| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 27, September 2024, pages 15-32
Lignans in Patrinia with various biological activities and extensive application value: A review
Xinjing Menga, b, Jiaojiao Qua, b, Xu Yanga, c, Shishi Zhanga, c, Yifei Zhanga, c, Beibei Yua, c, Zhenhua Liua, b, c, *, Gaixia Houa, d, *, Wenyi Kanga, b, c, *
aNational R & D Center for Edible Fungus Processing Technology, Henan University, Kaifeng 475004, China
bFunctional Food Engineering Technology Research Center, Henan, Kaifeng 475004, China
cJoint International Research Laboratory of Food & Medicine Resource Function, Kaifeng 475004, China
dCollege of Physical Education, Henan University, Kaifeng 475004, China
*Corresponding author: Zhenhua Liu, Gaixia Hou and Wenyi Kang, National R & D Center for Edible Fungus Processing Technology, Henan University, Kaifeng 475004, China. E-mail: liuzhenhua623@163.com(ZL), hgx715@163.com(GH), kangweny@hotmail.com(WK)
DOI: 10.26599/JFB.2024.95027384
Received: September 2, 2024
Revised received & accepted: September 29, 2024
| Abstract | ▴Top |
Lignans in Patrinia have attracted the attention of researchers due to their diverse structure and remarkable activity. We searched the PubMed database for articles published from 2003 to 2023 using appropriate search terms: Patrinia, Lignans, Biological activity, and Chemical structures. In this paper, the active lignans and their action mechanisms were summarized over the past 20 years. The results showed that 56 lignans have been isolated and identified from Patrinia, including furofurans, dibenzyltyrolactones, tetrahydrofurans, arylnaphthalenes, benzofurans and biphenyl derivatives. 45 lignans had anti-oxidant, anti-inflammatory, anti-tumor, cytotoxicity, enzyme inhibitor, anti-Alzheimer’s disease, neuroprotection, anti-bacterial, hepatoprotection and anti-diabetic activities. The anti-inflammatory mechanism involves AMPK, MAPK, NF-κB and JAK-STAT signaling pathways, and the antitumor mechanism involves Raf/MEK/ERK, Akt/JNK and AKT signaling pathways. Lignans in Patrinia are promising to be utilized in food and medicine.
Keywords: Lignans; Patrinia; Anti-inflammatory mechanism; Anti-tumor mechanism
| 1. Introduction | ▴Top |
There are about 20 species of Patrinia genus, mainly found in eastern to central Asia and northwestern North America. There are 10 species, 3 subspecies and 2 varieties in China, which are widely distributed throughout the country (The Editorial Committee of Chinese Flora, 1986).
Humans have been dependent on nature since ancient times for the fulfillment of their basic requirements such as shelters, foodstuffs and especially medicines (Adnan et al., 2020). It has a long history for the plant of Patrinia genus used as herb medicine in China, P. scabiosaefolia and P. villosa were first recorded in Shennong Materia Medica that have a pungent, bitter and slightly cold taste, and have the effects of clearing heat and detoxifying, eliminating carbuncle and discharging pus, removing blood stasis and relieving pain (Wang and Li, 2004).
In addition to its medicinal value, the young leaves of Patrinia can be eaten as wild vegetables, or picked and dried before flowering, such as P. scabiosaefolia, P. villosa, P. punctiflora and P. heterophylla, which have high nutritional value and are rich in a variety of vitamins, amino acids and minerals necessary for human body (Xiao et al., 2007).
In recent years, a series of lignans in Patrinia with diverse structures and significant activities have been found (Bai et al., 2018; Bai et al., 2017; Di et al., 2013; Gu et al., 2002; Huang et al., 2021; Jiang et al., 2017; Lee et al., 2018; Lee et al., 2020; Lee et al., 2016; Li et al., 2005; Li et al., 2003; Liu et al., 2015; Xiang et al., 2017; Yan et al., 2016; Zhang et al., 2020). Active lignans discovered in Patrinia genus could be used as dietary supplements or functional food ingredients for health promotion and disease risk reduction (Gülsüm and Zeliha, 2019; Zeliha, 2018). In order to better expand that application value of lignans in Patrinia, it is necessary to summarize their structures, activities and mechanisms of active lignans discovered in Patrinia genus in the past 30 years. It is hoped that more and more attention to focus on the application value of lignans in Patrinia, promoting the utilization of lignans in food, medicine and other industries.
