| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 26, June 2024, pages 72-79
Analysis of components and antioxidant activity of Angelica sinensis essential oil (AEO) extracted from supercritical carbon dioxide
Beibei Penga, Zihao Zhoua, Rongyuan Wanga, Chunmeng Fua, Wen Wanga, Yuan Lia, Xiaoyu Qina, Lingling Zhangb, *, Hui Zhaoa, *
aTianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China
bShandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University, Jinan 250014, China
*Corresponding author: Lingling Zhang, Shandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University, Jinan 250014, China., E-mail: zhanglingling0539@163.com; Hui Zhao, Tianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China. E-mail: zhaohui@tjcu.edu.cn
DOI: 10.31665/JFB.2024.18382
Received: June 6, 2024
Revised received & accepted: June 30, 2024
| Abstract | ▴Top |
Angelica sinensis is well documented as a traditional medicine for a broad spectrum of disease. Recently, supercritical carbon dioxide extraction has been developed as an emerging green technology for processing of Angelica sinensis essential oil (AEO). To investigate the volatile components and antioxidant activity of AEO both in vitro and in vivo, we conducted an analysis of its chemical composition using gas chromatography-mass spectrometry (GC-MS). The results showed that AEO was mainly composed of phthalides, such as (Z)-Ligustilide (12.85%), 2,4-di-tert-butylphenol (11.6%), and 3-Butylidenephthalide (3.16%). In vitro antioxidant experiments indicated that when the concentration of Vitamin C was 10 μg/mL, the scavenging capacity of 1,1-diphenyl-2picrylhydrazyl (DPPH) and 2,2-azino-bis-3-ethyl-benzothiazoline-6-sulfonic acid (ABTS) for free radicals approached 100%, while the required AEO concentrations were 0.5 mg/mL and 0.2 mg/mL, respectively. Additionally, the iron-reducing capacity also showed a concentration-dependent relationship with AEO. In vivo experiments showed that Saccharomyces cerevisiae was cytotoxic after treatment with AEO at concentrations greater than 0.2 mg/mL. Furthermore, in a model of oxidative stress under 2 mM hydrogen peroxide (H2O2) stress, AEO increased the resistance of yeast to H2O2-promoted oxidative stress and improved its survival rate. These results demonstrate that AEO has good antioxidant capacity, and we hope to actively expand the application of AEO in various fields to fully develop and utilize this plant resource.
Keywords: Angelica sinensis essential oil; Antioxidant; Oxidative stress; GC-MS; (Z)-Ligustilide
| 1. Introduction | ▴Top |
Free radicals are intermediates or by-products produced during cellular metabolism, mainly including reactive oxygen species (ROS), which have the ability to defend against viral infections and regulate redox processes (Valko et al., 2007). When ROS accumulate excessively, they can disrupt the redox balance of cells and exceed the antioxidant capacity of cells, leading to oxidative stress (Liu et al., 2014; Pourzand et al., 2022). Excessive ROS can extensively damage and inhibit the normal function of important biomolecules such as DNA, cellular lipids, and proteins, leading to aging, various human diseases, and even carcinogenesis (Valko et al., 2007; Csepanyi et al., 2017). At this point, the in vivo antioxidant defense system is unable to alleviate the effects of excessive ROS and must rely on exogenous antioxidants to mitigate this situation. However, common synthetic antioxidants such as butylated hydroxyanisole and butylated hydroxytoluene pose the risk of liver injury and cancer. Therefore, exploring safe and effective antioxidants to combat oxidative stress has become the focus of recent research (Choi et al., 2013; Zou et al., 2010).
Essential oils are a complex mixture of volatile odorants extracted from the flowers, stalks, seeds, roots, and other organs of plants. They are mainly composed of terpenes, alcohols, phenols, esters, ketones, and other compounds, which have good antioxidant activity and can be used as a natural alternative to synthetic antioxidants in the resistance to ROS-induced oxidative stress (de Sousa et al., 2023; Bolouri et al., 2022).
Angelica sinensis, a perennial herb in the Umbelliferae family, is a traditional Chinese medicinal herb that grows widely in Asia, and its roots are rich in volatile oils (Wei et al., 2016; Chen et al., 2013). The essential oil extracted from the dried rhizome of Angelica sinensis has a strong herbal aroma, and the main active ingredients are ligustilide, n-butyl phthalide, and ferulic acid (Yao et al., 2015; Li et al., 2007). Additionally, Angelica sinensis essential oil (AEO) has strong pharmacological activities, including anti-inflammatory, anti-cancer, cardioprotective effects, strengthening the lungs and liver meridians, lubricating the intestines, and regulating menstrual disorders in women. It has been used in a wide range of fields such as medical care, food, cosmetics, and tobacco (Chen et al., 2024; Tang et al., 2023). In recent years, CO2 supercritical extraction has been increasingly used in extraction of essential oils including AEO. However, whether AEO obtained by CO2 supercritical extraction possesses the antioxidant ability remain unclear.
