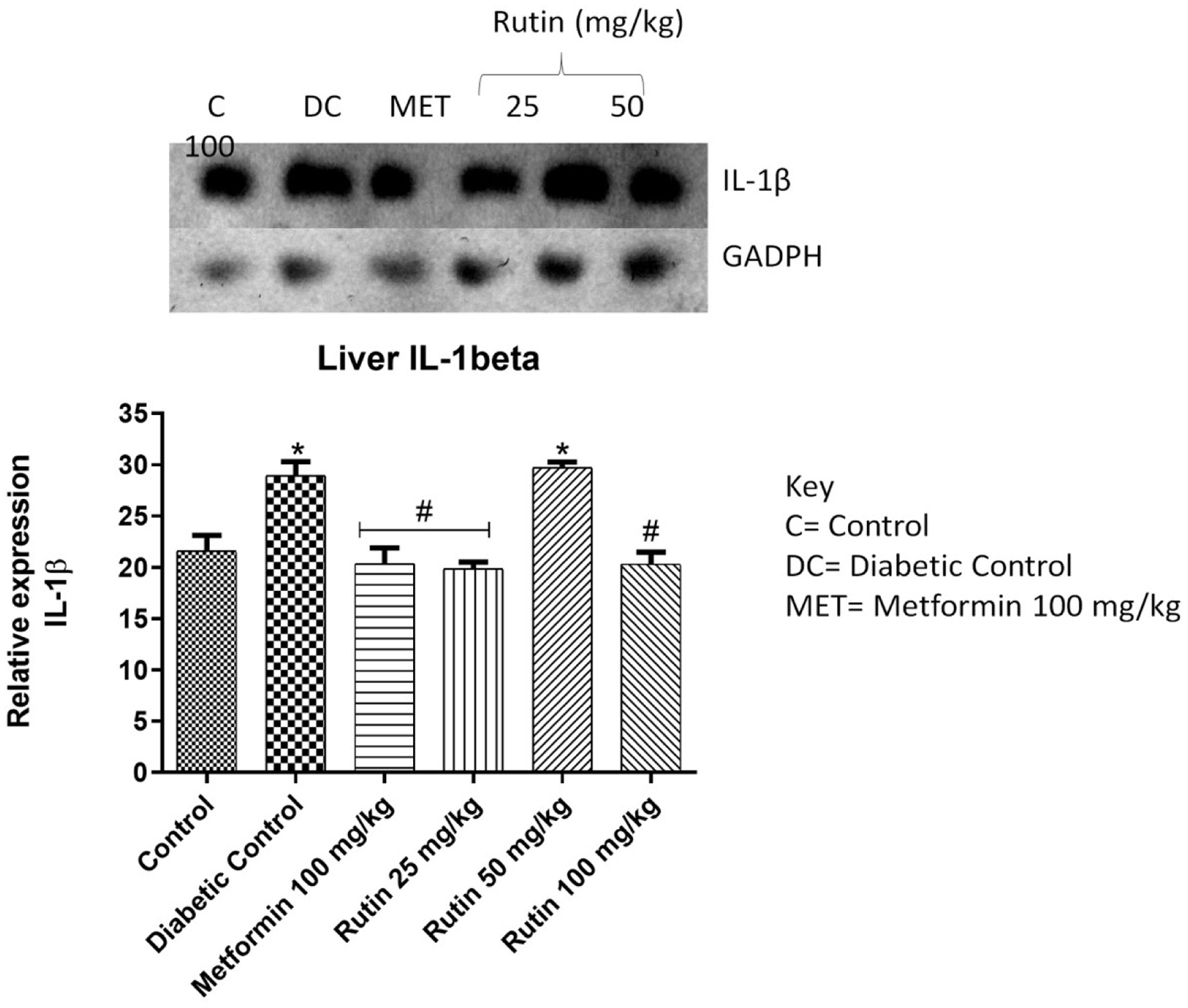 Click for large image | Figure 6. Pictorial view of RT-PCR AGE analysis of hepatic interleukin-1 beta expression in rats with STZ-induced diabetes treated with rutin (n = 3) followed by densitometric analysis. * and # represent a significant variation between the control group and the diabetic control group respectively at p < 0.05. |
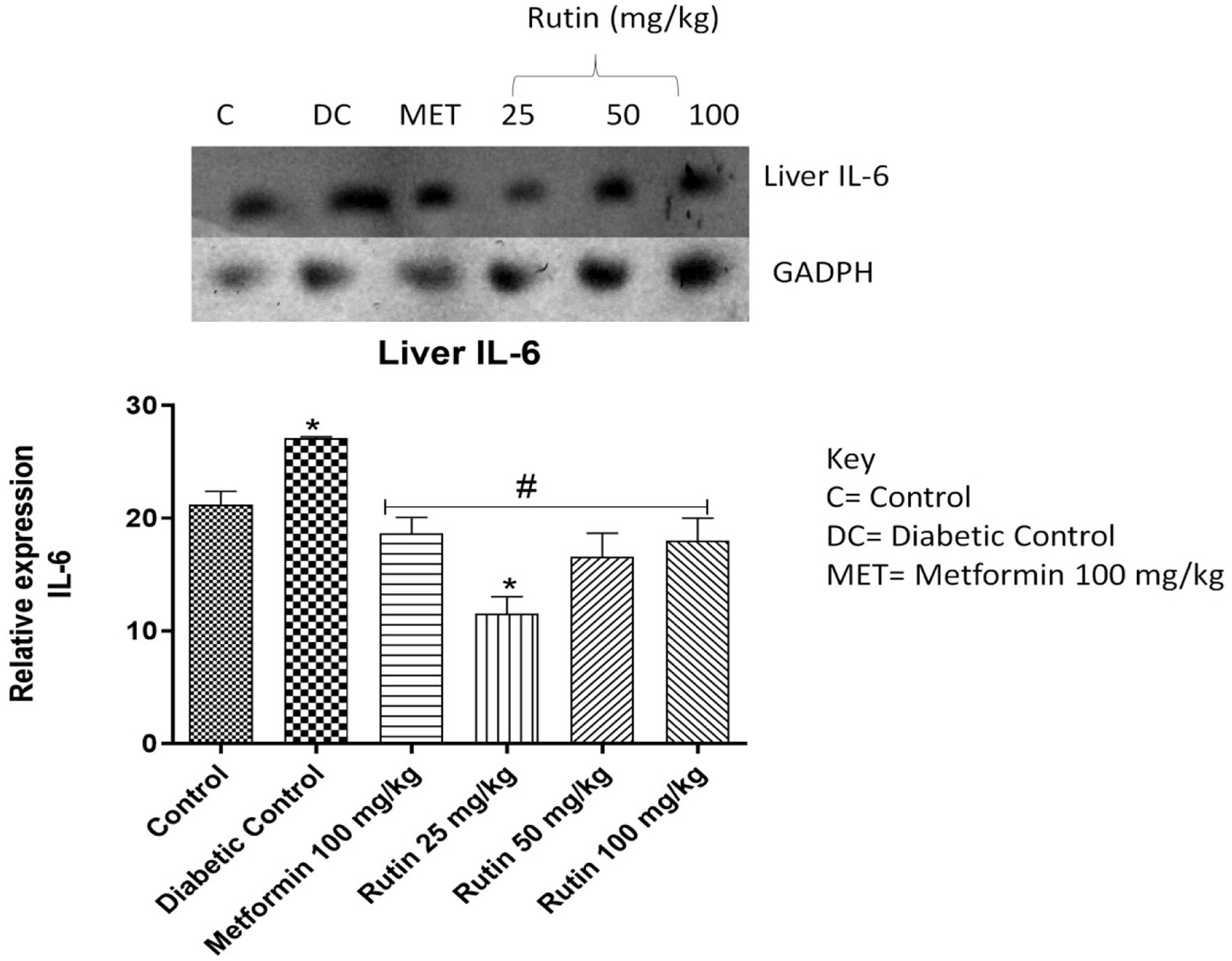 Click for large image | Figure 7. Pictorial view of RT-PCR AGE for interleukin-6 expression in rats with STZ-induced diabetes (n = 3) treated with rutin followed by densitometric analysis. * and # represent a significant variation between the control group and the diabetic control group respectively at p < 0.05. |
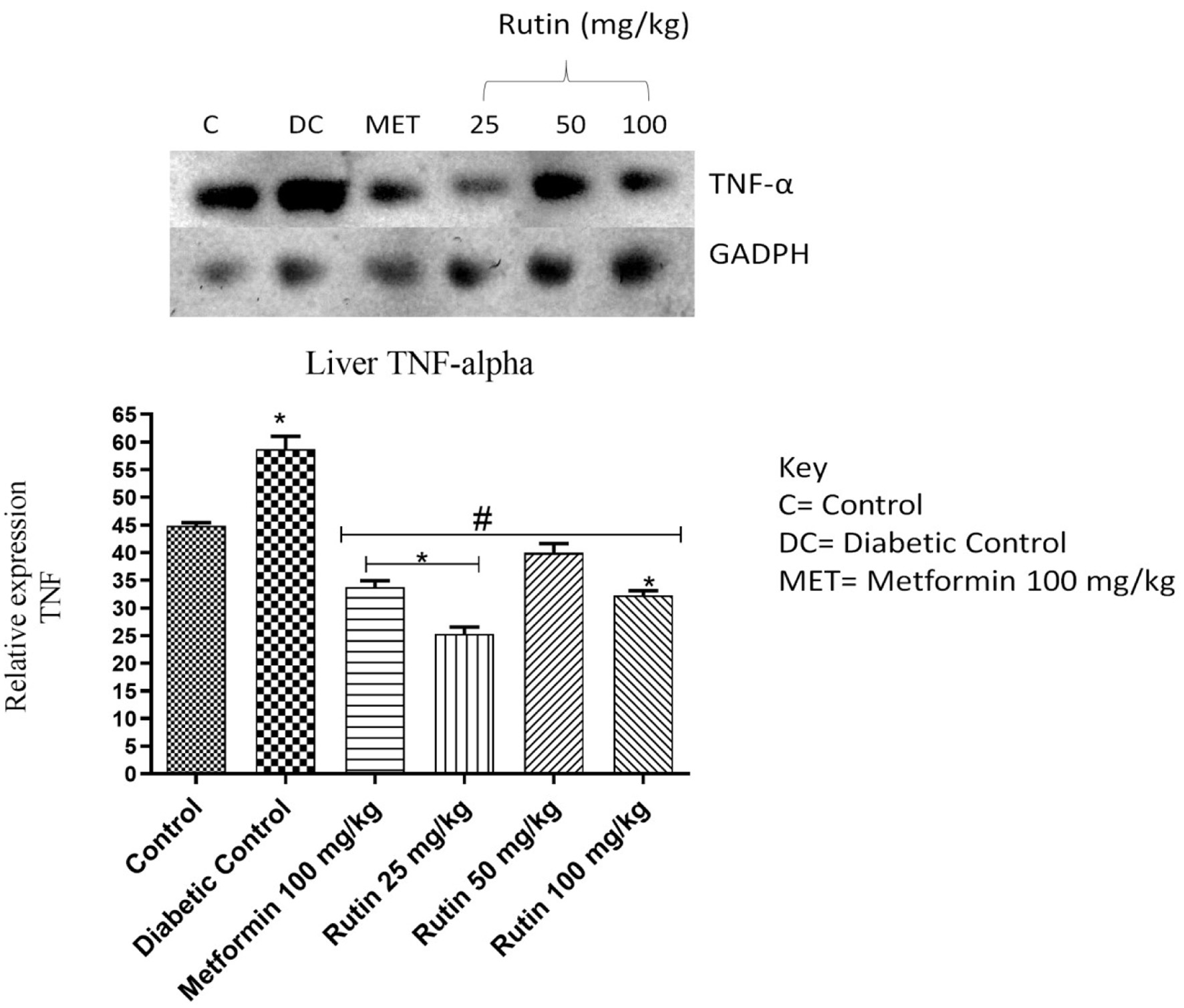 Click for large image | Figure 8. Pictorial view of RT-PCR AGE for hepatic tumour necrosis factor (TNF-α) in rats with STZ-induced diabetes treated with rutin (n = 3) followed by densitometric analysis. * and # represent a significant variation between the control group and the diabetic control group respectively at p < 0.05. |
3.6. Effect of rutin on insulin-sensitive genes in the pancreas of streptozotocin-induced diabetic rats
TGR-5, GLP-1 and DPP4 genes were investigated in the pancreas (Figures 9–11). These genes are associated with glucose metabolism. After 28 days of treatment, rutin (50 and 100 mg/kg) significantly upregulated TGR-5 expression relative to that in the diabetic control group. Metformin and rutin (25 and 50 mg/kg) significantly upregulated GLP-1 (p < 0.05) compared to that in the STZ-induced diabetic untreated group. When compared to the nondiabetic control group, the expression of dipeptidyl peptidase-4 (DPP4) was significantly upregulated in the diabetic control and metformin-treated groups, suggesting a disruption in glucose metabolism. However, rutin (25, 50, and 100 mg/kg) significantly corrected this abnormality, as shown by the downregulation of DPP4 expression in comparison to the diabetic control group (Figure 11, p < 0.05).