| 2. Phytochemistry | ▴Top |
Lignins are natural compounds derived from the polymerization of two phenylpropyl derivatives (C6-C3) (Zalesak et al., 2019). According to different polymerization methods, lignans can be divided into ordinary lignans involving dibenzylbutanes, dibenzyltyrolactones, arylnaphthalenes, tetrahydrofurans, furofurans and dibenzocyclooctenes, neolignans involving benzofuran, bicyclooctane, futoenone, biphenyl and norlignans (Zalesak et al., 2019). 56 lignans were isolated and identified (Figure 1), including furofurans (1–10), dibenzyltyrolactones (11–20), tetrahydrofurans (21–28), arylnaphthalenes (29–34), benzofurans (35–42), biphenyl derivatives (43,44), and others (45–56). In addition, most of the substituents of lignans isolated from Patrinia were hydroxyl and methoxyl, and most of the linked glycosides were β-D-glucose.
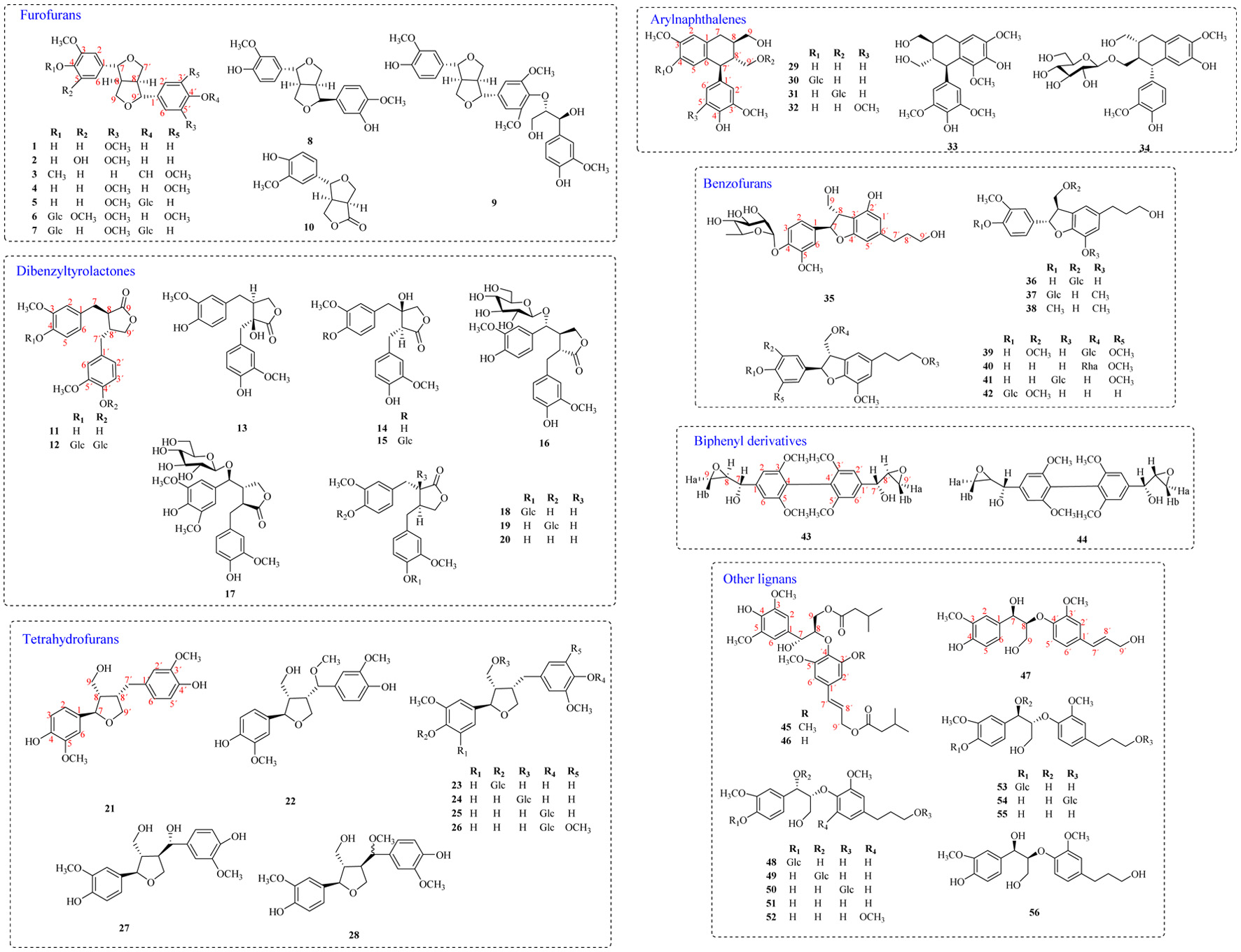 Click for large image | Figure 1. Structure of lignans in Patrinia. |
Lignans reviewed in this paper have various biological activity (Table 1) including 18 kinds of activities such as antioxidant (32), anti-inflammatory (10), cancer cell toxicity (7), anti-tumor (4), enzyme inhibitor (7), anti-Alzheimer’s disease (6), neuroprotection (5), antibacterial (4), hepatoprotection (3) and hypoglycemic (3) etc. The antioxidant activity is the main biological activity, followed by anti-inflammatory activity (Figure 2a). In addition, the relationship network between lignans and biological activities is established, which reflects the number of activities for each compound. Compound 1 showed eight biological activities, including cytotoxicity, anti-oxidation, hepatoprotection, enzyme inhibitor, anti-osteoporosis, anti-malaria, anti-inflammation, anti-Alzheimer’s disease, anti-tumor