Assessment of antioxidative capacity is important to explore potential phytochemicals. Traditionally, methods for antioxidative assessment including in vitro chemical methods such as the 1,1-diphenyl-2picrylhydrazyl (DPPH) and 2,2-azino-bis-3-ethyl-benzothiazoline-6-sulfonic acid (ABTS) free radical scavenging capacity and in vivo mammalian cell (e.g., HepG2 cells)-based methods. Opposite to HepG2 which acts as a simple mammalian cell, Saccharomyces cerevisiae represents a unicellular eukaryotic organism with greater integrity and possessing a high degree of homology to the human genome and specific protein functions, has been well established as an antioxidant model for assessing the activity of natural dietary products (Meng, et al., 2017; Kachroo, et al., 2015; Dani, et al., 2008).
In the present study, we aim to assess the antioxidant ability of AEO obtained by supercritical extraction. Gas chromatography-mass spectrometry (GC-MS) was used to isolate and characterize its chemical constituents. The in vitro antioxidative capacity of AEO was determined using DPPH and ABTS free radical scavenging capacity and ascorbic acid was used as a positive control. Saccharomyces cerevisiae under hydrogen peroxide (H2O2) stress was used as an oxidative stress model to evaluate the in vivo antioxidant capacity of AEO.
| 2. Materials and methods | ▴Top |
2.1. Materials and reagents
AEO was obtained by supercritical CO2 extraction and provided by Lihe Flavor (Qingdao) Food Co., Ltd (Qingdao, China) (Nie, et al., 2005). DPPH, Vitamin C (Sigma, USA), ABTS, and potassium ferricyanide were purchased from Beijing Solarbio Science & Technology Co., Ltd (Beijing, China). Trichloroacetic acid (TCA) and ferric chloride were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. H2O2 was supplied by Tianjin Fengchuan Chemical Reagent Science and Technology Co., Ltd.
2.2. Strain and culture media
The Saccharomyces cerevisiae strain used in this study was the wild-type strain BY4741 (MATa, his3, leu2, met15, ura3), purchased from Euroscarf. The YPD medium consisted of 10 g yeast extract, 20 g peptone, 20 g glucose, 20 g agar, and 1,000 mL distilled water. Yeast was grown in liquid YPD medium at 28 °C and 200 rpm in an oscillating incubator.
2.3. GC-MS analysis
The composition of AEO was determined using a GC-MS system (Thermo Fisher Scientific Inc. Waltham, USA). The chromatographic conditions were as follows: the carrier gas was helium, the column flow rate was 1 mL/min, and the column was an SPB-5 capillary column (30 m × 250 μm × 0.25 μm). The initial temperature was 50 °C, held for 3 min, then increased to 160 °C at 10 °C/min, held for 5 min, and finally increased to 280 °C at 10 °C/min. The injection volume was 1 μL, the injection port temperature was 280 °C, the split ratio was 30:1, and the solvent delay time was 5 min. The mass spectrometry conditions were: ionization mode EI, electron energy 70 eV, ion source temperature 250 °C, mass scan mode full scan, and scanning range 40 to 500 amu. Compounds were identified using the NIST 2017 database (Zhong et al., 2016; Wang et al., 2016; Zhou et al., 2023; Zhao et al., 2022).
2.4. DPPH assay
The DPPH radical scavenging method for AEO was established with reference to related literature (Joshi et al., 2007). Ethanol was used to prepare different concentrations of AEO. Equal volumes of DPPH solution(0.1 mmol/L) were mixed with different concentrations of AEO in a 1:1 ratio, vortexed, and allowed to react for 30 min at room temperature in the dark. Ethanol was used as a blank reference and Vitamin C solution as a positive control. Absorbance was measured at 517 nm (Bajpai et al., 2015). The scavenging activity was calculated using the following formula:
2.5. ABTS assay
The ABTS radical scavenging rate of AEO was determined by referring to the method of (Zhao et al., 2022). The working solution of ABTS(0.1 mmol/L) and different concentrations of AEO solutions were mixed in a 3:1 ratio, kept in the dark at room temperature for 10 min, and the absorbance was measured at 734 nm. Ethanol was used as a blank reference and Vitamin C solution as a positive control (Shang et al., 2018). The scavenging activity was calculated using the following formula:
2.6. Reducing power analysis
The total reducing power of AEO was determined by referring to the method established by (Costa et al., 2010). Different concentrations of AEO, phosphate buffer (pH 6.6, 0.2 M), and 1% potassium ferricyanide were mixed thoroughly and placed in a 50 °C water bath for 30 min. After cooling to room temperature, 1 mL of 0.1% ferric chloride and 2 mL of distilled water were added. The mixture was incubated at room temperature for 8 min. Distilled water was used as the blank control and Vitamin C solution as the positive control. Absorbance was measured at 700 nm. A higher absorbance of the reaction mixture indicated greater reducing power ability.
