| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 26, June 2024, pages 61-71
Exploring the therapeutic mechanisms of Astonia boonei in diabetes mellitus ligand-based virtual screening with TGR5 Receptor
Sunday Ayodele Alongea, b, Olusola Olalekan Elekofehintia, Moses Orimoloye Akinjiyana, Isaac Iseoluwa Ajayib
aBioinformatics and Molecular Biology Unit, Department of Biochemistry, Federal University of Technology, Akure, Ondo State, Nigeria
bDepartment of Biological Sciences, Bamidele Olumilua University of Education, Science and Technology, Ikere
*Corresponding author: Sunday Ayodele Alonge, Bioinformatics and Molecular Biology Unit, Department of Biochemistry, Federal University of Technology, Akure, Ondo State, Nigeria., E-mail: alongesunday0082@gmail.com
DOI: 10.31665/JFB.2024.18381
Received: April 14, 2023
Revised received & accepted: June 17, 2024
| Abstract | ▴Top |
The anti-diabetic effects of Astonia boonei have been demonstrated by several studies, but few have clarified the mode of action of compounds extracted from Astonia boonei as an agonist for the Takeda G protein-coupled receptor 5 (TGR5) pathways in the etiology of diabetes mellitus (DM). TGR5 is a membrane protein receptor that has been linked to increase insulin signalling and is an appropriate target for diabetes treatment. This study looked into A. boonei bioactive compounds that could be TGR5 agonists and hence have therapeutic value in DM treatment using Schrödinger suites. Investigations include molecular and induced-fit docking, MMGBSA, and ADMET studies. Chlorogenic acid, alstonidine and quercetin have a binding energy of −10.661, −9.982 and −9.661 kcal/mol and induced-fit scores of −533.89, −528.94 and −528.66 kcal/mol respectively. The MMGBSA free binding energy of chlorogenic acid, alstonidine and quercetin with TGR5 is −71.28, −88.62 and −55.21. Moderate indices for ADMET parameters of these hit compounds’ drug-likeness, pharmacokinetic, and toxicity features show they do adhere to Lipinski’s rule of five for druggability. The potential of A. boonei bioactive compounds might be explored as therapeutic options in the treatment of diabetes.
Keywords: Astonia boonei; Bioactive compounds; Diabetes mellitus; TGR5; Molecular docking; ADMET
| 1. Introduction | ▴Top |
Alstonia boonei (family: Apocynaceae) is a medicinal plant commonly called “devil tree”. It is a vast evergreen tree, seen throughout the tropical and rain forests of West and Central Africa including Nigeria. In Nigeria, it is referred to as “Awun” in the South-West, “Egbu-ora” in the South-East, and “Ukpukunu” in the South-Central (Akinnawo et al., 2017). The leaves, stem bark, and roots of the tree are commonly sold in local markets in Africa as remedies for a variety of illnesses (Bello et al., 2009; Bekoe et al., 2020). The ripe fruits are edible, and the bark and leaves can also be boiled in water and then taken for therapeutic purposes (Akinnawo et al., 2017; Akinmurele et al., 2023). It is reported to possess a number of beneficial bioactive compounds like rutin, isoquercetin caffeic, chlorogenic, ferulic acids, β-amyrin, and α-amyrin among others (Okoye et al., 2014; Kehinde et al., 2016; Atanu et al., 2021; Mollica et al., 2022). The stem bark’s anti-rheumatic, anti-malarial, anti-inflammatory and anti-helminthic qualities have all been reported (Kehinde et al., 2016). Rutin has been reported to possess hypoglycemic activity (Ghorbani, 2017; Hasanein et al., 2020).
Diabetes mellitus (DM) is a common risk factor for cardiovascular diseases and one of the world’s major causes of death (Arroyave et al., 2020; Agrawal et al., 2021), according to reports (Kehinde et al., 2016). Previous research indicates that diabetes mellitus is a metabolic disorder characterized by hyperglycemia brought on by insufficient insulin production (type 1 DM) (Bekoe et al., 2020; Kehinde et al., 2016). A World Health Organization (WHO) research states that diabetes is one of the most prevalent chronic diseases affecting about 500 million people globally (Arroyave et al., 2020).
