| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 27, September 2024, pages 70-78
Impact of light roasted coffee enriched with chlorogenic acid on lipid and glycaemic indices in healthy obese adults. A randomized, double-blinded, placebo-controlled clinical trial
Hui-Fang Chiua, Jyun-Syong Wangb, Kamesh Venkatakrishnanb, Chin-Kun Wangb, *
aDepartment of Chinese Medicine, Taichung Hospital Ministry of Health and Welfare, Taichung-40301, Taiwan, China
bDepartment of Nutrition, Chung Shan Medical University, 110, Sec. 1, Jianguo North Road, Taichung City-40201, Taiwan, China
*Corresponding author: Chin-Kun Wang, Department of Nutrition, Chung Shan Medical University, 110, Sec. 1, Jianguo North Road, Taichung City-40201, Taiwan, China. Fax: +886 4 22654529; E-mail: wck@csmu.edu.tw
DOI: 10.26599/JFB.2024.95027388
Received: July 4, 2024
Revised received & accepted: August 29, 2024
| Abstract | ▴Top |
Coffee contains phytocomponents including caffeine, chlorogenic acid (CGA), and ferulic acid. This study was intended to explore the impact of light roasted coffee rich in CGA on anthropometric parameters, lipid, and glycaemic indices, along with various lipid metabolizing molecules in healthy obese subjects. Forty healthy obese subjects (n = 40) were recruited and divided into 2 groups as experimental (CGA-340 mg/day and caffeine-220 mg/day; n = 20) and placebo (CGA-12.4 mg/day and caffeine-220 mg/day; n = 20). Daily consumption of the experimental sample resulted in a significant decrease (p < 0.05) in body weight, body fat, BMI, waist circumference, total cholesterol, and low-density lipoprotein cholesterol. Various lipid metabolism-related signaling molecules like AMPK-α and PPAR-α were improved (p < 0.05) after 12 weeks of supplementation with experimental samples as compared to the placebo group. The adiponectin levels were increased (p < 0.05) in the experimental group, levels of leptin, LXR-α, and PPAR-γ were decreased (p < 0.05). No significant difference was observed in any glycaemic indices or hepatic/renal markers in either the experimental or placebo group. The present outcome depicts that consumption of coffee (experimental/test sample) rich in CGA and caffeine (holistically) for 12 weeks could positively alter various lipid metabolizing signaling molecules and thereby lower the body fat accumulation and the incidence of obesity.
Keywords: Light roasted coffee; Chlorogenic acid; Caffeine; Body fat; Obesity
| 1. Introduction | ▴Top |
Obesity is a metabolic derangement, which resulted due to an imbalance between energy expenditure and energy intake, which leads to excessive accumulation of body fat especially visceral fat, and eventually ends up in obesity (Segula, 2014). Previous reports showcase that obesity directly or indirectly contributes to various health ailments like dyslipidemia, hypertension, diabetic mellitus, cardiovascular diseases, and cancer (Lu et al., 2019; Rodgers et al., 2012; Mokdad et al., 2003). A recent study conducted by World Health Organisation (WHO) shows that approximately 1.9 billion adults were overweight and 600 million adults were obese and also they predicted these numbers will be doubled by the year 2030 (WHO, 2013). The above data indicated that obesity could pose an enormous psychological, physical and social-economic burden (health concern) to the world and need to be addressed as soon as possible. Hence, recently many researchers are showing immense interest to negate and treat obesity as well as its related complications through developing novel therapy using functional foods/nutraceuticals (Nijhawan and Behl, 2020; Venkatakrishnan et al., 2019).