and anti-diabetes. Compound 11 also showed eight biological activities, including cytotoxicity, anti-inflammatory, anti-diabetes, hepatoprotection, anti-tumor, anti-inflammatory, antioxidant and neuroprotection. Compound 3 showed six kinds of activities, including cytotoxicity, lipid-lowering, anti-tumor, anti-bacterial, anti-inflammatory and enzyme inhibition (Figure 2b).
 Click to view | Table 1. Lignans and lignans with bioactivity in Patrinia |
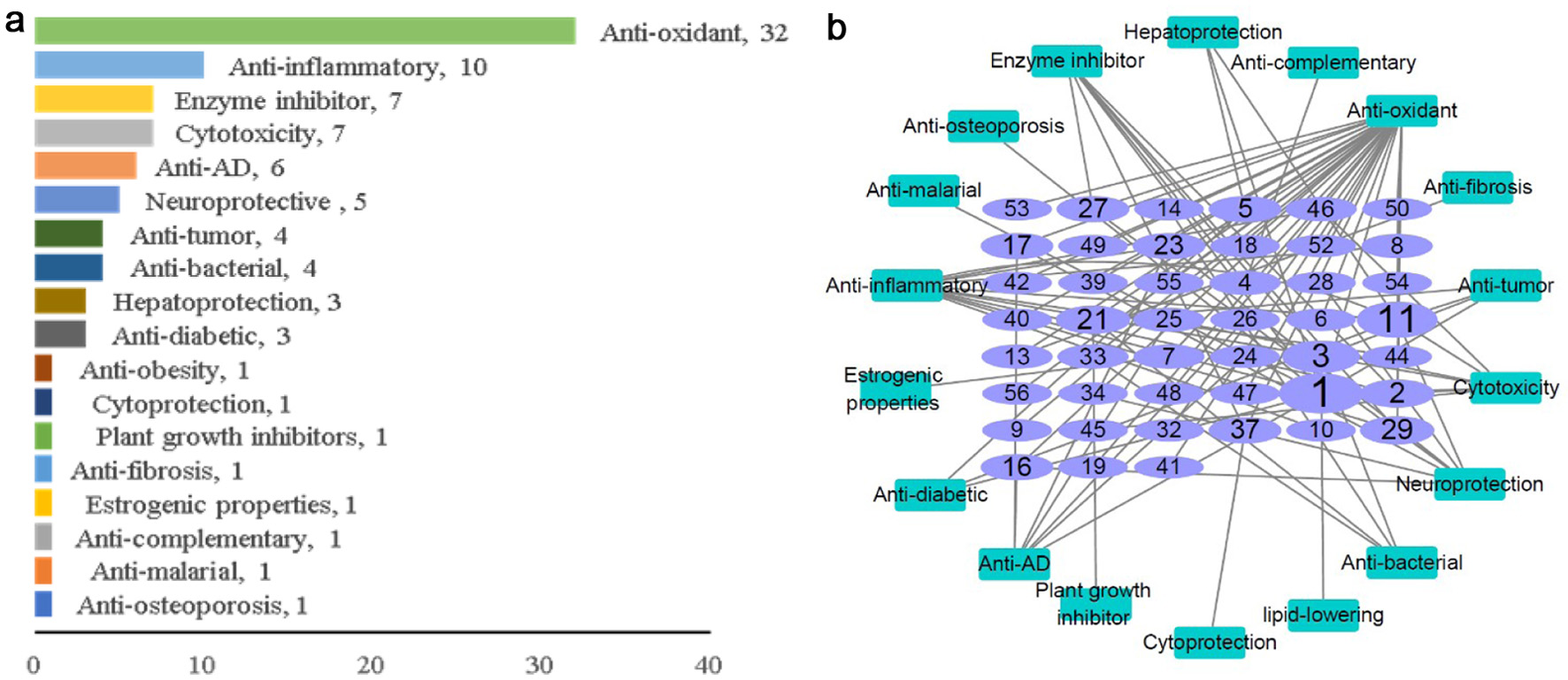 Click for large image | Figure 2. (a) The bioactivity of lignins. (b) The relationship network between lignins and biological activity. (The size of the ellipse represents the amount of biological activity of the compound, the green square box represents the biological activity, and each side line represents the biological activity of the compound). |
| 3. Active lignans | ▴Top |
3.1. Antioxidant activity
Antioxidants have been shown to reduce the risk of chronic diseases including cancer, liver injury (Selamoglu et al., 2015) and cardiovascular system by some scientific studies (Selamoglu et al., 2017). 32 lignans in Patrinia showed antioxidant activity with IC50 values ranging from 0.3 to 61.9 μM (Table 2). DPPH radical scavenging assay, ABTS cationic radical scavenging assay, metal ion reducing antioxidant capacity assay (CUPRAC, FRAP), thiobarbiturate reactive substance (TBARS) assay and modified irradiated riboflavin/ethylenediaminetetraacetic acid (EDTA)/Nitroblue tetrazolium (NBT) system were used to evaluate their antioxidant activities in vitro.
 Click to view | Table 2. Lignans with antioxidant activity |