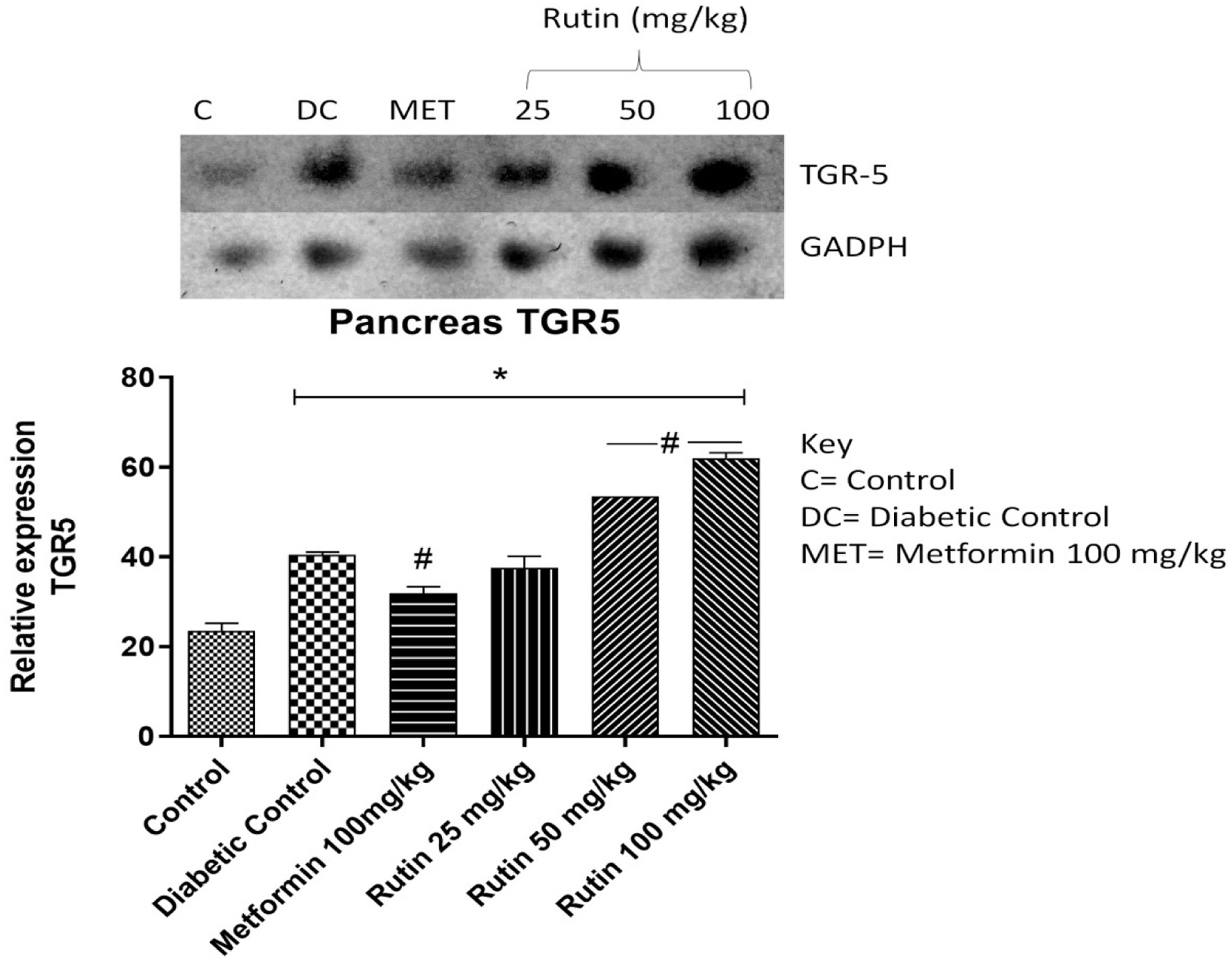 Click for large image | Figure 9. Pictorial view of RT-PCR AGE for pancreatic Takeda G-protein-coupled receptor 5 (TGR5) in rats with STZ-induced diabetes treated with rutin (n = 3) followed by densitometric analysis. * and # represent a significant variation between the control group and the diabetic control group respectively at p < 0.05. |
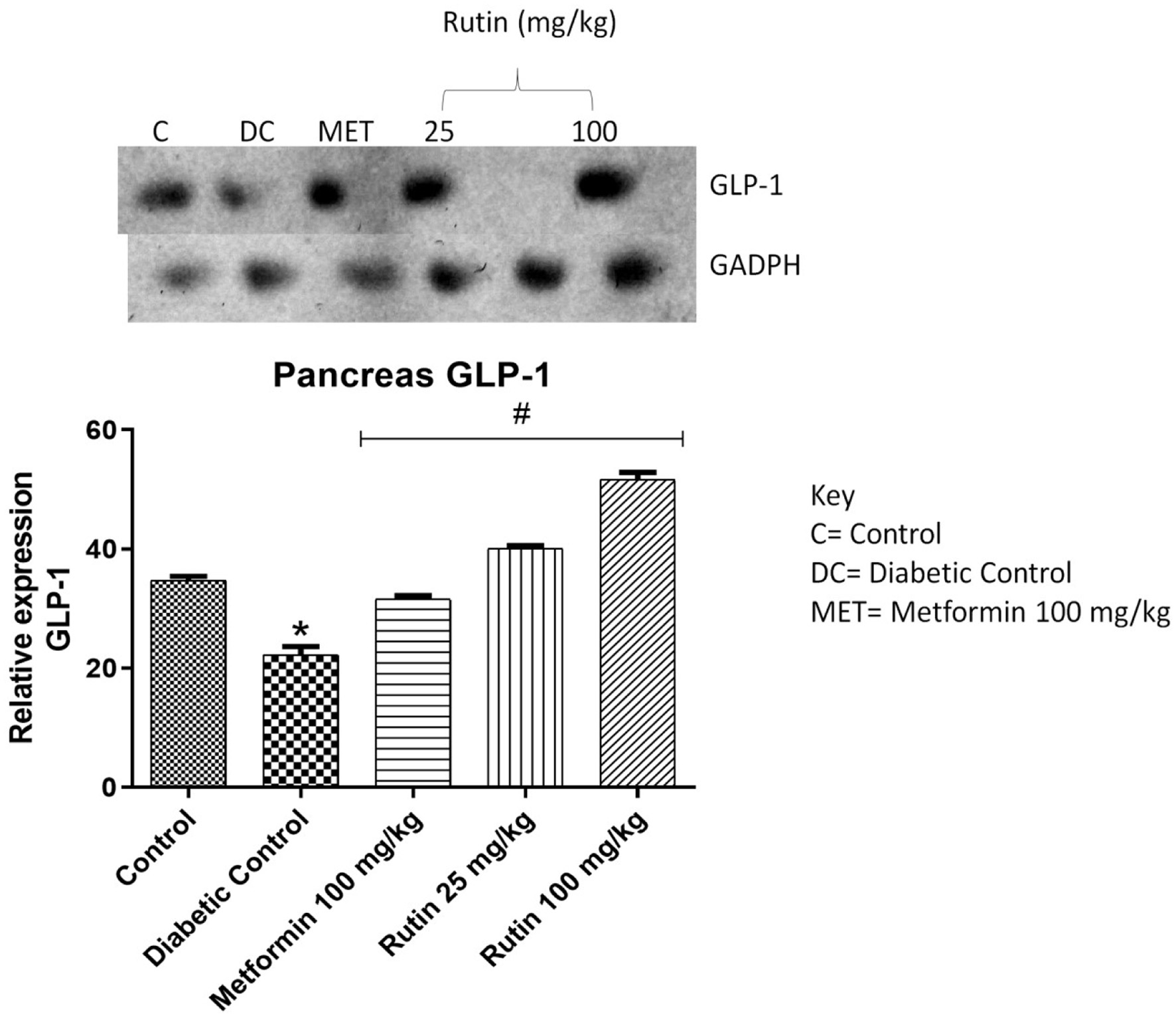 Click for large image | Figure 10. Pictorial view of RT-PCR AGE for pancreatic glucagon-like peptide-1 (GLP-1) in rats with STZ-induced diabetes treated with rutin (n = 3) followed by densitometric analysis. * and # represent a significant variation between the control group and the diabetic control group respectively at p < 0.05. |