2.7. Cytotoxicity assay for AEO
The activated Saccharomyces cerevisiae strain was inoculated into YPD medium and incubated overnight at 28 °C, 200 rpm on a shaker. The culture was centrifuged at 4 °C, 6,000 rpm for 2 min, the supernatant discarded, and the precipitate resuspended in sterile water. The OD value was measured with a spectrophotometer and adjusted to OD600 = 1. One milliliter of the adjusted culture was added to 9 mL of liquid medium, different concentrations of AEO were added, mixed well, and incubated at 28 °C for 2 h on a shaker. The dilution-coated plate method was used to dilute the medium appropriately and then spread onto solid medium. Plates were incubated at 28 °C for 72 h, and colony counts were recorded (Nenadis et al., 2022). The survival rate of untreated yeast was recorded as 100%.
2.8. AEO In vivo antioxidant activity assay
The activated yeast strains were incubated on a shaker overnight. Different concentrations of AEO were added to the YPD broth, mixed well, and incubated at 200 rpm and 28 °C for 2 h. Then, 2.0 mM H2O2 was added, mixed, and incubated under the same conditions for 1 h (Lopes et al., 2023). Colony counts were recorded as in the above method of counting on coated plates.
2.9. Statistical analysis
All experiments were performed at least three times in parallel. The experimental data were analyzed by one-way analysis of variance (ANOVA) with Duncan’s post hoc multiple comparison test using SPSS 27.0 software. GraphPad Prism software 8.0 was also utilized for graphing, and a p-value of less than 0.05 indicated statistical significance.
| 3. Results | ▴Top |
3.1. Compounds of AEO
The volatile components of AEO were analyzed using GC-MS, and the total ion current diagram is shown in Figure 1. The peak times of the substances were mainly within 10 to 60 minutes.
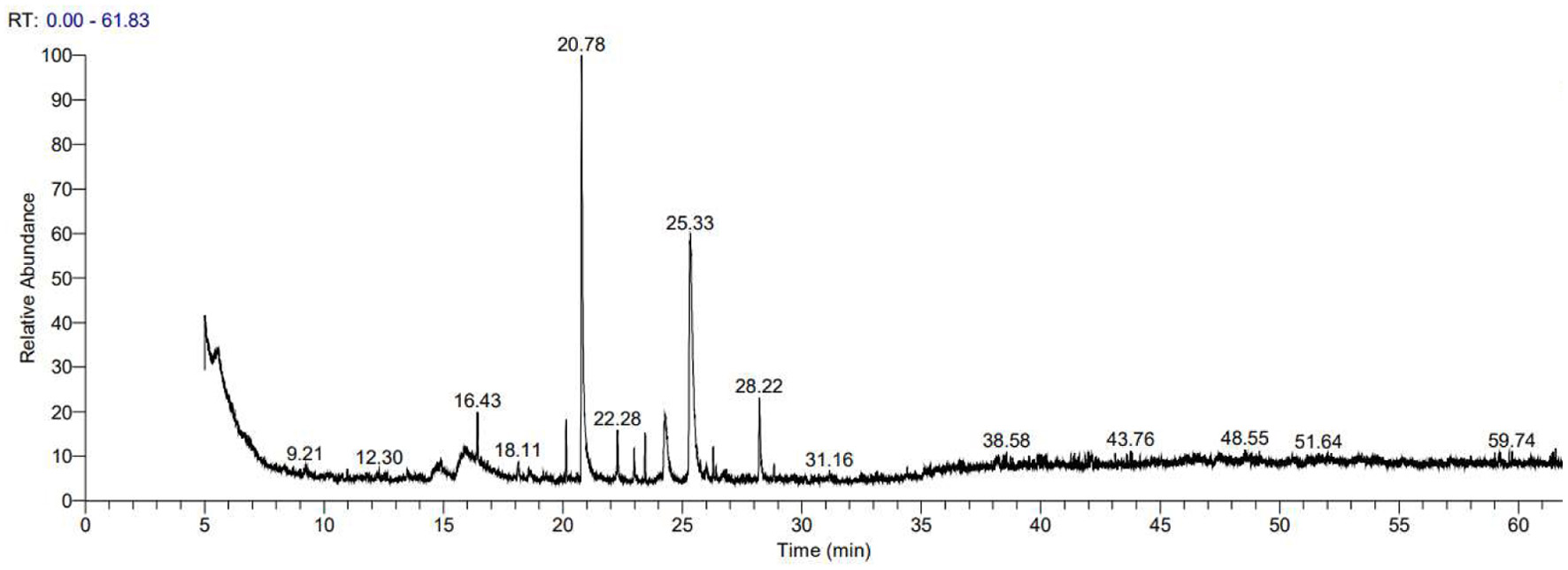 Click for large image | Figure 1. GC-MS diagram of Angelica sinensis essential oil. |
Through GC-MS compositional analysis of AEO, we identified 55 major components. The detailed identification results are shown in Table 1. The major components include phthalides, esters, terpenes, and ketones, with (Z)-Ligustilide (12.85%), 2,4-Di-tert-butylphenol (11.6%), and 3-Butylidenephthalide (3.16%) being the most abundant, which is consistent with relevant literature reports (Zhou et al., 2023).