By comparing the structure and activity of lignans, it was found that active lignans contain a large number of phenolic hydroxyl groups, which may be related to its antioxidant capacity (Youssef et al., 2020). In general, the presence of phenolic hydroxyl groups increases the antioxidant capacity of a molecule, especially when they are located in positions where they can form intramolecular or intermolecular hydrogen bonds. Compound 7 had no hydroxyl group in its structure, and its IC50 was 61.9 μM, showing the weakest antioxidant activity. With the increase of hydroxyl group, the antioxidant activity increased, and compound 29 had four hydroxyl groups at the 4, 4′, 9 and 9′ positions. The IC50 value of compound 29 was 0.3 μM, indicating the strongest antioxidant activity. In addition, the action mechanism Showed that compound 11 played an antioxidant role by inhibiting the expression of antioxidant proteins Nrf2 and HO-1 (Wu et al., 2021), and compound 21 played an antioxidant role by activating p38 to up-regulate the NRF2-mediated HO-1 expression (Bajpai, Alam, et al., 2017).
3.2. Anti-inflammatory activity
Antibiotics are commonly used to treat inflammation, however, their clinical utility is limited by toxic side effects and drug resistance (Fan et al., 2023). The mechanism of natural products is complex, which can reduce drug resistance and side effects (Zou et al., 2023). 10 lignans in Patrinia showed anti-inflammatory activity (Table 3). Its anti-inflammatory activity was evaluated by measuring the release of NO and the production of pro-inflammatory factors (IL-6, IL-8, and NF-κB) in lipopolysaccharide (LPS)-induced mouse mononuclear macrophage leukemia cells (RAW264.7), the superoxide anion production of human neutrophils induced by N-formyl-methioyl-leucine-phenylalanine/cell relaxant B (fMLP/CB) and the release of NO from MH7A cells induced by (tumor necrosis factor-α) TNF-α.
