| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 27, September 2024, pages 33-43
Recent advances in the anticancer molecular mechanisms of bioactive components from tea: A review
Jie Pana, Chuanai Chenb, Liang Baia, *, Hui Zhaoa, *
aTianjin Key Laboratory of Food and Biotechnology, College of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China
bTianjin Blood Center, Tianjin 300110, China
*Corresponding author: Liang Bai and Hui Zhao, Tianjin Key Laboratory of Food and Biotechnology, College of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China. E-mail: bailiang20101@163.com (LB) and zhaohui@tjcu.edu.cn (HZ)
DOI: 10.26599/JFB.2024.95027385
Received: August 22, 2024
Revised received & accepted: September 4, 2024
| Abstract | ▴Top |
Tea is one of the most popular health drinks around the world, especially in China, Japan, and Southeast Asia. Drinking tea has become a traditional culture and healthy lifestyle for preventing diseases in these countries and regions. Recent scientific researches indicate that tea has good efficacy in anti-cancer. The bioactive components and related anti-cancer mechanisms of tea are constantly being supplemented. Drinking tea can reduce the incidence of cancers. However, there is a lack of a systematic summary of the current tea anticancer research process to date. Here, we conduct an update on the bioactive components and r anti-cancer mechanisms of tea.
Keywords: Tea; Epidemiological studies; Bioactive components; Anticancer; Molecular mechanisms
| 1. Introduction | ▴Top |
Tea (Camellia sinensis), generally contains the leaves and buds of the tea tree and is the most consumed healthy drink in the world. Tea drinking is very popular in Asian countries such as China, Japan, and Indonesia, and has gradually aroused interest in Western countries (Tang et al., 2019). According to the different processing technologies, tea can be divided into six categories, green tea, black tea, white tea, oolong tea, yellow tea, and dark tea (Abudureheman et al., 2022). Research in recent years has shown the beneficial effects of tea, including anti-oxidation, lowering blood sugar, anti-bacteria, and anti-cancer (Ni et al., 2020; Wang et al., 2014). Tea contains many effective functional ingredients and has been widely used in beverages, foods, and medicines (Xu et al., 2023).
In recent years, cancer gradually become a high fatality rate disease worldwide and has become a focus of attention and research in developed and developing countries (Sung et al., 2021). An accumulating body of research shows that tea drinking can decrease the risk of obesity, metabolic syndrome, cancer, and other diseases, and improve the body’s immunity (Moreira et al., 2022; Yu et al., 2023; Zhao et al., 2021). Previous reviews have shown that tea may be a potentially effective substance for reducing the risk of cancer (Yang and Wang, 1993; Yang, 1999). This article reviews the main bioactive components in tea and advances in anticancer molecular mechanisms, emphasizes the importance of bioactive components and related mechanisms in tea to reduce the occurrence of cancer, and provides new ideas for the prevention and treatment of cancer.
| 2. Tea reduces the risk of cancer | ▴Top |
Cancer has become a non-hereditary disease with a high fatality rate worldwide and the leading killer of human health, including lung cancer, breast cancer, prostate cancer, and colorectal cancer (Avgerinos et al., 2019; Mizrahi et al., 2020). Tea drinking associated with the risk reduction of cancer has been confirmed by researchers (Liu et al., 2021; Filippini et al., 2020). Especially in Southeast Asia and other areas where drinking tea is popular, people who drink tea regularly have a much lower risk of cancer than normal people (Oh et al., 2019). The last five years of epidemiological studies on tea and cancer showed that regular tea drinking may reduce the risk of a variety of cancers, including liver cancer, breast cancer, brain cancer, bladder cancer, and other cancers (Li et al., 2023; Al-Zalabani et al., 2022; Zheng et al., 2023; Song et al., 2019; Martimianaki et al., 2022; Chen et al., 2022; Sen et al., 2019; Zhang et al., 2019), Table 1 shows the summary and comparison of recent epidemiological articles of tea and cancer.
 Click to view | Table 1. Summary and comparison of recent epidemiological articles of tea and cancer |
Bioactive components in tea have shown good anti-cancer effects, such as tea polyphenols, tea pigments, and theanine (Figure 1). At present, there are two main aspects of tea to reduce the occurrence of cancer. One is to kill cancer cells or inhibit their reproduction, and the other is the antioxidant mechanism (Li et al., 2023). Different bioactive components in tea play a preventive role in different mechanisms.