 Click to view | Table 1. Compounds identified in n-hexane solution of sweet almond oil by GC-MS technique |
3.2. DPPH radical scavenging activity of AEO
The above data showed that AEO contains a high abundance of phthalides, implying that AEO may have good antioxidant activity. Therefore, we first assessed the antioxidant capacity of AEO using the DPPH radical assay. DPPH is a stable free radical whose solution is purple, and it turns yellow when it reacts with antioxidants, allowing absorbance to be measured (Costa et al., 2010). In Figure 2a, the Vitamin C-equivalent antioxidant capacities (VCEAC) standard curve was calculated using absorbance versus Vitamin C concentration (R2 = 0.9920), demonstrating that Vitamin C scavenges DPPH radicals in a concentration-dependent manner. As shown in Figure 2b, 0.5 mg/mL of AEO is equivalent to the DPPH scavenging capacity of 10 μg/mL VCEAC, with the scavenging rate approaching 100%. This indicates that AEO has strong DPPH radical scavenging ability.
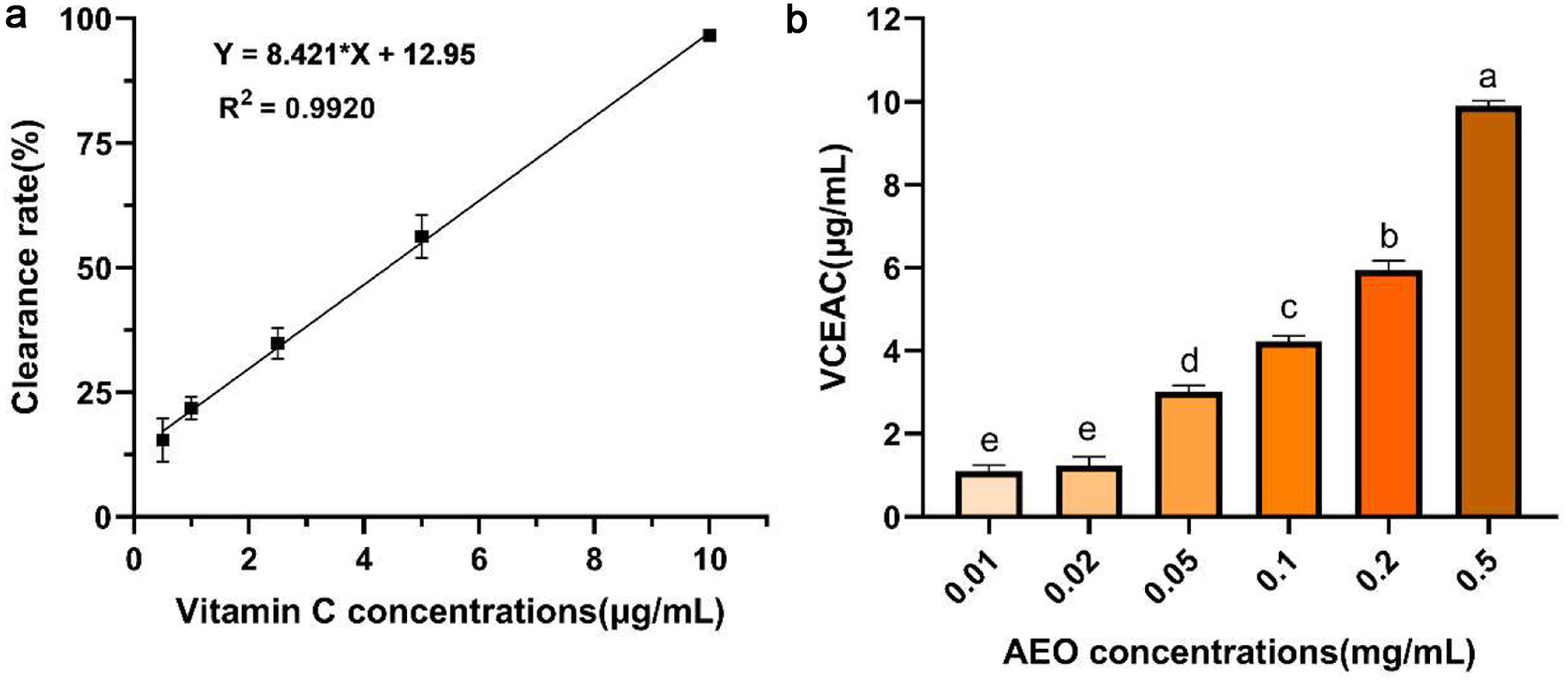 Click for large image | Figure 2. DPPH radical scavenging activity of AEO. (a)Standard curve of Vitamin C scavenging ability of DPPH, (b) Vitamin C-equivalent antioxidant capacities (VCEAC) of different concentrations of AEO on DPPH. Different letters indicate statistically significant differences in the results of each oxidative stress group; P < 0.05. |
3.3. ABTS radical scavenging activity of AEO