 Click to view | Table 3. Lignans with anti-inflammatory activity |
Structure-activity relationship analysis showed that lignans containing furan rings had the best anti-inflammatory activity, and the anti-inflammatory activity of lignan aglycones was better than that of lignan glycosides (Wang et al., 2011). Among them, compounds 1, 2, 3, 8 and 10 contain two furan rings as furofurans, indicating that lignans containing furan ring structure have more prominent anti-inflammatory activity. Anti-inflammatory effects are mainly achieved by regulating AMPK, MAPK, NF-κB and JAK-STAT signaling pathways (Figure 3). Compound 1 significantly reduced the phosphorylated protein levels of protein kinase B (Akt) and c-Jun N-terminal kinase (JNK) (Yang et al., 2021). Compound 2 decreased the expression of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells (Chang et al., 2019). Compound 11 attenuated the phosphorylation of MAPK, JNK and NF-κB in CLP rats and LPS-stimulated microglia in a concentration dependent manner, and upregulates Nrf2 and HO-1 (Al-Sayed et al., 2020). Compound 23 inhibitd the NF-κB pathway, thereby reducing the expression of influenza virus-induced pro-inflammatory cytokines IL-6, TNF-α, IL-8, MCP1, IP-10, and IFN-α (Li et al., 2015). The anti-inflammatory activity of compound 46 was regulated by the phosphorylation of the Janus kinase (JAK)/ signal transductor and the transcription 1/3 activator (STAT1/3) signaling pathway (Gülsüm and Zeliha, 2019).