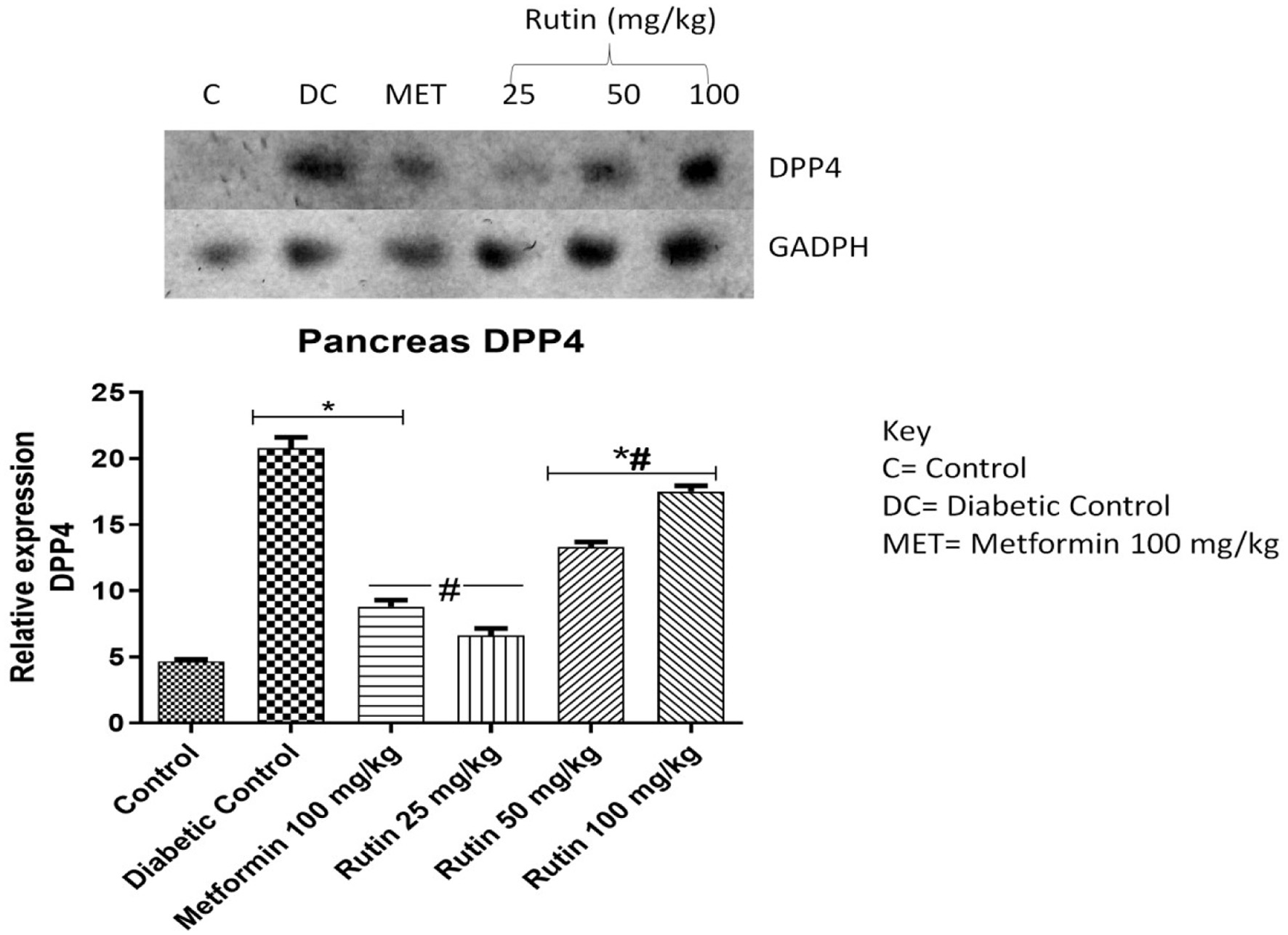 Click for large image | Figure 11. Pictorial view of RT-PCR AGE for pancreatic dipeptidyl peptidase-4 (DPP4) in rats with STZ-induced diabetes treated with rutin (n = 3) followed by densitometric analysis. * and # represent a significant variation between the control group and the diabetic control group respectively at p < 0.05. |
3.7. In Silico molecular interactions of rutin with selected proteins
A molecular docking simulation of rutin was performed with receptor targets associated with insulin sensitivity, such as GLP-1, TGR-5 and DPP4 (Figures 12–17). The results revealed that rutin is a potent ligand of these targets (Table 2). The binding affinities of TGR-5 and GLP-1 were -12.407 kcal/mol and -12.723 kcal/mol, respectively, when rutin was docked to their 3D structures. The phytochemicals’ binding position and binding site with the insulin receptor are depicted in Panel A (3D image) of the