Coffee is a one of the most popular and highly consumed beverage, which is rich in various bioactive phytocomponents including polyphenols and alkaloids like caffeine, chlorogenic acid (CGA), caffeic acid, ferulic acid, caffeol, and diterpenes (kahweol) with numerous health benefits (Chen, 2019; Suzuki et al., 2019). Especially the CGA and caffeine from coffee are considered as the major phytocomponents, that might have contributed to various biological functions and hence they are recommended as functional food/nutraceuticals (Karuppagounder et al., 2021; Santana-Galvez et al., 2017). Light roasted coffee has rich content of CGA and caffeine than medium or high roasted coffee. Due to the high content of CGA, many researchers are showing immense interest in light roasted coffee to explore the better health benefits. CGA is an ester formed between quinic acid (5-caffeoyl quinic acid) and caffeic/ferulic acid or trans-cinnamic/coumaric acid. Its various active metabolite includes caffeic acid -3-O-sulfate and dihydro-ferulic acid-4-O-sulfate (Pimpley et al., 2020; Li et al., 2020). CGA displays a wide range of biological properties including antioxidant, anti-inflammatory, anti-microbial, anti-hyperlipidemic, anti-cancer, anti-hypertensive, anti-obesity (thermogenesis), and anti-diabetic activities as well as possessing neuroprotective, hepatoprotective and cardio-protective properties (Suzuki et al., 2019; Wang et al., 2019). Moreover, caffeine (moderate consumption) is also reported to stimulate the nervous system (neuro-stimulant), promote blood circulation, mood enhancer, antioxidant and anti-obesity (thermogenesis) activities as well as neuroprotective and cardioprotective properties (Clark et al., 2019; Tabrizi et al., 2019; Golzarand et al., 2018; Sugiura et al., 2012).
Previous cell line and animal models have demonstrated that administration of CGA and caffeine individually or in combination, would considerably regulate lipid/fat metabolism and thus lower the risk of obesity and its related complications (Xu et al., 2019; Zhao et al., 2017; Zheng et al., 2014). Although CGA and caffeine have been certified as FOSHU, clinical trials investigating their effects on lipid metabolism (Sarria et al., 2020; Watanabe et al., 2019) have yielded variable results, highlighting the need for further research to better understand their impact of the exact mechanism behind regulating various lipid metabolizing molecules. Therefore, this novel pilot clinical trial was designed to check the effect of light roasted coffee rich in CGA and caffeine on anthropometric parameters, lipid profile, and various lipid metabolizing molecules in healthy obese subjects.
| 2. Materials and methods | ▴Top |
2.1. Sample
Both placebo and experimental coffee samples were provided by Chateau No. 26 company (Tainan, Taiwan, China). Nutrient contents in one pack (10 g) of experimental sample includes 0.7 g carbohydrates, 0.6 g protein, and 0.12 g of protein. The duration of light roasted coffee was 13 minutes under 185 °C. Both placebo and experimental samples were provided Chateau No. 26 premium coffee powder (Manor No. 26 Premium Coffee Shop, Tainan, Taiwan, China). The experimental pack (light roasted coffee powder-10 g) contains 170 mg of CGA and 110 mg of caffeine and the placebo pack contains coffee flavored powder, which contains only 6.2 mg of CGA and 110 mg of caffeine. Both sample drinks were prepared by mixing the total pack content (10 g) in 90–95 °C hot drinking water.
2.2. Subject enrolment
The current trial was conducted at Chung Shan Medical University Hospital, Taichung, Taiwan, China from Feb to June 2021. The human clinical trial ethical review committee board affiliated with Chung Shan Medical University Hospital, Taichung, Taiwan, China (IRB No: CS1-20073) in accordance with the Declaration of Helsinki has approved this clinical trial. All the participants are enrolled into this trial based on various inclusion and exclusion criteria. Inclusion criteria: Only the healthy obese subjects, whose BMI ≥ 27 and body fat (male 20–25%; female 25–30%) aged between 20 to 70 were recruited for this trial. Several exclusion criteria such as subjects who are pregnant and breastfeeding women, Without any major cardiovascular diseases, hepatic or renal disorders as well as subjects under health supplementation especially CGA or caffeine and heavy coffee or alcohol drinkers. Volunteers were recruited through, advertisement based on the above-mentioned inclusion, and exclusion criteria by telephone. Eligible subjects were requested to visit Chung Shan Medical University Hospital for further screening by basic biochemical analysis and anthropometric measurements, followed by documenting their medical history. Before enrolling in the trial all the participants were requested to sign the written consent.