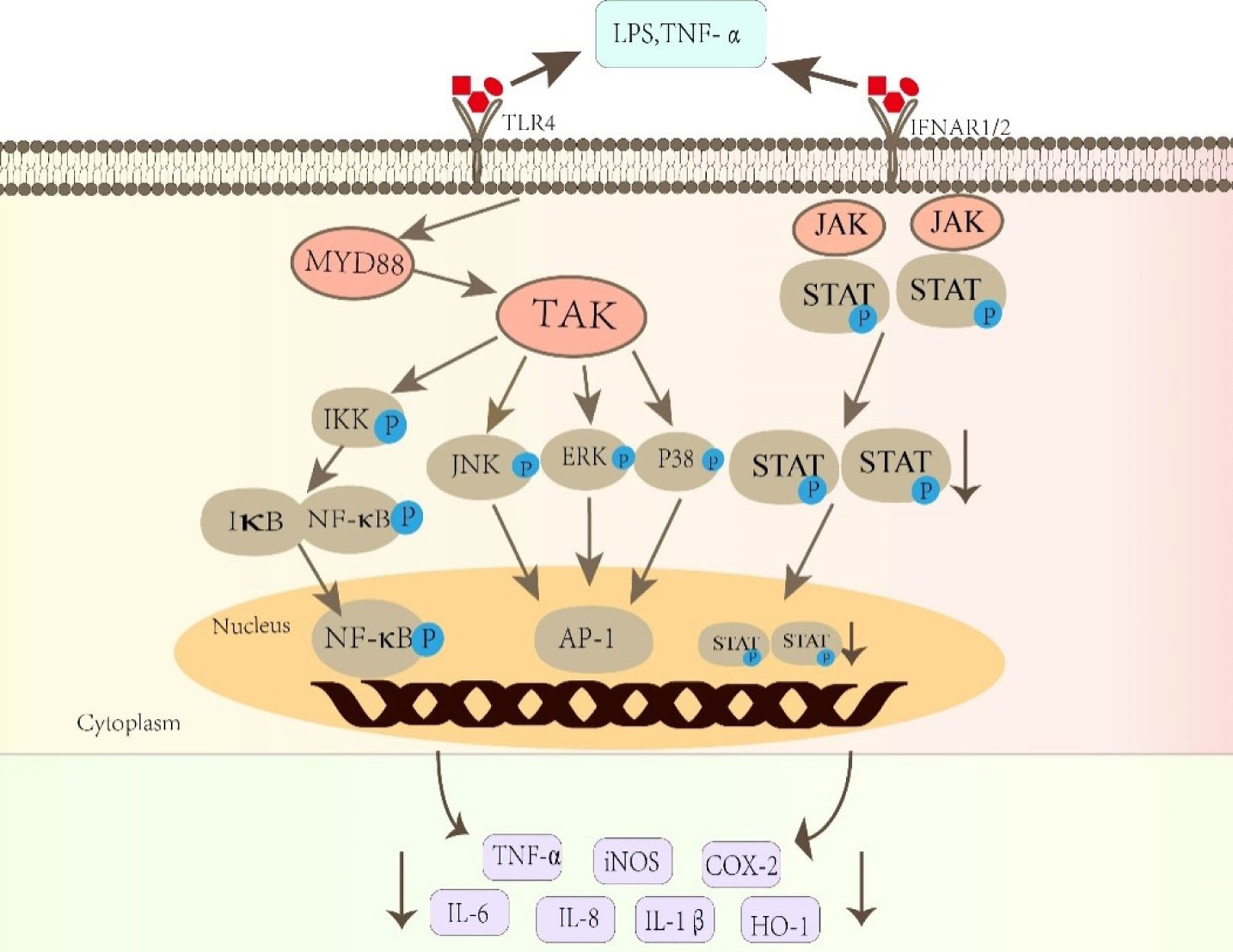 Click for large image | Figure 3. Anti-inflammatory pathways of active ingredients in Patrinia. IL-6 (Interleukin-6); IL-8 (Interleukin-8); TNF-α (Tumor Necrosis Factor-α); IL-1β (Interleukin-1beta); NF-κB (nuclear factor kappa-B); COX-2 (Cyclooxygenase-2); iNOS (Inducible nitric oxide sythase); HO-1 (Heme oxygenase 1); ERK (Extracellular regulated protein kinases); JNK (c-Jun N-terminal kinase); STAT Signal transducer and activator of transcription);MyD88 (Myeloid differentiation primary response protein 88); TAK (Transforming growth factor β-activated kinase); IκB (Inhibitor of NF-κB); IKK (Inhibitor of kappa B kinase); JAK (Janus kinase). |
3.3. Anti-tumor and cytotoxic lignans
3.3.1. Anti-tumor activity
Cancer is a disease seriously endangering people’s life and health worldwide, whose mortality rate is the second in all diseases (Zeng et al., 2022). There are four lignans in Patrinia showed antitumor activity. compound 1 had anti-ovarian (Ning et al., 2019) and cervical tumor (Zhou et al., 2022), Compound 3 had anti-nasopharyngeal (Yu et al., 2019) and lung tumor (Jiang et al., 2017), compound 11 inhibited the proliferation of pancreatic tumor cell lines (Lee et al., 2022), significantly reduced the activity of breast tumor and prostate tumor cell lines (Mahajan et al., 2021), and compound 21 had anti-human liver tumor activity (Lee et al., 2016).
Anticancer activity is mainly mediated by the regulation of Raf/MEK/ERK, Akt/JNK and AKT signaling pathways (Figure 4). Compound 1 inhibited phosphoric acid (p)-MEK and (p)-ERK expression in a concentration-dependent manner (Ning et al., 2019). Compound 3 significantly reduced EZH2 expression by inhibiting Akt signaling (Yu et al., 2019). Compound 3 induces apoptosis through the Akt/ JNK signaling pathway (Jiang et al., 2017). Compound 11 inhibited the expression of invasion genes through the MAPK and AKT signaling pathways and weakens the migration ability of cancer cells (Yang and Wang, 2022). Compound 21 induced apoptosis by inhibiting cell proliferation, possibly by activating the mitochondria-mediated apoptosis pathway (Ma et al., 2018).