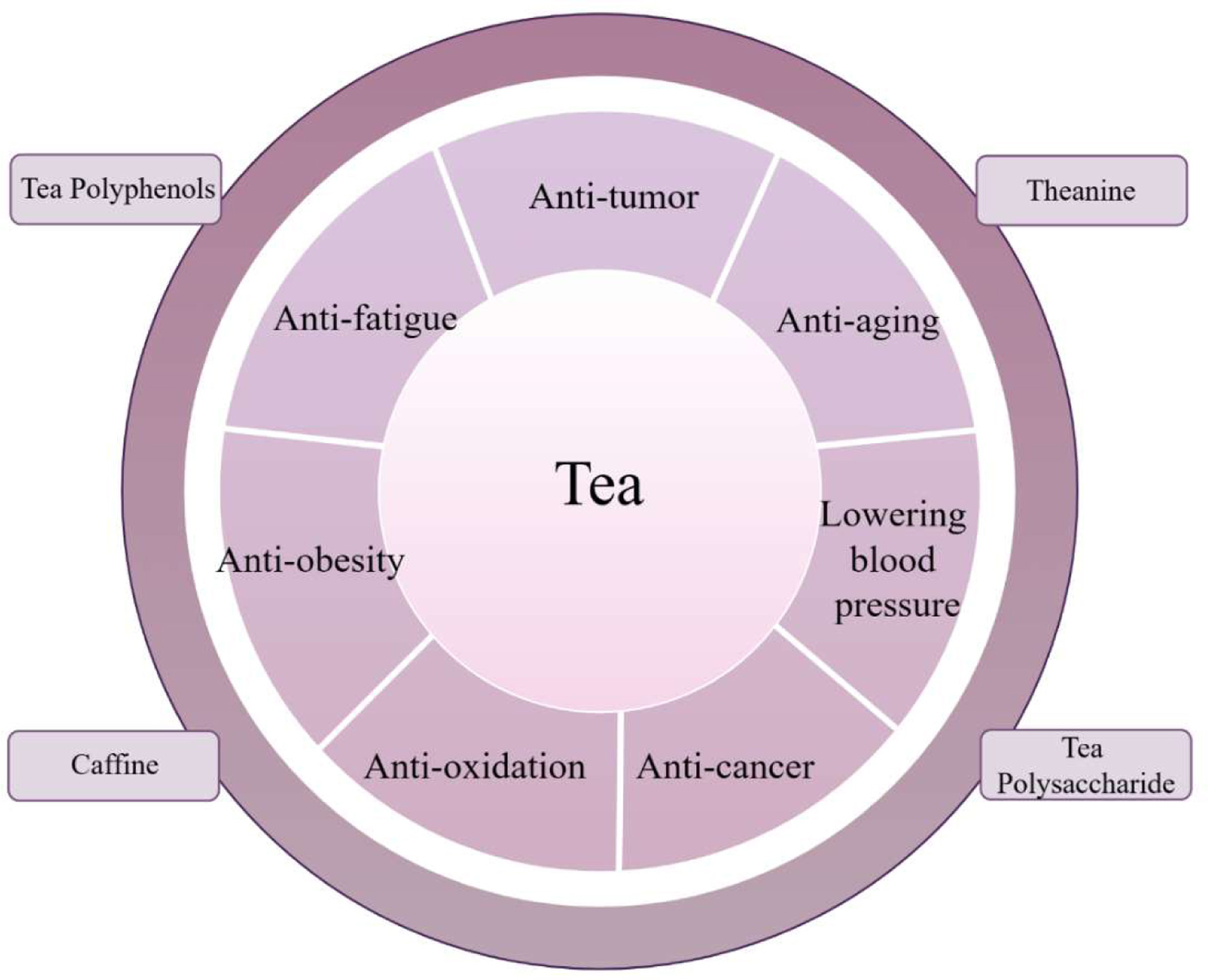 Click for large image | Figure 1. The bioactive components and bioactivities of tea. |
| 3. Bioactive components and their anticancer effects | ▴Top |
3.1. Tea polyphenols
Tea polyphenols is the general term for polyphenols in tea, and it is one of the main functional substances in the tea industry. Catechins are typical polyphenols, including epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG) and epigallocatechin gallate (EGCG) (Han et al., 2023), their chemical structure is given in Figure 2a. A lot of research shows that tea polyphenols have varying degrees of preventive and alleviative effects on a variety of cancers (Xing et al., 2019). The occurrence and development of cancer are related to the excessive production of oxygen-free radicals in the organism. Tea polyphenols and catechins have strong antioxidant activity and can inhibit reactive oxygen species in oxidative stress animal models. Thereby, playing an important role in inhibiting the occurrence and development of tumors. Studies show that EGCG can reduce lipid peroxidation and protein carbonylation, and lower the risk of the occurrence of reactive oxygen species and toxicity (Zhang et al., 2019; Guo et al., 2019). According to Wu and colleagues’ research, green tea polyphenols from (GTPs) suppressed melanoma progression by regulating circ MITF/miR-30e-3p/HDAC2 axis (Wu et al., 2022). Moreover, cellular function assays revealed that GTPs inhibited melanoma cell proliferation, migration, invasion, epithelial-mesenchymal transition (EMT), and promoted apoptosis in vitro.