The ABTS radical scavenging ability of AEO was also evaluated. As shown in Figure 3a, the standard curve of Vitamin C’s scavenging ability of ABTS radicals was established (R2 = 0.9902). The ABTS radical scavenging ability of AEO was compared using the VCEAC method at six different concentrations (Figure 3b). The results showed that the ABTS radical scavenging capacity of AEO increased in a dose-dependent manner and stabilized at 0.2 mg/mL. At this concentration, the AEO scavenging capacity was close to 10 μg/mL of VCEAC, reaching nearly 100%. Compared to an equivalent amount of DPPH radical scavenging capacity, a lower concentration of AEO was required, indicating a stronger ABTS radical scavenging capacity than DPPH.
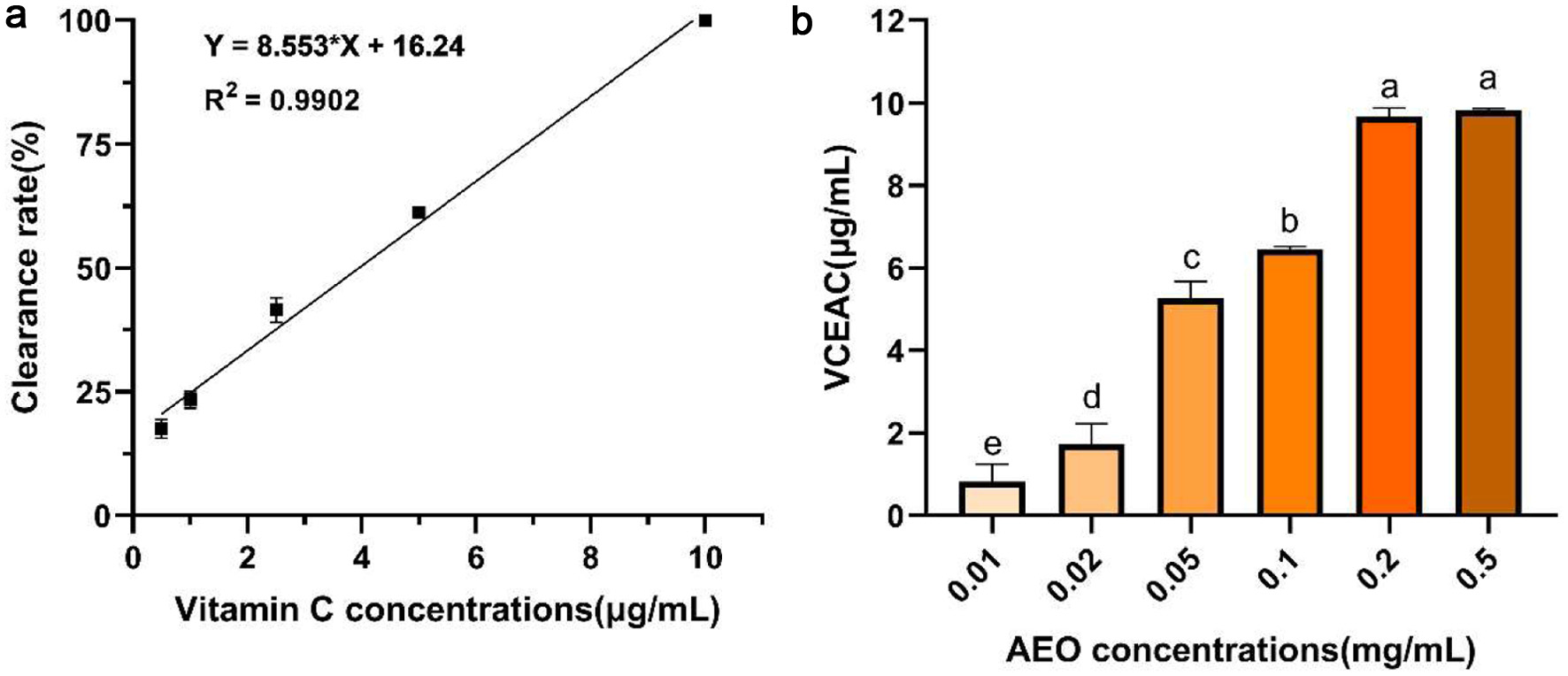 Click for large image | Figure 3. ABTS radical scavenging activity of AEO. (a) Standard curve of Vitamin C scavenging ability of ABTS, (b) Vitamin C-equivalent antioxidant capacities (VCEAC) of different concentrations of AEO on ABTS. Different letters indicate statistically significant differences in the results of each oxidative stress group; P < 0.05. |
3.4. Ferric-reducing antioxidant power (FRAP) of AEO
To comprehensively evaluate the antioxidant activity of AEO, we assessed its total reducing capacity using the FRAP method. In Figure 4a, the absorbance standard curve of iron reduction capacity by Vitamin C (R2 = 0.9955) was established, and the reducing capacity of AEO was compared using the VCEAC method (Figure 4b). The results showed that absorbance at 700 nm increased with higher AEO concentrations, indicating AEO’s strong ability to reduce ferric ions to ferrous ions, thus demonstrating good antioxidant activity.