2.3. Experimental design/grouping
Based on the advertisement, a total of 62 subjects visited Chung Shan Medical University Hospital for initial screening, but only 40 subjects are eligible were included in this trial. All the healthy obese subjects (n = 40) were divided equally into 2 groups experimental and placebo groups. Experimental group subjects (n = 20) were requested to consume 2 packs of coffee powder rich in CGA (340 mg) and caffeine (220 mg) every day for 12 weeks by dissolving in hot water. Placebo group subjects (n = 20) were requested to consume a coffee flavored drink with CGA (12.4 mg) and caffeine (6.4 mg) every day for 12 weeks. Subjects can decide to drink before or after breakfast and lunch at their choice/discretion. Subjects have the freedom to withdrew from this trial at any time. After 12 weeks of intervention with experimental or placebo sample, followed by 2 weeks of the follow-up period (confirmed the impact of light roasted coffee- experimental sample). Every four weeks subjects are requested to visit Chung Shan Medical University Hospital for every four weeks to get samples as well as to record dietary intake (crosscheck CGA or coffee intake) as well as blood samples were collected for various biochemical analyses. Moreover, subjects are enquired about any side effects or discomfort after experimental or placebo drink consumption. Figure 1 shows the schematic representation of present clinical trials.
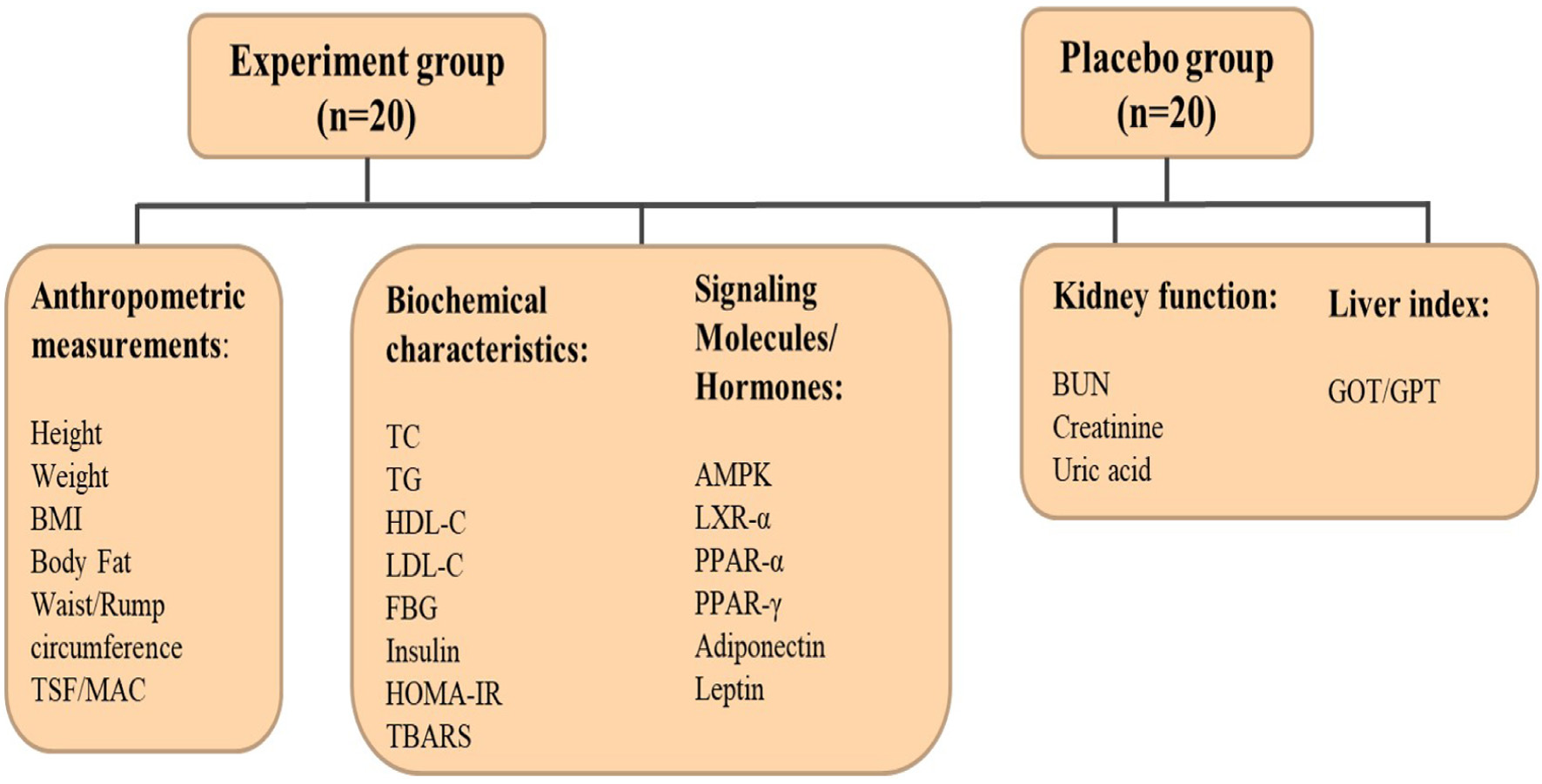 Click for large image | Figure 1. Shows the schematic representation of present clinical trials. |
2.4. Sample collection and processing
After recruitment, subjects are requested to visit the hospital on 0, (baseline/initial), 4th, 8th, 12th (last week of treatment), and 14th (Follow up) weeks, and the blood sample was collected in the heparinized tube for plasma sample and non-heparinized tube for serum preparation. Both plasma and serum samples were stored at −80 °C until biochemical analysis. Likewise, the various anthropometric parameters were also conducted on the 0, (baseline/initial), 4th, 8th, 12th, and 14th (Follow up) weeks. The body weight, height, body fat, and