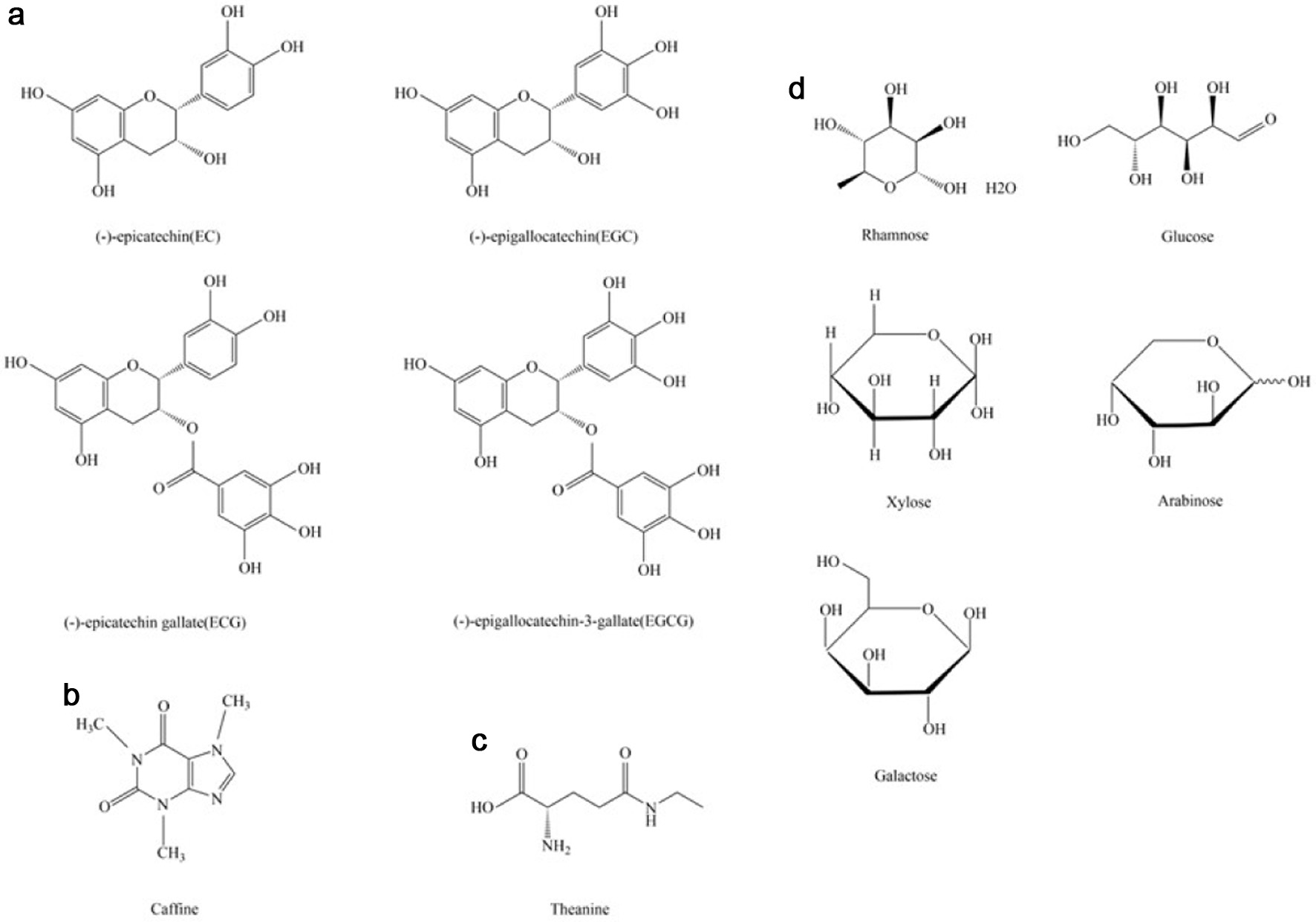 Click for large image | Figure 2. The structure of the main components in tea. (a) Catechins. (b) Caffine. (c) Theanine. (d) Tea polysaccharides. |
3.2. Caffeine
Caffeine is one of the most natural components presented in tea (van Dam et al., 2020). Figure 2b shows its chemical structure. Caffeine is an important methylxanthine that has been extensively studied, which has multiple positive biological activities including alleviative effects on nervous diseases, respiratory diseases, diabetes, and cancer (Monteiro et al., 2016). Recent studies have shown that caffeine exhibits synergistic effects in combination with many anticancer drugs. The research from Tran et al demonstrated that the cholesterol-lowering drug simvastatin can inhibit neuroblastoma growth by blocking the mevalonate pathway (Tran et al., 2023). Due to the activity, caffeine is used as an adenosine receptor antagonist. It can synergize with simvastatin to inhibit the growth of neuroblastoma cells and patient-derived xenograft (PDX) tumors and block the feedback activation of the statin-induced mevalonate pathway. Cisplatin is a widely used anticancer agent and has shown efficacy in a variety of cancers, including prostate cancer, lung cancer, ovarian cancer, and esophageal cancer. Caffeine can enhance the anticancer effect of cisplatin by inhibiting FANCD2 monoubiquitination, which demonstrated that caffeine may regulate DNA repair and cell apoptosis by inhibiting the FA pathway in HepG2 human hepatocellular carcinoma cells (Oda et al., 2017). Another research showed all concentrations of caffeine inhibited the activity of the anticancer drug paclitaxel and promoted the growth of paclitaxel-treated cancer cells (Xu et al., 2020). In conclusion, caffeine has a promising combined anticancer effect, but whether it can be used as a combined anticancer agent needs further study.