following Figures 12 to 17, while Panel B (2D image) depicts the ligand rutin’s molecular interaction with amino acid residues inside the protein structures’ binding pocket.
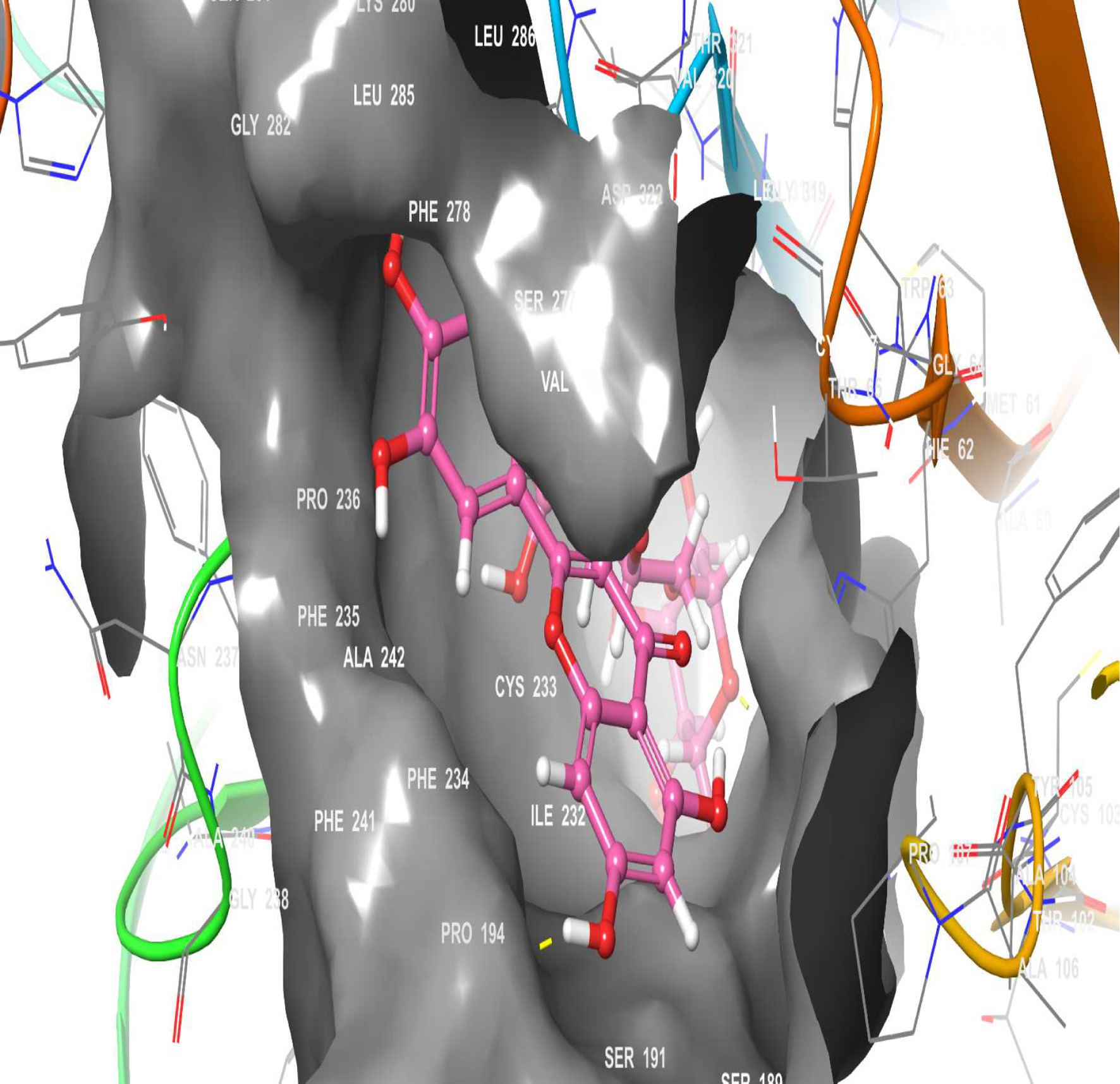 Click for large image | Figure 12. The 3D chemical relationship of Rutin with TGR5 with amino acid residues. |
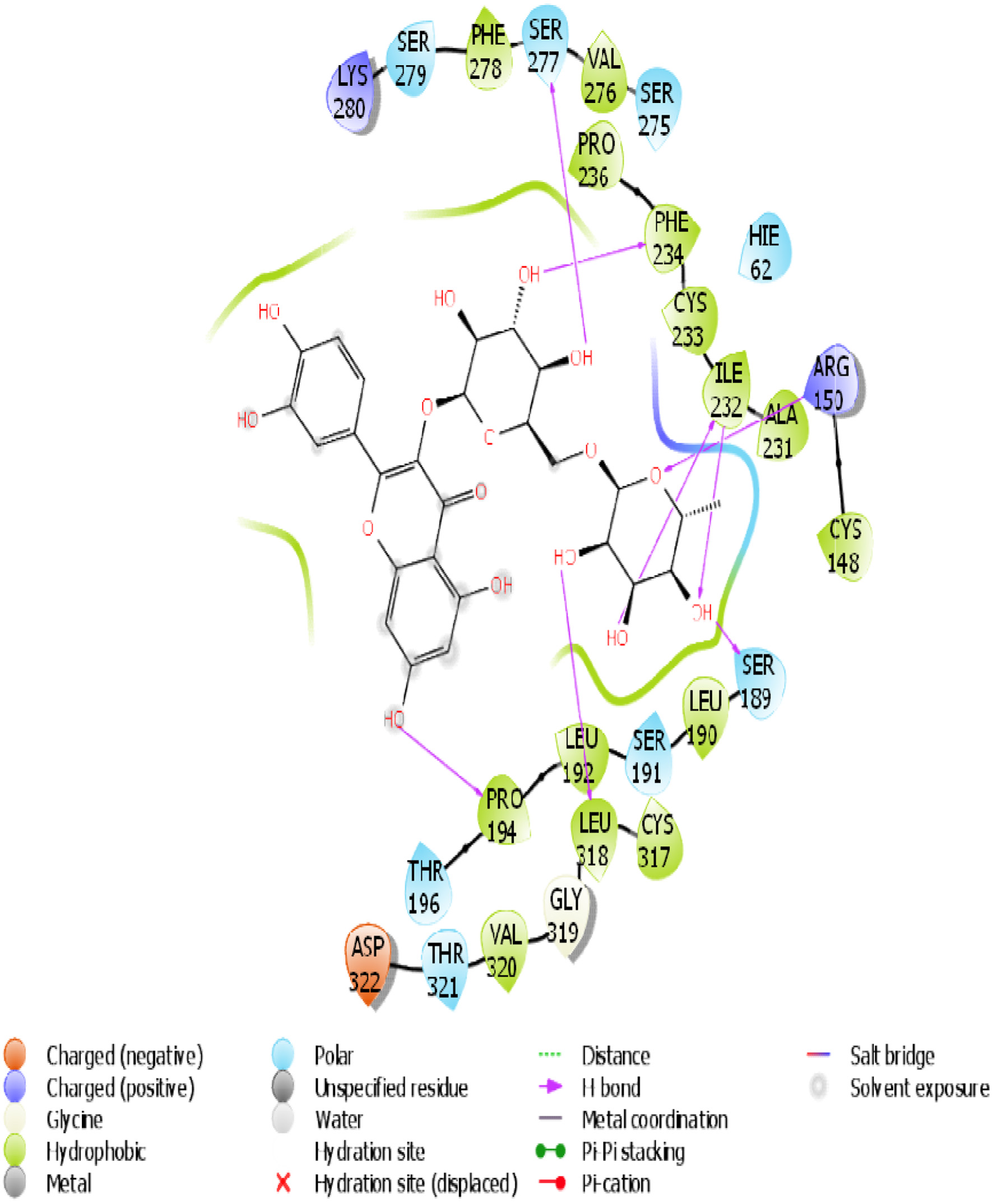 Click for large image | Figure 13. The 2D chemical relationship of Rutin with TGR5 with amino acid residues. |
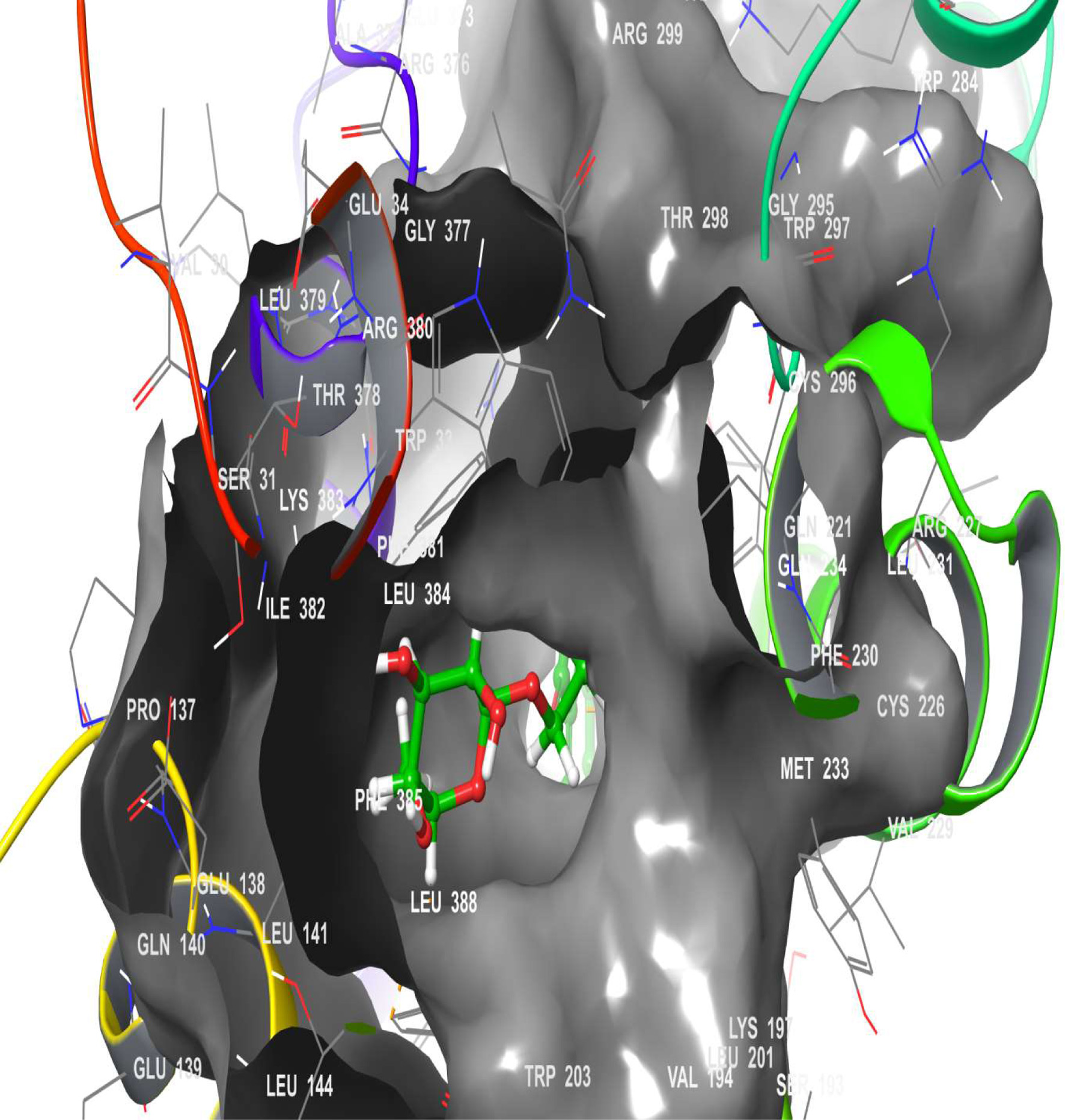 Click for large image | Figure 14. The 3D chemical relationship of Rutin with GLP-1 with amino acid residues. |
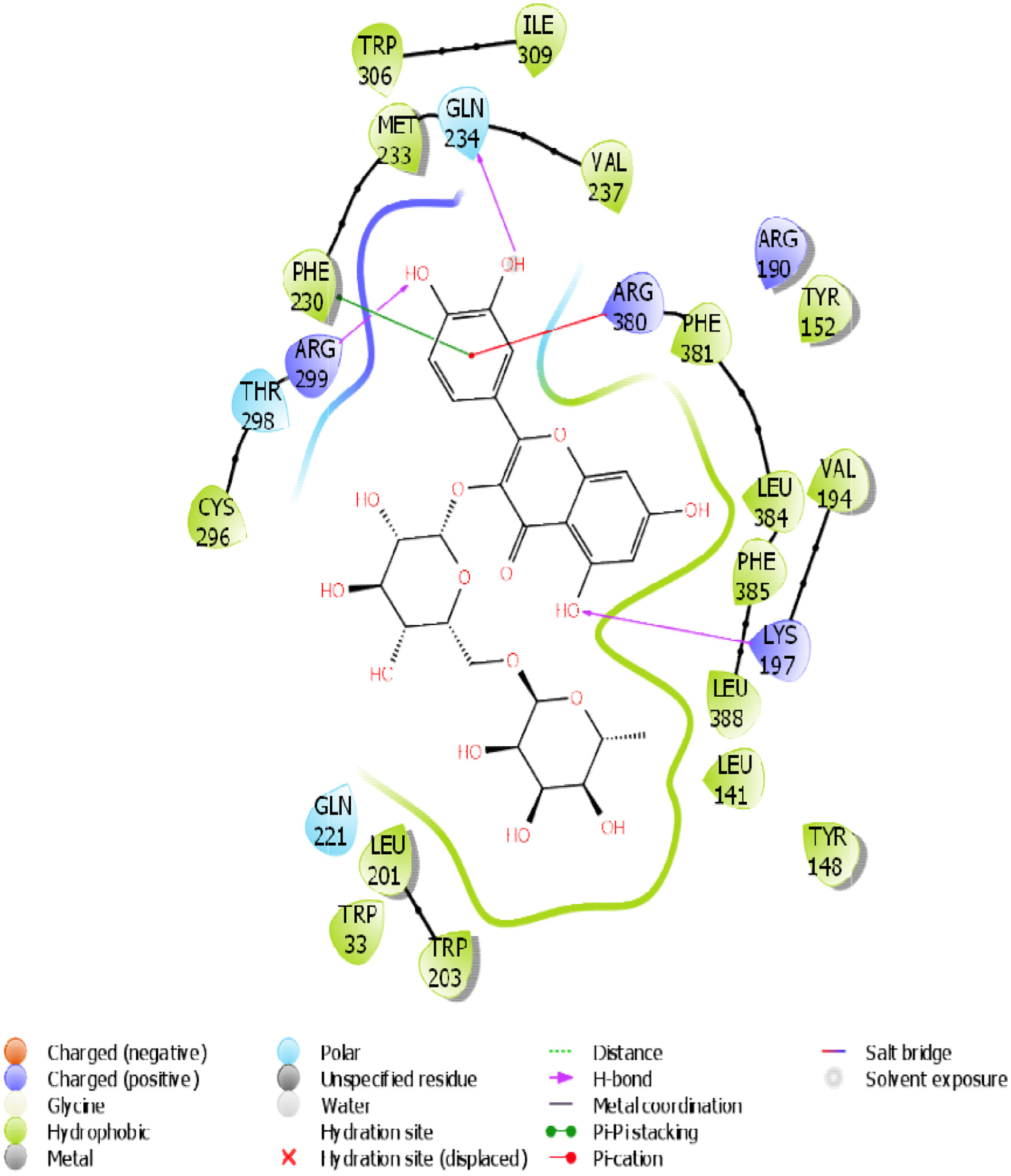 Click for large image | Figure 15. The 2D chemical relationship of Rutin with GLP-1 with amino acid residues. |