body mass index (BMI) were measured with Omron Karada Body Scan/analyzer (HBF:370). Whereas, Waist circumference (WC) and Rump circumference (RC) were measured using inch tape on bare skin. Also, the triceps skinfold (TSF), was measured with Holtain T/W skin caliper (Holtain Ltd., Crymych, UK). Mid-arm circumference (MAC) was measured as indicated in our previous studies (Lu et al., 2019). The basic characteristics of subjects including age, gender, height, weight, BMI, and dietary intake (macronutrient and energy) of all subjects are furnished in the supplementary document.
2.5. Biochemical Parameters
Various lipid profiles including total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-c) were estimated by a commercial kit bought from Roche Diagnostics (Mannheim, Germany). However, low-density lipoprotein cholesterol (LDL-c) was calculated using the Friedewald method (1972). Serum adiponectin and leptin levels were determined using an ELISA kit bought from MyBioSource (CA, USA). Also, various lipid metabolizing signaling molecules (transcription factors/proteins) like Adenosine monophosphate-activated protein kinase-alpha (AMPK-α), peroxisome proliferator-activated receptor alpha (PPAR-α), Liver X receptor alpha (LXR-α) and peroxisome proliferator activated receptor gamma (PPAR-γ) were measured in blood samples using ELISA kit bought from MyBioSource, CA, USA. The lipid peroxidation products like thiobarbituric acid reactive substance (TBARS) were evaluated in plasma by the methods of Draper and Hadley, 1990. The total protein contents were estimated using a commercial bicinchoninic acid (BCA) kit (Thermo Fisher Scientific, IL, USA). The fasting blood glucose (FBG), insulin, and HbA1c levels were determined using a commercial kit (Thermo Scientific Inc., MO, USA). The homeostasis model assessment of Insulin resistance (HOMA-IR) was calculated by the formula insulin × FBG/405. The hepatic markers like glutamic pyruvic acid transaminase (GPT), glutamic oxaloacetic acid transaminase (GOT), as well as renal markers like Blood urea nitrogen (BUN) and creatinine (Cr), were assessed using a commercial kit from AppliedBio (CA, USA).