3.3. Theanine
Theanine is the characteristic amino acid of tea, which is present in green tea and naturally exists as L-Theanine. Theanine has important biological activities, such as lowering blood pressure, anti-fatigue, anti-obesity, and neuroprotective effects (Zhao et al., 2020), Figure 2c shows its chemical structure. Recently, some studies have shown that theanine also has significant anti-cancer activity. L-Theanine could inhibit melanoma cell migration and promote melanoma cell death by enhancing the expression of brain and muscle Arnt-like protein 1 (BMAL1), a clock gene in melanoma cells. Moreover, research further proved that L-Theanine can raise the transcriptional activity of p53 by enhancing the expression of BMAL1 (Zhang et al., 2022). Researchers have discovered that theanine showed a chemoprotective effect against colorectal cancer by inhibiting oxidative stress and inflammatory reactions. Their experiments demonstrated that the theanine significantly reduces the level of prostaglandin (PGE2), cyclooxygenase-2 (COX-2), and myeloperoxidase (MPO) in 1,2-dimethylhydrazine (DMH)-induced colorectal cancer. And it also decreases oxidative stress by inhibiting the level of malonaldehyde (MDA) and enhancing the levels of SOD, GPx, CAT, and GR (Ma et al., 2022). Prostate cancer (PCa) is an epithelial malignant tumor that occurs in the prostate. The metastasis rate of prostate cancer is high and the metastasis is harmful, even leading to death. L-theanine could suppress invasion, migration and increase cell-cell adhesion of prostate cancer cells in vitro and in vivo. L-theanine also could inhibit the EMT process in PCa, downregulating MMP9, N-cadherin, Vimentin, Snail and upregulating E-cadherin. In addition, L-theanine inhibited transcription of MMP9 and snail by significantly suppressing the ERK/NF-κB signaling pathway and the binding activity of p65 (Fan et al., 2021). Furthermore, oral administration of cystine and theanine exhibits relieved therapy of the side effects of capecitabine-based adjuvant chemotherapy for colorectal cancer patients (Hamaguchi et al., 2020). These results suggest that Theanine may be considered a potential alleviative agent for cancer treatment or chemotherapy side effects mitigation.
3.4. Tea polysaccharides
Tea polysaccharides is a complex polysaccharide with various biological activities, like anti-oxidation, anti-aging, anti-bacterial, and anti-tumor. It is composed of rhamnose, xylose, glucose, arabinose, and galactose, and is one of the main bioactive components in tea (Fan et al., 2022), Figure 2d shows their chemical structures. Research showed that miR-93 promoted the progression and metastasis of prostate cancer by inhibiting DAB2 and activating the Akt/ERK1/2 pathway (Yang et al., 2019). Green tea polysaccharides could promote the expression of DAB2 protein in PC-3 cells by inhibiting the metastasis of PC (Zhou et al., 2021). Tea polysaccharide (TP) could enhance autophagy, lysosome, and cell death signaling pathways in HCCT116 cells through activating EB(TFEB), a key nuclear transcription factor modulating autophagy and lysosome biogenesis, and inhibit mTOR activity and promoted LAMP1 expression. TP significantly promoted lysosomal abundance, lysosomal acidification, and cathepsin activity, and significantly inhibited cell viability to HCCT116 at a concentration of 800 μg/mL. However, there was a slight effect on rat normal intestinal epithelial cell line IEC-6, which indicated that TP might serve as an anticancer agent with minimal side effects. Researchers isolated a water-soluble homogeneous polysaccharide (DTP-1) from black tea bricks and tested its anti-tumor activity in vitro. The results showed that DTP-1 can significantly inhibit the viability of A549 and SMMC7721 at a concentration of 600 μg/mL, but has little effect on normal cells (Liu et al., 2022). In a word, tea polysaccharides may have great potential for cancer treatment.
| 4. Molecular mechanisms underlying the anticancer effects of tea common cancers | ▴Top |
4.1. Lung cancer
Lung cancer is a common primary malignant tumor of the lung. Recently, the incidence and mortality of lung cancer have increased significantly. It is one of the most threatening malignancies to human health and life (Han et al., 2023; Xing et al., 2019; Zhang et al., 2019). Living habits and behaviors are cancer occurrence key factors. The results of a meta-analysis showed that maintaining the habit of drinking tea can effectively reduce the risk of lung cancer among non-smokers, and keeping one or more cups of tea a day can reduce the risk of lung cancer in smokers (Guo et al., 2019).