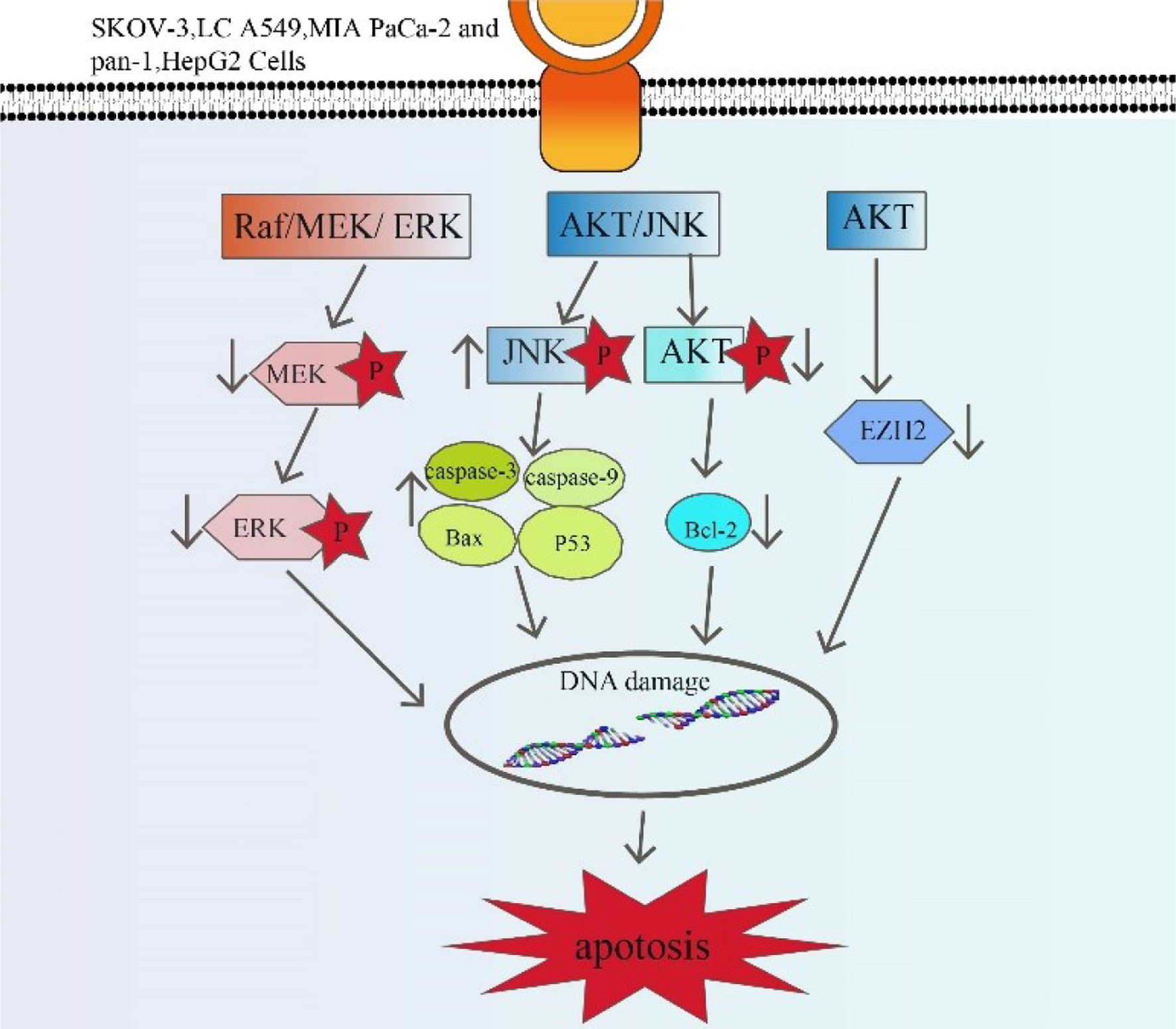 Click for large image | Figure 4. Anticancer pathways of active ingredients in Patrinia. MEK (Mitogen-activated extracellular signal-regulated kinase); BAX (BCL2-Associated X); BCL2 (B-cell lymphoma-2); EZH2 (Enhancer of zeste homolog). |
3.3.2. Cytotoxic lignans
Seven lignans isolated and identified from Patrinia were cytotoxic to 28 different cancer cell lines, with IC50 values ranging from 1.8 to greater than 100 μM (Table 4). Compound 1 showed cytotoxicity against human breast cancer cell line (MCF-7) (Deveci et al., 2019), compound 2 showed cytotoxicity against human non-small cell lung cancer cell lines (A549) (Ma et al., 2020), compound 3 showed cytotoxicity against human colon cancer cell line (HCT-116) (Zhang et al., 2020). Compound 11 showed cytotoxicity against human hepatocellular carcinoma cell line (HepG2) (Al-Sayed et al., 2020), and compounds 1, 2, 11, 25 showed cytotoxicity against human skin melanoma cells (SK-MEL-2) (Lee et al., 2016). Compound 45, 46 showed cytotoxicity against human cervical cancer cells (HeLa) and human gastric cancer cells (MNK-45) (Di et al., 2013) (Table 4).
 Click to view | Table 4. Lignans with cytotoxic activity |
3.4. Other active lignans
In addition to the above activities, the lignans in Patrinia have enzyme inhibition, anti-AD, neuroprotection, antibacterial, Hepatoprotection, hypoglycemic, anti-osteoporosis, anti-malaria, lipid-lowering, anti-complementary, estrogen, anti-fibrosis, plant growth inhibition, and cytoprotection.