2.6. Statistical analysis
All values are expressed as mean ± standard deviation (SD) and p < 0.05 is considered statistically significant. The Significant difference between the initial (0 week) and after 12 weeks of treatment [0 vs 12th weeks] and follow up (14th week) [12 vs 14th weeks] in the same group was calculated by paired t-test using Statistical Package for Social Sciences (SPSS) version 21 (IBM Inc, NY, USA). Bonferroni correction post hoc test (one-way ANOVA) and student t-test was performed pairwise for multiple comparisons between experimental vs placebo group at a different time interval (0 or 12th or 14th week).
| 3. Results | ▴Top |
3.1. Changes in Anthropometric parameters
The impact of experimental and placebo samples on various anthropometric parameters was epitomized in Table 1. After 12 weeks of intervention with experimental sample rich in CGA and caffeine showed a significant reduction (p < 0.05) in body weight, BMI, body fat, WC, and RC. Especially in the 8th week of intervention the fat reduction was prominent in the experimental group. The placebo group also showed a slight decrease in body weight, BMI, body fat, WC, and RC. But not that significant as compared to the experimental group.
 Click to view | Table 1. Effect of experimental and placebo samples on various anthropometric parameters |
3.2. Changes in Lipid profile
The effect of experimental and placebo samples on various lipid profiles was showcased in Table 2. Lipid profiles including TC, TG, LDL-c, and HDL-c did not show any significant impact after 12 weeks of placebo intervention. However, TC and LDL-c levels were significantly lowered (p < 0.05) in obese subjects who consumed the experimental sample (CGA and Caffeine rich), but no significant changes were observed in TG and HDL-c (but showed a lower trend). Overall, 12 weeks of supplementation with CGA and Caffeine rich coffee drinks display a considerable decrease (p < 0.05) in TC and LDL-c as compared to a placebo group. Also, a significant difference (p < 0.05/p < 0.01) was noted in the baseline (0 weeks) between the placebo and experimental group in all lipid profiles.
 Click to view | Table 2. Effect of experimental and placebo samples on various lipid profile |
3.3. Changes in lipid metabolizing related signaling molecules (hormone/transcription factors)
Table 3, shows the changes in lipid metabolizing related signaling molecules (hormone/transcription factors) after 12 weeks of drinking coffee rich in CGA and caffeine. As compared to a placebo group, the experimental group showed a significant increase (p < 0.05) in the levels of AMPK-α, PPAR-α, and adiponectin. Whereas, the levels of PPAR-γ, LXR-α, and leptin were substantially decreased (p < 0.05) in obese subjects who consume experimental sample rich in CGA and caffeine. This may indicate a distinct metabolic response associated with obesity. In non-obese (healthy) subjects, the changes in these markers were less pronounced and did not reach statistical significance. Nevertheless, the levels of various lipid metabolizing related signaling molecules like AMPK-α, PPAR-α, PPAR-γ, LXR-α, leptin, and adiponectin did not show any significant changes in the placebo group. In addition, on the 12th week of supplementation, a significant difference (p < 0.01) was noted between the experimental and placebo group, which indicates the efficiency of experimental samples on various lipid metabolizing related signalling molecules.
 Click to view | Table 3. Effect of experimental and placebo samples on various lipid metabolizing related signalling molecules |
3.4. Changes in glycaemic and oxidative indices
There was no significant difference in the levels of glycaemic indices like FBG, insulin, HbA1c, and HOMA-IR in the experimental and/or placebo group (Table 4). Nevertheless, the lipid peroxidation products (oxidative indices) like TBARS was significantly declined (p < 0.05) in the experimental group of obese subjects. Also, a considerable change in the levels of TBARS is noted between the experimental and placebo group (exp vs placebo) on the 4th, 8th, 12th, and 14th weeks.