Researchers found that EGCG could inhibit the expression of lung cancer-related gene STAT1 in A549 cells (Li et al., 2023) (Table 2). Oxidized tea polyphenol (OTP-3), an oxidized derivative of EGCG, could bind to the extracellular domain of EGFR and combine with nimotuzumab to significantly inhibit tumor growth by degrading EGFR in vivo. It can also enhance the effect of nimotuzumab in the treatment of NSCLC, providing evidence for new cancer treatment ideas (Huang et al., 2022). The brown polyphenol oxidized polymer TBS(TBS-C) from tea polyphenols could inhibit the activation of PI3K/AKT/mTOR pathway in A549 and H2030 cells, promote autophagy, and also up-regulate the expression of AKT downstream gene p21, leading to cell cycle arrest in G1 phase. TBS-C significantly inhibited tumor growth by inhibiting the PI3K/AKT/mTOR pathway without toxic damage to other organs in xenograft NSCLC nude mice (Wang et al., 2022). In the experiments of a recent study, EGCG at all concentrations could inhibit the proliferation and migration of A549, HCC827, and H1975 cells. The inhibition of proliferation and migration of A549 and HCC827 cells by EGCG was related to the EGFR signaling pathway. However, the inhibitory effects of EGCG on the proliferation and migration of H1975 cells were not related to the EGFR signaling pathway, suggesting that EGCG can treat NSCLC through multiple mechanisms (Minnelli et al., 2021). The synthesized dimeric-(-) -Epigallocatechin-3-Gallate (PBOG) can inhibit EGFR phosphorylation by binding to extracellular domain proteins of epidermal growth factor receptor (EGFR), a marker of NSCLC. Thus, it induces apoptosis, which may be a new idea for the treatment of lung cancer (Sun et al., 2022). There is a study showed that a high concentration of EGCG could inhibit the proliferation and migration of A549 and H1299 cells and induce apoptosis by inhibiting the NF-κB signaling pathway, which showed significant synergistic effects when EGCG and NF-κB inhibitor BAY11-7082 were used together (Zhang et al., 2019).
 Click to view | Table 2. The bioactive components in tea and related anticancer molecular mechanisms |
Golden-flowered tea can effectively inhibit the growth of non-small cell lung cancer (NSCLC) cells, and the inhibitory effect may be related to cytokine-cytokine receptor interaction, PI3K-Akt signaling pathway, and MAPK signaling pathway (Wang et al., 2022). Furthermore, the brownie inhibited the proliferation of p53-deficient NSCLC(H1299) cells and P53 wild-type NSCLC(A549) cells by phosphorylating the MAPK/JNK pathway, down-regulating epithelial-mesenchymal transition-related genes and anti-apoptotic molecules, and up-regulating metastasis-related genes HSPA6 and pro-apoptotic molecules. This is a p53-independent mechanism, indicating that the foci are a safe and effective agent for the treatment of NSCLC (Xiao et al., 2022). It is suggested that there may be many bioactive components in tea that can be used as natural anticancer agents to enhance the efficacy of anticancer drugs.
4.2. Colorectal cancer
Colorectal cancer is one of the most frequent and lethal malignancies in Europe and the United States. Studies have shown that increasing natural foods such as fruits and vegetables can reduce the incidence of colorectal cancer (Chapelle et al., 2020). In recent years, there have been increasing studies on the effects of tea and its bioactive components on the prevention and treatment of colorectal cancer.