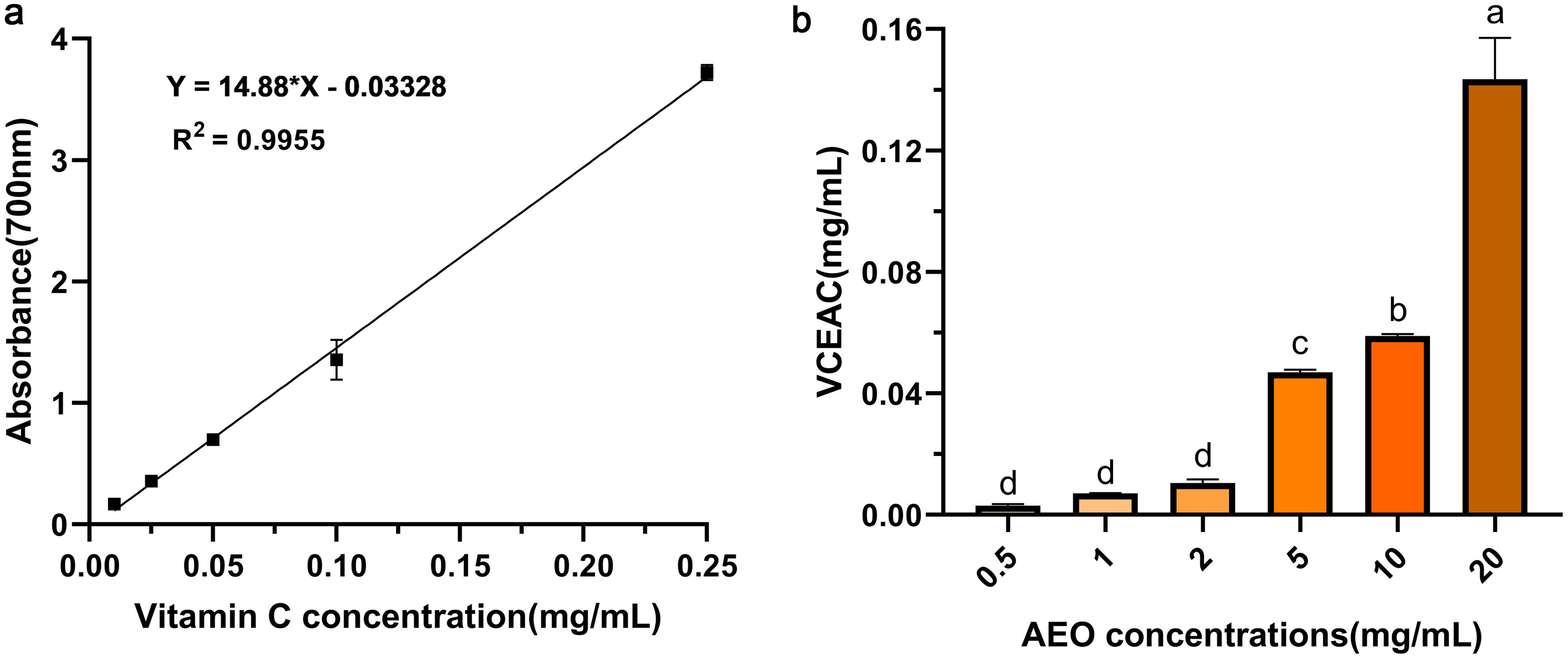 Click for large image | Figure 4. Ferric-reducing antioxidant power (FRAP) of AEO. (a) Standard curve of absorbance of Vitamin C iron reducing power, (b) Vitamin C-equivalent antioxidant capacities (VCEAC) of iron reducing power of different concentrations of AEO. Different letters indicate statistically significant differences in the results of each oxidative stress group; P < 0.05. |
3.5. Effect of AEO on cell survival of Saccharomyces cerevisiae under H2O2 stress
Having determined the in vitro antioxidant capacity of AEO, we further explored its in vivo antioxidant capacity using a wild-type strain of saccharomyces cerevisiae We first tested whether AEO caused any cytotoxicity to the yeast strain. As shown in Figure 5a, AEO at concentrations greater than 0.2 mg/mL significantly inhibited cell viability. Interestingly, AEO concentrations below 0.2 mg/mL showed no cytotoxicity and even significantly improved cell viability. Therefore, AEO concentrations below 0.2 mg/mL were selected for in vivo antioxidant experiments. H2O2 acted as an oxidative stress inducer, generating superoxide radicals and inducing oxidative stress (Ribeiro et al., 2015). As shown in Figure 5b, yeast cell survival was significantly reduced to about 20% after oxidative stress induced by H2O2. In contrast, AEO intervention reversed this trend, and yeast survival rates increased with higher AEO concentrations. These results suggest that AEO has moderate antioxidant capacity.