 Click to view | Table 4. Effect of experimental and placebo samples on various glycaemic and oxidative indices |
3.5. Changes in various biochemical parameters
Effects of experimental and placebo samples on various biochemical parameters like hepatic and renal markers were indicated in Table 5. GOT, GPT, and renal markers BUN and Cr showed a decreasing trend after 12 weeks of intervention with the experimental sample, although not all changes were statistically significant. The above data infer that holistically CGA and caffeine did not alter any other enzyme activity and thus maintain normal physiology/ chemical reaction.
 Click to view | Table 5. Effect of experimental and placebo samples on various hepatic and renal markers |
| 4. Discussion | ▴Top |
Obesity is one of the major health issues with a complex pathophysiological impact, which is tightly associated with various health complications like hypertension, diabetic mellitus, and cardiovascular diseases (Lu et al., 2019; Rodgers et al., 2012). Obesity and its related complication can be abolished by using various nutraceuticals/function foods, one such popular nutraceuticals/function food is CGA and caffeine. This is the very first pilot clinical trial conducted to explore the impact of light roasted coffee rich in CGA and caffeine on anthropometric parameters, lipid profile, and various lipid metabolizing molecules in healthy obese subjects. Also, the in-depth molecular mechanism behind the lipid-lowering effect of CGA and caffeine in obese subjects was explained in this trial with proper evidence.
Various anthropometric parameters were checked to confirm the impact of light roasted coffee rich in CGA and caffeine in healthy obese subjects. The mean body weight, BMI, body fat, WC, and RC levels were substantially decreased after 12 weeks of consumption with experimental samples (light roasted coffee rich in CGA and caffeine). However, a significant difference was observed in all anthropometric parameters between the placebo and experimental group in all time frames (exp vs placebo on 0, 4th weeks) as the mean values of the experimental and placebo group are considerably different. But, overall 12 weeks of drinking experimental sample showed a significant decline in various anthropometric measurements like body weight, BMI, body fat, WC, and RC. The above data are in line with the results of a clinical trial conducted by Sarria and his co-worker (2020), who in his study confirmed that intake of coffee rich in CGA and caffeine showed a significant decrease in body weight, body fat, BMI and WC in hypercholesterolemic subjects. Recently, a systematic review is done by Asbaghi and his colleagues (2020), also concluded that drinking green coffee extract rich in CGA and caffeine could considerably lower various anthropometric parameters. A handful of studies demonstrated that CGA and caffeine would trigger thermogenesis and lipogenesis and thereby suppressing fat accumulation and eventually lower various anthropometric parameters (Xu et al., 2019; Zhao et al., 2017).
Dyslipidaemia (hyperlipidemia/hypercholesterolemia) is one of the crucial factors which might result in obesity and cardiovascular diseases (CVDs). Hence, the levels of various lipid profile levels (TC, TG, LDL-c, and HDL-c) were determined. After 12 weeks of drinking light roasted green coffee (rich in CGA and caffeine) the levels of LDL-c and TC were significantly decreased without altering TG or HDL-c. Present data are in correspondence with the findings of Xu and his co-worker (2019), who also indicated that combinational treatment with CGA and caffeine significantly decreased the levels of TC and LDL-c by altering the AMPK and LXR signaling pathways. Moreover, CGA active metabolites like 5-caffeoyl quinic acid and caffeic/ferulic/coumaric acid are reported to positively alter microbiota and thereby increasing short chain fatty acids (SCFA) production, which resulted in improved lipid metabolism (Xie et al., 2021; Li et al., 2020; Madrid-Gambin et al., 2016).