EGCG can inhibit the formation of neutrophil extracellular traps (NETs) and inhibit colon cancer cell migration and invasion by regulating the STAT3/CXCL8 signaling pathway (Zhang et al., 2023) (Table 2). EGCG has anti-proliferation and anti-migration effects on colorectal cancer cells SW480, SW620, and LS411N by inhibiting the expression of STAT3, indicating that EGCG may be a natural substance with bioactivity for anti-colon cancer (Luo et al., 2020). EGCG enhances the sensitivity of colorectal cancer cells to the chemotherapy agent 1-FU by inhibiting the GRP5/NF-κB/miR-78-155p/MDR5 pathway, suggesting that EGCG may be used as a natural chemical sensor for further study (La et al., 2019). Researchers treated colorectal cancer (CRC) model mice with theabrownine (TB) and found that TB significantly reduced phosphatidylinositol 3 kinases (PI3K), protein kinase B (Akt) phosphorylation, as well as downstream mechanism targets of rapamycin (mTOR) and cyclin D1 protein expression. This indicates that TB acquisition can inhibit tumor cell proliferation or promote tumor cell apoptosis. TB also increased the abundance of short-chain fatty acids and short-chain fatty acid-producing related bacteria and decreased the Bacteroides associated with CRC, suggesting that TB can inhibit tumor formation (Leung et al., 2022). In addition, TB may induce the apoptosis of human colon cancer cells HT-29 by inducing intracellular redox imbalance (Yuan et al., 2022). In the study mentioned earlier, tea polysaccharides were also shown to significantly promote the death of colon cancer cell CT26 (Zhou et al., 2021). Theanine alone inhibited tumorigenia in a rat model of dimethylhydrazine (DMH) induced colon cancer by down-regulating the Akt/mTOR and JAK2/STAT3 pathways and increasing Smad2 tumor suppressor (Shojaei-Zarghani et al., 2021). Several researchers have extracted theaflavins and thearubins from black tea and demonstrated that both induce apoptosis in a dose-dependent manner (Imran et al., 2019).
Huang qin tea (HQT) can regulate the expression of inflammatory cytokines, increase the production of IFN-γ, and inhibit inflammation by inhibiting the expression of IL-1β, IL-6, IL-10, and TNF-α. HQT can also regulate intestinal microbial composition and metabolic pathways, inhibit the formation of aberrant crypt foci (ACF) in precancerous colon ACF rat models, and achieve the effect of inhibiting the formation of colorectal cancer (Shen et al., 2020). Natural nanotherapeutics (NTS) from tea leaves inhibited inflammation by increasing the secretion of the anti-inflammatory factor IL-10, inhibiting the expression of pro-inflammatory factors, and significantly reducing the incidence and growth of colon tumors by oral administration (Zu et al., 2021). Chinese Hakka stir-fried green tea (HSGT) extracts from five different years showed that all concentrations and years of Chinese HSGT could inhibit the proliferation of HT-15 and enhance the apoptosis of HT-1 cells. They demonstrated that Chinese-aged tea could inhibit the proliferation and cell cycle progression of colon cancer cells and promote the apoptosis of colon cancer cells by blocking the transmission of PI3K/AKT signal (Zhang et al., 2022). Besides, cocoa tea (Camellia ptilophylla) can inhibit the phosphatidylinositol -3 kinase (PI3K)/Akt pathway and activate the mitochondrial apoptosis pathway by promoting ROS production in human colon cancer HCT116 cells, and the proliferation of HCT116 cells was inhibited and the apoptosis of HCT116 cells was promoted (Gao et al., 2020).
4.3. Prostate cancer (PCa)
Prostate cancer (PCa) is a disease with extremely complex pathogenesis, including environmental, physiological, and genetic. Relevant data show that PCa is one of the highest prevalences of cancer in the male. Recent studies show bioactive components in tea can prevent or treat prostate cancer (Wasim et al., 2022; Filippini et al., 2020).
Polyphenon E has a dose-dependent inhibitory effect on PC-3 cells (Carastro et al., 2022). Green tea may exert its anti-prostate cancer activity by up-regulating miRNA-181a expression and inducing cell apoptosis (Safari et al., 2022) (Table 2). Epicatechin gallate can down-regulate the expression of acetyl-CoA carboxylase, ATP citrate lyase, and fatty acid synthase in prostate cancer cells and prostate xenograft tissues. Epicatechin gallate could reduce fatty acid synthesis. Further research showed that epicatechin gallate could inhibit the expression of adipogenic genes by inhibiting the PI3K/AKT/mTOR signaling pathway to reduce fatty acid synthesis and attenuate prostate cancer cell metastasis (Chen et al., 2021). (-)-epicatechin can mediate proapoptotic effects on prostate cancer cells PC-3 and MDA-MB-468 breast cancer cells through ZIP9. When the (-)-epicatechin concentration was 100-200 nM, it showed strong agonist activity against cancer cells (Thomas and Dong, 2021). EGCG induced an increase in Ca2+ concentration in PCa cells in a dose-dependent manner, which may be related to sulfhydryl (SH) oxidation. The imbalance of intracellular calcium ion concentration was directly related to the apoptosis of PCa cells, indicating that EGCG could induce the apoptosis of PCa cells (Marchetti et al., 2020). LEGCG, a fat-soluble derivative of EGCG, using the enzymatic esterification reaction of lauric acid and EGCG, could stop the cell cycle in the G0/G1 phase by activating p53/p21. Therefore, the expression of cyclin D1 and CDK4 in DU145 human prostate carcinoma cells is down-regulated, and cell proliferation is inhibited. At the same time, LEGCG also induced apoptosis by increasing the Bax/Bcl2 ratio, cytochrome release, and caspase cleavage in DU145 cells, showing the alleviative effect on PCa from two aspects including inhibiting the proliferation of PCa cells and promoting the apoptosis of PCa cells (Chen et al., 2019).