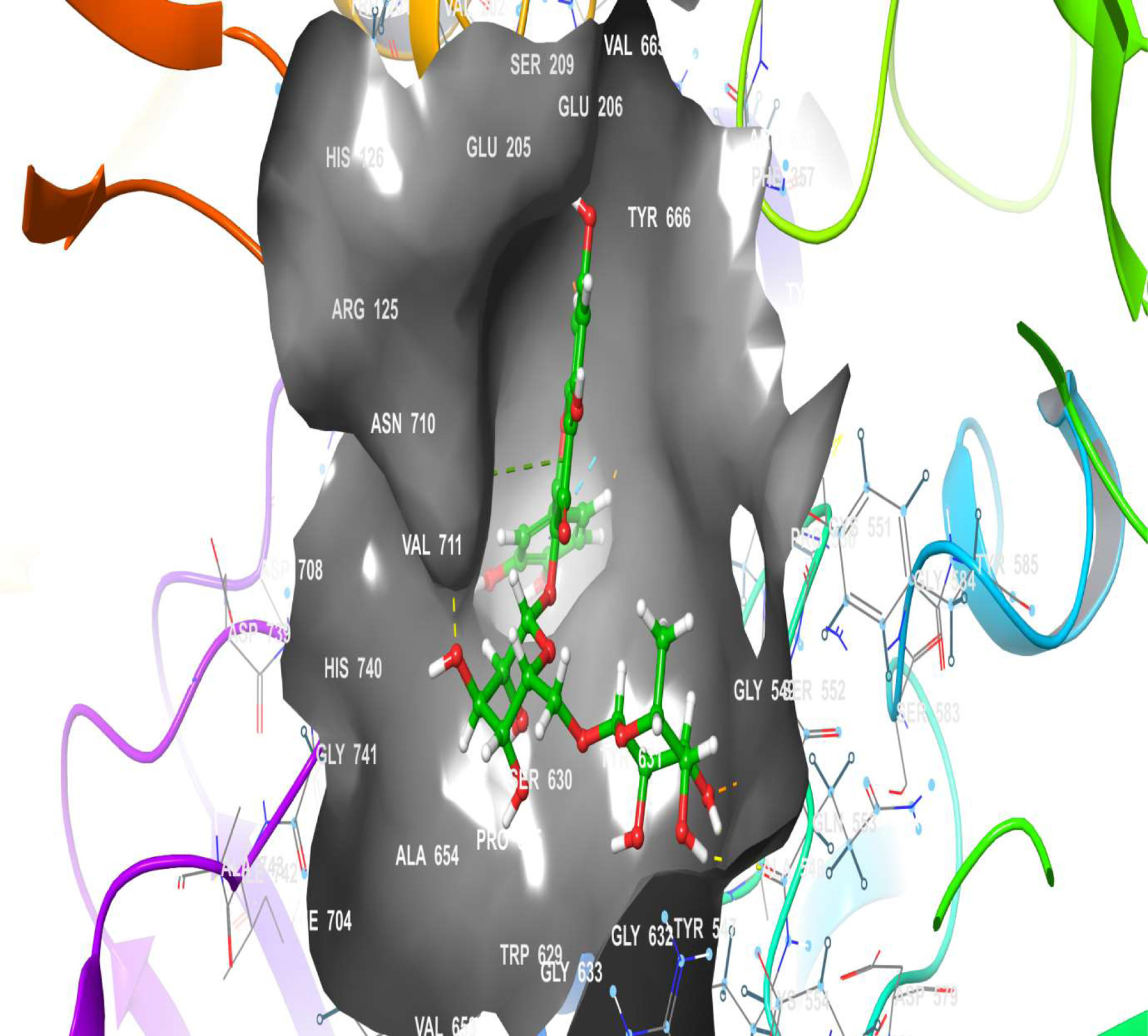 Click for large image | Figure 16. The 3D chemical relationship of Rutin with DPP4 with amino acid residues. |
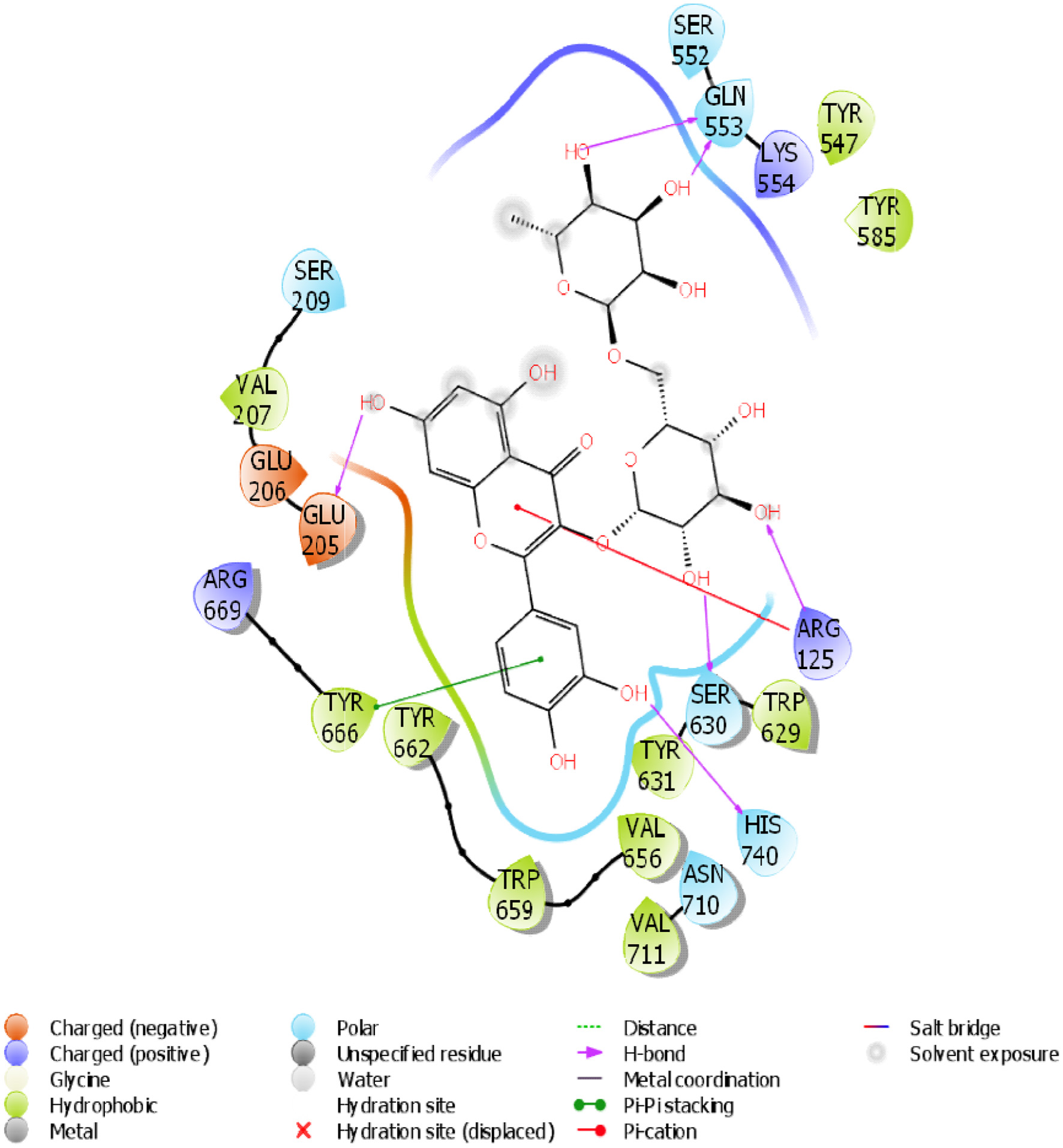 Click for large image | Figure 17. The 2D chemical relationship of Rutin with DPP4 with amino acid residues. |
 Click to view | Table 2. Binding affinity, H-bonding, and hydrophobic interactions of rutin with selected receptor targets |
Table 2 shows the binding affinities of rutin with the listed protein and receptor associated with insulin sensitivity. This figure also shows the amino acids involved in the hydrophobic interactions. A number of hydrogen bonds and Pi-Pi.
| 4. Discussion | ▴Top |
Mitochondria are highly dependent on glucose as a substrate for their energy-generating activities (Anderson et al., 2019). The electron transport chain and oxidative phosphorylation are two key reactions that occur in mitochondria. Nicotinamide adenine dinucleotide (NADH) is needed for the electron transport chain and oxidative phosphorylation, which occurs in the mitochondria for adenosine triphosphate (ATP) or energy generation (Elekofehinti et al., 2024).NADH is generated in the cytosol during glycolysis. However, NADH production is reduced or made impossible in diabetic patients because of glucose intolerance (Deshpande and Mohiuddin, 2021; Wu et al., 2017). This eventually leads to mitochondrial degeneration and shutdown. Eventually, cell death can occur, especially in the beta-cells of the pancreas, since the powerhouse of the cell has been shut down. The co-enzymes and co-factors above are found and abundant in the glycolytic pathway which can be initiated by the hormone insulin. Insulin secretion in turn can be initiated by the TGR5 which then signals the GLP-1.