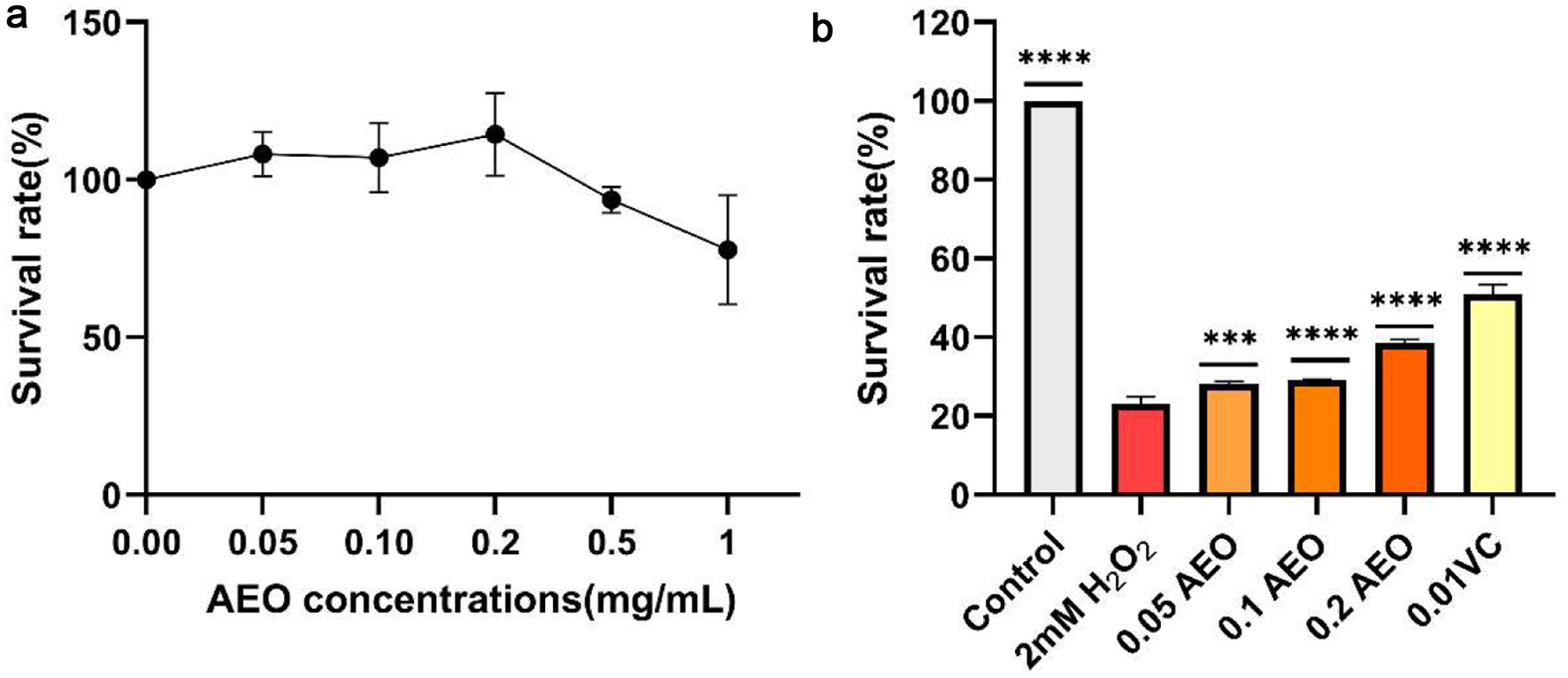 Click for large image | Figure 5. Effect of AEO on cell survival of Saccharomyces cerevisiae under H2O2 stress. (a) Survival of yeast strains under different concentrations of AEO stress, (b) Effect of different concentrations of AEO on yeast cell viability under the stress of 2.0 mM H2O2. Data are presented as the mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 against 2.0 mM H2O2. |
| 4. Discussion | ▴Top |
Oxidative stress, an imbalance between oxidant and antioxidant molecules, is associated with aging and the progression of various diseases (Sánchez-Rodríguez and Mendoza-Núñez, 2019). Plant foods, as natural reservoirs of antioxidants, act as free radical scavengers and offer protective effects against chronic and degenerative diseases (Kim and Lee, 2004; Ndhlala et al., 2010). Angelica sinensis, a traditional Chinese herb, is a natural antioxidant (Wei et al., 2016). In this study, the volatile components of AEO were detected and analyzed using GC-MS, identifying 55 major components, with (Z)-Ligustilide, 2,4-Di-tert-butylphenol, and 3-Butylidenephthalide being the most abundant. Further in vitro antioxidant experiments showed that AEO had significant antioxidant effects, with DPPH, ABTS free radical scavenging rates, and reducing power absorbance showing good positive correlations with AEO dosage within a certain range before stabilizing.
We analyzed the antioxidant activity of AEO in relation to its rich content of phthalides and terpenes. The GC-MS results showed that the highest content compounds were (Z)-Ligustilide, 2,4-Di-tert-butylphenol, and 3-Butylidenephthalide, all belonging to the class of phenolics. (Z)-Ligustilide is a natural benzoquinone derivative found in herbal medicines such as Angelica sinensis and has various pharmacological effects, including anti-inflammatory, anti-apoptotic, anti-tumor, and neuroprotective activities (Xie et al., 2020; León et al., 2017; Wu et al., 2020; Alshehri et al., 2023; Wu et al., 2022). 2,4-Di-tert-butylphenol is found in the volatile oils or essential oils of many seed plant species and is used as an intermediate in the preparation of antioxidants and UV stabilizers, pharmaceuticals, and fragrances, with significant antioxidant activity (Choi et al., 2013; Zhao et al., 2020). Although natural phthalide compounds exhibit significant pharmacological activity, their targets and mechanisms of action require further investigation (León et al., 2017).
Additionally, AEO contains various terpenes, such as monoterpenes (D-Limonene, Cedrene, and Cuparene) and tetraterpenes (Lycopene and Astaxanthin). Studies have shown that terpenoids possess strong antioxidant properties, with their double bonds preemptively reacting with free radicals to form stable hydroperoxides. These antioxidant radical molecules, due to their high stability, do not participate in other reactions, thus terminating the free radical chain reaction and exerting antioxidant effects (González-Burgos and Gómez-Serranillos, 2012; Enchev et al., 2024).
| 5. Conclusion | ▴Top |
Overall, we conclude that AEO is rich in natural antioxidant substances, exhibits significant antioxidant effects and offers new perspectives for the comprehensive development of AEO.
This work was supported by the College Student Innovation grant (202210069039).
Conflict of interest
The authors declare no conflict of interest.
| References | ▴Top |