Theaflavin-3,3′-gallate (TF-3), an important bioactive ingredient in black tea, can reduce the expression of 67 kDa laminin receptor (67LR) in prostate cancer PC-3 cells and prostate cancer mouse models. The receptors have excessive expression in a variety of tumors and play an important role in tumor growth and metastasis. They also found that TF-3 inhibits the proliferation of prostate cancer cells by regulating the PKCδ/aSMase signaling pathway. Moreover, the tumor volume significantly reduced after TF-3 treatment in vivo, indicating that TF-3 may have alleviative effects on human prostate cancer (Sun et al., 2022). Metabolic disorders caused by overexpression of lipogenic genes are one of the typical features of prostate cancer. Catechin nanoemulsion from oolong tea waste can increase the activity of Caspase-8, Caspase-9, and Caspase-3 in mouse prostate cancer cells DU-145, and block the cell cycle in the S phase and G2/M phase. Moreover, when the concentration of catechin nanoemulsion was 20 μg/mL, the effect on the volume and weight of mouse tumors was the most obvious (Lin et al., 2021). As mentioned earlier, L-theanine can inhibit the invasion, and migration and increase intercellular adhesion of prostate cancer cells both in vivo and in vitro. It indicates that L-theanine has an ideal effect on the treatment of prostate cancer (Ma et al., 2022). Green tea extract to treat N-methyl-N-nitrosourea-induced prostate cancer rats and the results showed that compared with the prostate cancer model group, the prostate weight of rats in the green tea extract group (CEx) was significantly reduced. Moreover, the expressions of NF-κB and p53 in the CEx group were significantly decreased compared with those in the model group, which once again indicated that tea and its bioactive components may have potential preventive and alleviative effects on prostate cancer (Saedmocheshi et al., 2019).
4.4. Breast cancer
Breast cancer is a cancer with high morbidity and mortality in women all over the world. It has been widely discussed by scholars for many years. Studies have shown that the risk of breast cancer is related to lifestyle, including diet and physical exercise. While some natural ingredients in foods may have preventive and alleviative effects on breast cancer (Marín et al., 2023).
EC could reduce tumor volume and improve survival in a triple-negative breast cancer (TNBC) tumor model mouse. In addition, they demonstrated that flavanol EC significantly inhibited the proliferation of 4T1 cells in a dose-dependent manner but had no significant effect on the survival rate of C2C12 cells. The migration of 4T1 cells was also significantly inhibited. The results showed that EC inhibited the proliferation, metastasis, and invasion of mouse breast cancer cells, suggesting that EC may be a new agent for the treatment of breast cancer without side effects (Pérez-Durán et al., 2023) (Table 2). EC31, a derivative of tea polyphenols, could reverse P-gp-mediated taxol resistance in a P-glycoprotein (P-gp) overexpressing LCC6MDR cell line breast cancer xenograft model. It also increased intratumoral paclitaxel levels in LCC6MDR xenografts 6-fold without affecting the pharmacokinetic activity of paclitaxel, suggesting that the tea polyphenol derivative EC31 may be an effective combined anticancer agent (Sun et al., 2023). In the previous study, (-)-epicatechin in green tea could promote apoptosis in MDA-MB-468 breast cancer cells and exhibit receptor agonist activity (Marchetti et al., 2020). Proline dehydrogenase (PRODH) treating TNBC cells found that PRODH could induce cancer cell epithelial-mesenchymal transition and promote cell proliferation. EGCG could significantly inhibit the expression of PRODH and its regulatory proteins in TNBC cells and 50 mg/EGCG could significantly inhibit tumor growth of patient-derived xenograft (PDX) mice. This study again provides evidence for the anti-tumor effect of EGCG, especially in TNBC (Lee et al., 2021). EGCG could significantly reduce the cell survival rate and increase the apoptosis rate of myeloid-derived suppressor cells (MDSCs) through typical signaling pathways such as Arg-1/iNOS/NOX2/NF-κB/STAT3 and atypical signaling pathways such as ECM-receptor interaction and focal adhesion. EGCG significantly reduced the accumulation of MDSCs and increased the proportion of CD4 and CD8 T cells in the spleen and tumor site of 4T1 breast tumor mice to improve immunosuppression and inhibit the growth of 4T1 breast cancer cells. A concentration of 0.5–5 μg/mL of EGCG was needed to have a significant effect (Xu et al., 2020). EGCG significantly reduced the methylation status of the tumor suppressor gene SCUBE2 by reducing the expression and activity of DNA methyltransferase (DNMT), enhancing its inhibitory effect on breast cancer development, again suggesting the significance of EGCG in the treatment of breast cancer (Sheng et al., 2019).