The blood sugar levels of the experimental mammals were significantly (p < 0.05) elevated three days after STZ induction, as shown in Figure 1. This could be due to streptozotocin (STZ), which destroys the beta cells of the pancreas, crippling its ability to produce insulin for glucose metabolism and resulting in hyperglycemia. This finding is corroborated by the studies of Wu et al. (2016). who reported the cytotoxic effects of STZ on beta cells. Rutin (25 and 50 mg/kg) significantly (p < 0.05) reduced blood glucose in diabetic rats after 28 days of oral administration. This could be because rutin can resuscitate and proliferate the beta cells of the pancreas. Compared with those in the noninduced group, the weights of the rats in the STZ-induced and metformin-treated groups decreased as the study progressed for 28 days (Figure 2). This further supports weight loss in hyperglycemic patients and the side effects of diabetic drugs that have been reported (Oyetayo et al., 2021)
Pancreatic and duodenal and homeobox 1 (PDX-1) stimulate insulin synthesis and is essential for the survival of pancreatic β-cells. The maturation and differentiation of common pancreatic precursor cells are determined by PDX-1, a crucial regulator of insulin gene expression and an essential component of normal pancreatic development (Ghorbani et al., 2019; Butler et al., 2007). The expression of PDX-1mRNA in diabetic control rats was significantly lower (p < 0.05) than in control rats, as depicted in Figure 3. This could be because β-cell death is caused by mitochondrial degeneration resulting from hyperglycemia-induced glucose intolerance. STZ may also induce reactive oxygen species (ROS) production in pancreatic beta cells, leading to a reduced expression level of PDX-1. This is also supported by the work of (Kim and Hebrok, 2001) . STZ has also been reported to be associated with mitochondrial dysfunction [18]. Upon treatment with rutin at 25 mg/kg, the expression of the PDX-1 gene was significantly upregulated (p < 0.05) relative to that in the diabetic control group, which could be due to the regeneration of mitochondria and β-cells, which in turn activates the PDX-1 gene and insulin gene, which aid in glucose metabolism. This probably shows the resuscitative and biogenesis effects of rutin on the mitochondria of pancreatic cells.
The expression of tumour necrosis factor α (TNF-α) and interleukin-1 beta (IL-1β) was studied in the pancreas (Figures 4 and 5). These genes encode proinflammatory cytokines that help to regulate immune and inflammatory events (Siddique and Awan, 2016). These genes were significantly (p < 0.05) upregulated in diabetic control rats relative to control rats, both in the liver and the pancreas, as observed in this study, which indicates toxicity. Tumor necrosis factor-alpha (TNF-α) is involved in the pathogenesis of diabetes mellitus and catalyzes multiple signalling cascades that result in β-cell apoptosis in the pancreas (Zhong et al., 2017; Perez-Matute et al., 2009; Faloon et al., 2011). Beta-cells (β-cells) apoptosis could be due to their chronic exposure to circulating inflammatory cytokines induced by excessive release of reactive oxygen species, which further reduce insulin secretion of these cells and facilitate their apoptosis (Ahrens, 2011). Treatment of diabetic rats with 25 mg/kg rutin helped to downregulate the expression of these cytokine genes relative to that in diabetic control rats in the pancreas (Figures 4 and 5). This could be due to the ablilty of rutin to reduce the amount of reactive oxygen species in the pancreas; this finding revealed the anti-inflammatory effect of rutin (Staurengo-Ferrari et al., 2019).
The expression of IL-1β, IL-6 and TNF-α were studied in the liver as seen in Figures 6–8 respectively. The upregulation of these cytokines in the liver observed in the diabetic group could be a consequence of caspase-3 induction. This is an important enzyme in the apoptotic pathway that causes the death of hepatocytes (liver cells) resulting in hyperglycemia as liver play important role in glucose homeostasis (Faloon et al., 2011). Rutin (25, 50 and 100 mg/kg) significantly downregulated the expressions of these cytokines relative to diabetic control in the liver. This suggests that rutin has hepatoprotective potential.
TGR5 is a membrane-found G-protein-coupled receptor, and its activation induces GLP-1 release in endocrine cells (Javed et al., 2012; Maruthur et al., 2016). The expression of TGR5 in diabetic rats was significantly lower than that in control rats (Figure 9). This decrease in TGR5 mRNA expression might be due to insulin resistance and the high glucose and/or fatty acid levels usually observed in diabetic patients. Insulin resistance and mitochondrial degeneration have been linked (Hunt et al., 2020). Rutin (50 and 100 mg/kg) significantly (p < 0.05) upregulated TGR5 expression relative to that in the diabetic control group. This could be due to rutin preventing oxidative stress and lipid accumulation. Glucagon-like peptide 1 (GLP-1) helps enhance pancreatic β-cell proliferation, boosts insulin production, and protects β-cells against apoptosis (Alonge et al., 2024). As shown in Figure 10, GLP-1 expression was significantly lower (p < 0.05) in the diabetic control group than in the control group. This could be a result of the death of beta cells (a source of the GLP-1 gene) caused by diabetes-induced inflammation. The oral administration of metformin and rutin at 25 and 100 mg/kg significantly upregulated (p < 0.05) GLP-1 expression relative to that in the diabetic control group. This could be a result of the resuscitative effect of rutin on the pancreas leading to the activation and proliferation of beta cells. This finding is in agreement with the work of Oyetayo et al. (2021) who reported that flavonoids can boost the production of pancreatic beta cells.
As a peptidase, Dipeptidyl peptidase-4 (DPP4) hydrolyses and cleaves the incretin hormone GLP-1, which helps to increase excess glucose in the blood by converting it to glycogen (Elekofehinti et al., 2018). DPP4 expression was significantly upregulated in diabetic control rats compared to nondiabetic control rats in this study (Figure 11). This impairs glucose metabolism, resulting in increased blood sugar levels, as observed in this study. A half-life-shortening effect of DPP4 on hormones that ensure glucose homeostasis has been reported (Omar et al., 2014; Oyetayo et al., 2021). Rutin (25, 50, and 100 mg/kg) significantly downregulated the expression of DPP4 gene relative to that seen diabetic control group. This suggests that rutin may help to boost the actions of hormones that ensure euglycemia.
To further investigate the antidiabetic effect of rutin, the three-dimensional structures of TGR-5, GLP-1, and DPP4 were docked into the Rutin structure or compound. The docking results (Table 2) showed that TGR-5 and GLP-1 were highly correlated, with binding energies of -12.407 kcal/mol and -12.723 kcal/mol, respectively, when docked with the Rutin ligand (Figures 12–15). During the docking simulation of DPP4 with rutin, six H-bond formulations and 7 residues (GLN 553, SER 630, ARG 125, HIS 740, TYR 666, GLU 205) contributed to the hydrophobic interactions around the active site (Table 2, Figures 16 and 17). The outcome of this in silico study suggests that rutin is a potent ligand of these receptors and capable of modulating them for its normoglycemic activity as seen in the glucose and gene expression results. This finding could further affirm the potential of rutin to exert its anti-inflammatory and antidiabetic effects, as shown in this study.
| 5. Conclusion | ▴Top |
This study further affirms and supports the use of rutin in diabetes management as it reduced the blood glucose levels of diabetic rats after 28 days. Rutin also modulated the expressions of insulin-related genes and downregulated the expression of proinflammatory genes in the liver and pancreas of diabetic rats. It effectively binds to insulin-sensitive receptors. The work suggests that the most potent dosage of rutin in diabetes treatment is a low dosage.
Additional genes associated with glucose metabolism and inflammation may still be studied in rutin-adminstered diabetic rats. More research on various rutin doses could still be conducted to establish the most effective dosage and possibly clinical trials.
Acknowledgments
The authors appreciate the staff of Teady Bioscience Laboratory, Akure, Ondo State, Nigeria for their support and use of their facilities for the study.
No funding was received for this work
Conflict of interest
The authors declare that they have no known competing financial interests or personal interest
| References | ▴Top |