Ample amount of studies indicated that the AMPK and PPAR signaling pathways are the major signaling pathway as they regulate the metabolic homeostasis (cellular energy sensor) and subsequent lipid/fat metabolizing downstream signaling molecules (Zhao et al., 2017; Ahn et al., 2008). Moreover, few researchers revealed that a combination of CGA and caffeine might holistically activate the AMPK and PPAR signaling pathway in an effective way (Vasileva et al., 2020; Zheng et al., 2014). Based on the above data, the author speculates that a combination of CGA and caffeine (light roasted coffee) might effectively lower body weight and positively regulate lipid metabolism via triggering the AMPK (p-AMPK) and PPAR signaling pathway. PAMPK is a heterotrimeric kinase contains catalytic α subunit and regulatory β and γ subunits. AMP activate AMPK by binding to the γ subunit and induce a confirmational change which relieves the autoinhibition γ domain from the kinase protein and allows phosphorylation at Thr172 residue of the α subunit. The activated AMPK- α is the critical regulator of short-term fatty acid oxidation. Hence AMPK- α is used a marker of AMPK activity (Hawley et al., 1996; Sozio et al., 2011). AMPK, a key regulator of cellular energy status, is predominantly active within cells, particularly in response to metabolic stress where it is activated via phosphorylation. The presence of AMPK in the bloodstream does not necessarily indicate its active phosphorylated state or functional role but may reflect a general response to metabolic alterations or, in some cases, tissue damage or cellular turnover (Steinberg and Kemp, 2009). This has to be understood in the way that the systemic presence of these proteins is the indirect insights but must be interpreted with caution, particularly in distinguishing between normal metabolic signaling and potential tissue leakage or damage.
Present data confer our speculation that light roast coffee (rich in CGA and caffeine) intervention significantly improved the levels of AMPK-α, and PPAR-α, which in turn also suppress the levels of PPAR-γ, LXR-α. CGA and caffeine (experimental sample) would activate AMPK-α (p-AMPK-α) which triggers the further activation of PPAR-α. Activated PPAR-α could inhibit LXR-α and thus increase LDL-c receptor (lower LDL-c circulation) as well as enhance β- oxidation and inhibit endogenous cholesterol synthesis (downregulate HMG-CoA reductase). The fat storage ability of adipocytes is orchestrated by PPAR-γ. The accumulation of PPAR-γ facilitates accumulation of fat in the subcutaneous tissues (Dixon et al., 2021). PPAR-γ also involves in the fat acid metabolism by influencing free fatty acid transporters, lipogenic and lipolytic genes which helps in the uptake of free fatty acid (Frohnert et al., 1999). LXR ability to regulate the inflammatory response is depends on the changes in lipid metabolism. The role of LXR in inflammation is studied by LXR agonists, that inhibit the entry of nuclear factor-kappa B (NF-κB) into nucleus by negatively influencing the phosphorylation and ubiquitin-dependent inhibitory κB proteins degradation (Bi et al., 2016). Supporting this evidence, our results showed that experimental group subjects exhibited significantly reduced PPAR-γ and LXR in week 12.
CGA and caffeine are reported to increase cholesterol excretion through bile (through feces) and also regulate lipid metabolism (trigger lipolysis and lower fat accumulation), and thus promote anti-obesity activity (Xu et al., 2019; Zhao et al., 2017; Hoang et al., 2012). This combination (CGA + caffeine) could be an alternative and safe way to lower body weight and abdominal obesity (Asbaghi et al., 2020; Zheng et al., 2014). Furthermore, the author checked the levels of two major adipokines like leptin and adiponectin. Since both adipokines work opposite to each other during fat/lipid metabolism. Leptin was directly proportionate to fat level, but adiponectin was inversely proportionate to fat level. Hence many scientists focus on these adipokines to check the anti-obesity activity of any drug of interest (Fruhbeck et al., 2018). Our results showed that 12 weeks of drinking experimental samples rich in CGA and caffeine showed a significant increment in the levels of adiponectin and a decline in the levels of leptin. Accumulating evidence has conferred that administration of unroasted/light roasted coffee rich in CGA and caffeine and its metabolites could substantially lower leptin circulation along with increase adiponectin circulation. Thus, effectively alter various lipid metabolism-related molecules and thereby suppress fat deposition (Xu et al., 2019; Lee et al., 2017; Zheng et al., 2014).