The extracted and purified exosome-like NTs (TLNTs) from tea leaves, in vitro experiments showed that TLNTs could increase the ROS level in 4T1 cells, which in turn caused mitochondrial damage, cell cycle arrest, and apoptosis (Chen et al., 2023). Both oral and intravenous TLNTs were found to be effective in the treatment of breast cancer via direct pro-apoptosis or microbiota modulation in mice. Oral administration of TLNTS did not cause significant liver and kidney toxicity or immune activation. It is suggested that it may be used as an oral drug for the treatment of breast cancer with fewer side effects. A study on the inhibitory effect of natural nanovehicles from tea flowers (TFEN) on breast tumors showed that TFEN could trigger apoptosis of breast cancer cells and inhibit tumor growth and metastasis in vivo (Chen et al., 2022). The isolated Gypensapogenin I (Gyp I) from Gypenia pentaphylla tea showed that Gyp I could inhibit the proliferation of human breast cancer cell line MDA-MB231 through the AKT/GSK3β/β-catenin signaling pathway. Their study showed that Gyp I could regulate the closure of Notch-1 and AKT/GSK3β/β-catenin signaling pathways in TNBC and inhibit the growth of xenograft tumors in nude mice in a dose-dependent manner (Tan et al., 2022). Green tea extract (GTE) was selectively toxic to breast tumor cells MCF-7, but not to non-tumor cells MCF-10A. GTE exerted its detrimental effect on MCF-7 cells by stimulating the expression of p53 and p21 in MCF-7 cells, and also significantly inhibited the cell migration of MCF-7 and MDA-MD-231 cells, suggesting that green tea may have an alleviative effect on breast cancer (Santos et al., 2021). PTP1B, a tyrosine phosphatase regulated by oxidative stress, is involved in the pathogenesis of a.o. Proto-oncogenic pathways for breast cancer formation. Epigallocatechin, epigallocatechin gallate, epicatechin, and epicatechin gallate, derived from green tea, reduced PTP1B phosphatase activity and MCF-7 cell survival, once again proved that tea has a potential antioxidant effect (Kuban-Jankowska et al., 2020).
| 5. Limitations and expectations | ▴Top |
Although most studies have shown that tea has preventive and mitigating effects on a variety of cancers at both in vivo and in vitro levels as well as at the cellular level, some epidemiological studies have also shown that there is no significant association between tea consumption and cancer risk. Some studies have even shown that tea consumption can increase the risk of gastric cancer among regular smokers and drinkers (Sen et al., 2019; Li et al., 2019). Therefore, more definitive evidence is still needed to prove the role of tea in the prevention and alleviation of cancer
In general, the anti-cancer effect of tea is mainly achieved by inhibiting the production of carcinogens, inhibiting tumor cell proliferation and metastasis, tumor growth, and enhancing the body’s cellular immunity level.
Studies have shown that certain components of tea can be used as combined anticancer agents. EGCG in combination with cisplatin significantly inhibited cell proliferation and increased apoptosis in NSCLC cell lines and xenograft tumors in vivo in a synergistic manner (Wang et al., 2019). Theaflavin-rich black tea (BT) could improve the sensitivity of A549 cells to the anticancer drug cisplatin by regulating the level of nuclear factor erythroid 2-related factor 2 (NRF2) in A549 cells. It also reduced the resistance of A549 cells to anticancer drugs (Datta et al., 2023). In addition, we mentioned above that caffeine has the potential as a combined anticancer agent due to its activity (Tran et al., 2023), and oral administration of cystine and theanine exhibits relieved therapy of the side effects of capecitabine-based adjuvant chemotherapy for colorectal cancer patients (Hamaguchi et al., 2020). All of this evidence suggests that tea may be able to combine with anticancer drugs to enhance their anticancer activity or reduce their side effects, but more evidence is still needed to prove whether this is feasible.
| 6. Conclusions | ▴Top |
Current studies have shown that tea, especially green tea has shown some preventive and alleviative effects against common cancers such as breast, endometrial, colon, prostate, and lung cancer. Tea has many other benefits for cancer patients, such as aiding digestion, increasing appetite, and strengthening physique. However, doctors warn that although tea has a certain anti-cancer effect, the effect is limited, and it cannot completely replace surgery and chemotherapy drugs. Therefore, cancer patients should still go to the hospital for routine treatment, supplemented by tea, so that cancer treatments complement each other.
Acknowledgments
The graphical abstract and Figure 1 were drawn using the Figuredraw platform. We thank Figuredraw for providing a convenient platform for scientific research illustration.
| References | ▴Top |