3.4.1. Lignans of enzyme inhibition
Seven lignans from Patrinia show enzyme inhibitory activity. Compound 1 has anticholinesterase activity (Deveci et al., 2019), compound 1, 2, 4 has the inhibitory activity of lipoxygenase (LOX) (Salleh et al., 2019), compound 3 inhibits the activities of glucuronic transferase 1A1 and 1A3 in human liver microsomes (Park et al., 2021), compound 4 has the inhibitory activity of α-amylase (Timalsina et al., 2021). Compound 27 inhibits glycogen synthase kinase-3β (Ohtsuki et al., 2012), compound 29 inhibits dipeptide peptidase 4 (Lunder et al., 2019), and compound 37 inhibits tyrosinase (Hong et al., 2014). (Table 5)
 Click to view | Table 5. Lignans with other activity |
3.4.2. Anti-AD lignans
The deposition of amyloid-beta (Ab) peptide in neuronal cells is a defining feature of the diagnosis of Alzheimer’s disease (Lee et al., 2022). Compound 1 could improve memory impairment in cholinergic block-induced dementia models (Yu et al., 2019), and compounds 16, 17, 23, 24 and 26 could inhibit deposition of amyloid-beta (Ab) peptide in neuronal cells (Liu et al., 2015). (Table 5)
3.4.3. Neuroprotective lignans
Six lignans from Patrinia showed neuroprotective activity. Compound 11 had a protective effect against oxaliplatin (LOHP)-induced neurotoxicity (Yi et al., 2019), compound 16, 23, 27, 28 had neuroprotective activity (Lee et al., 2018), and compound 29 had neuroprotective activity against L-glutamate-induced HT22 cell damage (Cheng et al., 2020). (Table 5)
3.4.4. Anti-fungal lignans
Four lignans from Patrinia showed antibacterial activity. Compound 3 had an inhibitory effect on the growth of Helicobacter pylori (Yang et al., 2018). Compound 14 was resistant to Candida albicans (Li et al., 2003) and had antibacterial effects on Escherichia coli by destroying and disturbing the cytoplasmic membrane (Heejeong Lee et al., 2016). Compound 21 had antibacterial activity against Staphylococcus aureus and E. coli (Hwang et al., 2011)and could damage the fungal plasma membrane against Candida albicans, Trichosporon beigelii and Malassezia furfur (Bajpai, Shukla, et al., 2017). Compound 33 had anti-trichomoniasis vaginalis activity (Moo-Puc et al., 2014).
In addition, Compounds 1 and 5 had liver protective activities (Kim et al., 2019; Youssef et al., 2020)to relieve liver fibrosis (Badr et al., 2019). Compounds 5 and 11 had hypoglycemic activities (Youssef et al., 2020; Yang and Wang, 2022), compound 1 also had anti-osteoporosis (Jiang et al., 2019) and antimalarial activities (Hashim et al., 2021). Compound 3 blocked adipogenesis by inhibiting S6K1 signaling pathway (Nam et al., 2018). Compound 4 had Anti-complementary activity (Hou et al., 2017), compound 7 could activate the transcription of estrogen response reporter gene and induce the expression of estrogen response gene (pS2) mRNA (Wang et al., 2011). Compound 13 could improve bleomycin-induced fibrosis (Pemmari et al., 2018). Compound 21 had the activity of inhibiting plant growth (Nakano et al., 2002), and compound 37 had a protective effect on HepG2 cell damage induced by acetaminophen (Wang et al., 2017) (Table 5).
| 4. Conclusion and prospects | ▴Top |
Lignans are widely distributed in natural plants (flaxseed, sesame, Schisandra chinensis, Magnolia officinalis and forsythias), and their edible and medicinal values are also being continuously developed. Literature studies have shown that lignans play important roles in imparting the biological activities to plants of Patrinia (Bai et al., 2018; Liu et al., 2023a). It is evident from the discussed literature that lignans in Patrinia have abundant biological activities, mainly showing antioxidant, anti-inflammatory, anti-tumor, anti-Alzheimer’s disease and neuroprotective activities, etc. The anti-inflammatory and anti-tumor mechanisms showed the characteristics of multi-pathway and multi-target. Among them, antioxidant is the main biological activity of the lignans. In addition, the research on the chemical constituents of the Patrinia was mainly focused on P. s cabiosifolia, P. villosa, and P. scabra. It is hoped that researchers should use new science and technology to quickly explore the active ingredients and action mechanism of Patrinia plants (Liu et al., 2023), which will be conducive to better exploitation and utilization of Patrinia resources.
Acknowledgments
This work was funded by National Key R&D Program of China (2022YFF1100300), National Natural Science Foundation of China (31900292), Science and Technology Development Program of Henan Province (212102110469, 222102520035), and Research on Precision Nutrition and Health Food, Department of Science and Technology of Henan Province (CXJD2021006).
| References | ▴Top |