CGA and caffeine were reported to improve various endogenous antioxidants as well as lower inflammatory cascade by improving adiponectin via positively modulating the AMPK signaling pathway (Xu et al., 2019; Zheng et al., 2014). The present trial also showed the decrease in the levels of lipid peroxidation products like TBARS were significantly reduced after 12 weeks of supplementation with light roasted green coffee in healthy obese subjects. Similarly, green coffee extract supplementation would considerably improve antioxidant capacity as well as lower lipid peroxidation product formation in dyslipidemic subjects (Salamat et al., 2019; Martinez-Lopez et al., 2019). However, the author has not found any significant changes in term of glycaemic indices (FBG, insulin, HbA1c, and HOMA-IR) and other biochemical parameters like GOT, GPT, and renal markers BUN, Cr. But, all the above said parameters were in a downtrend from baseline (0 week) to the 12th week, which indicated that CGA and caffeine showed moderate anti-glycaemic activity. GOT and GPT levels in the serum is a direct marker of fatty liver or liver injury due to wide range of etiologies. The inflammation induced due to obesity disturb the liver function and may cause the release of GOT and GPT in serum and are considered as a marker for hepatic dysfunction (Sookoian and Pirola, 2015). In our study, the decreasing trend in both GOT and GPT from week 0 till week 12 and also follow up visit in the experimental group indicates that the CGA and caffeine might reduce the liver upset.
In addition, both CGA and caffeine did not pose any adverse impact on renal or hepatic functions. A few limitations of this trial include no separate group for CGA or Caffeine alone group as well as the author failed to include a few lipid metabolizing markers/enzymes/hormones and antioxidants/inflammatory markers. Since it’s a pilot study, the author included only major parameters but would like to add more parameters in upcoming trials. The major advantage of this trial includes the assessment of various lipid metabolizing enzymes/hormones/signaling molecules to explore the in-depth possible mechanism behind the anti-obesity effect of CGA and caffeine. Also, the follow-up period (2 weeks) was included to confirm the impact of CGA and caffeine after 12 weeks of intervention.
| 5. Conclusion | ▴Top |
The present pilot clinical trial demonstrates that drinking light-roasted coffee rich in chlorogenic acid and caffeine (holistic effect) for 12 weeks would considerably lower various anthropometric parameters (body weight, body fat, BMI, WC), lipid profile (TC and LCL-c) as well as positively modulate various hormones and lipid metabolizing molecules such as leptin, adiponectin as well as AMPK-α, PPAR-α, LXR-α, and PPAR-γ and thus effectively promote body weight reduction and suppress visceral fat accumulation (regulating lipid profile). Based on the above study, the author recommends that drinking light-roasted coffee rich in chlorogenic acid and caffeine along with a healthy lifestyle (exercise and intake of healthy food) would help to lower obesity (control body weight) and its related cardiovascular complication. Further, longer (more than 4 months) and large clinical trial (more than 100 participants, including high BMI obese subjects) would be planned to explore whether the light-roasted coffee rich in chlorogenic acid and/or caffeine has a positive impact on the regulation of lipid metabolism, blood pressure, and blood sugar. However, our study faces certain limitations in the study of AMPK-α in the blood. As because, while the concentrations of these AMPK-α in the blood can provide indirect insights into cellular metabolic processes, they do not necessarily reflect the exact levels or activation states within specific target tissues. Emphasizing that while circulating levels offer valuable information, they may not fully represent intracellular dynamics. For this a more detailed molecular study of gene expression may be more beneficial.
| References | ▴Top |