The variable condition known as type 1 diabetes mellitus is marked by a reduction in the activity of the beta cells in the pancreas that generate insulin (Elekofehinti, 2015). The main goals of type 1 diabetes treatments are to enhance insulin secretion and reduce the synthesis of glucose in the liver (Kehinde et al., 2016; Oyetayo et al., 2021). Activation of TGR5 can regulate glucose homeostasis, by inducing GLP-1 to produce more insulin (Kumar et al., 2016; Lun et al., 2024). TGR5 works by increasing insulin-mediated insulin receptor tyrosine kinase activity, which in turn activates post-receptor insulin signaling pathways. It might result from changes in membrane fluidity in hyperglycemic circumstances. Because TGR5 is a good target for bioactive compounds that can have an anti-diabetes effect, it is an essential agonist target (Hasan et al., 2022).
There have been many approaches by scientists over the years to combat DM. The mechanisms involve glycemia control, enhancement of glucose transporters and insulin therapy. The two most common strategies used in these treatments are medication and diet plans. These medications include metformin, acarbose, sulfonylureas, thiazolidinedione, and meglitinides. However, synthetic medications used to treat DM come with some side effects and are relatively costly (Padhi et al., 2020). Thus, the need to look at plants’ bioactive components as another option. This study aims to investigate the molecular interaction of the bioactive compounds of Alstonia boonei with TGR5 which signally help in insulin release.
The computational method known as “molecular docking” examines the conformations of tiny molecules at protein binding sites and uses scoring functions and algorithms to determine how binding between the two molecules occurs and which conformation is most likely based on the binding energy values (Elekofehinti et al., 2021). It is the study of the interactions between two or more molecular structures, an enzyme or protein. The process of finding new medications is multifaceted and dependent on in-silico tools (Cheng, 2018; Bekoe et al., 2020). Finding the most likely ligand-protein binding mechanism is the aim of ligand-protein docking. Ligand-based simulation or docking decreases the number of animals used in research, lowers expenses, increases the success rate of studies, and aids in the comprehension of drug-protein interactions. In research on pharmaceuticals and drug development, molecular docking facilitates the rapid identification of any molecules with significant therapeutic potential. To assess the pharmacological characteristics of Alstonia boonei compounds against diabetes mellitus while taking into account its bioactive activities, this analytical investigation uses a computational method (Perino et al., 2014; Ajiboye et al., 2017; John et al., 2012).
| 2. Materials and methods | ▴Top |
2.1. Preparation of protein and ligand
The isolated compounds from Astonia boonei leaves and bark were obtained from literature and then transformed and minimized into 3D structures using LigPrep wizard Schrodinger maestro (v11.1). With the help of Epik, a possible ionization state was generated at target pH 7.0 ± 2.0 for precise tautomer enumeration and to determine the protonation state in biological status. By keeping certain chiralities, stereoisomers were created, up to a maximum of 32 per ligand. OPLS3 was selected as the force field. The RCSB Protein Data Bank (PDB) was used to obtain the membrane protein receptor target protein, TGR5 (PDB ID: 7BW0), for the docking process. The production wizard in Schrodinger Maestro (v11.1) was used to create the protein’s 3D structure. In order to generate disulfide bonds, assign bond orders, add hydrogens, remove zero-order links to metal water molecules beyond 5°A from het groups, and leave the het state at its default pH (7.0 ± 2.0) using Epik, the CCD database was consulted. Lastly, heavy atoms are converged to an RMSD of 0.30 Å using the OPLS3 force field, which is used for restrained minimization.
2.2. Identification of the active site of the receptor
A drug’s propensity to bind to a specific region or target on the surface of a receptor and cause conformational changes that result in a pharmacological response is known as an active site (Hasan et al., 2022). Molecular docking assisted in determining the ligand-receptor binding active site, and the PockDrug server was utilized to ascertain the druggability influence of the target protein’s active site (Hasan et al., 2022).
2.3. Generation of receptor grid
The bounding box was selected to cover the whole target site for docking simulation, and the default values for the partial charge cutoff and van der Waals radius scaling factor are 0.25 and 1, respectively.
2.4. Molecular Docking Simulation
Following completion of the prerequisite procedures, the docking simulation was run in Schrodinger maestro’s SP glide (v11.1) (Cheng, 2018; Hasan et al., 2022), taking into account the penalties applied to non-cis/trans amide bonds. In this case, the partial charge cutoff was set to 0.15 and the van der Waals scaling factor was set to 0.80. Energy-minimized posture was used to calculate the final result, which is known as the glide score. Every activity’s score for the chemical was noted (Figure 1).
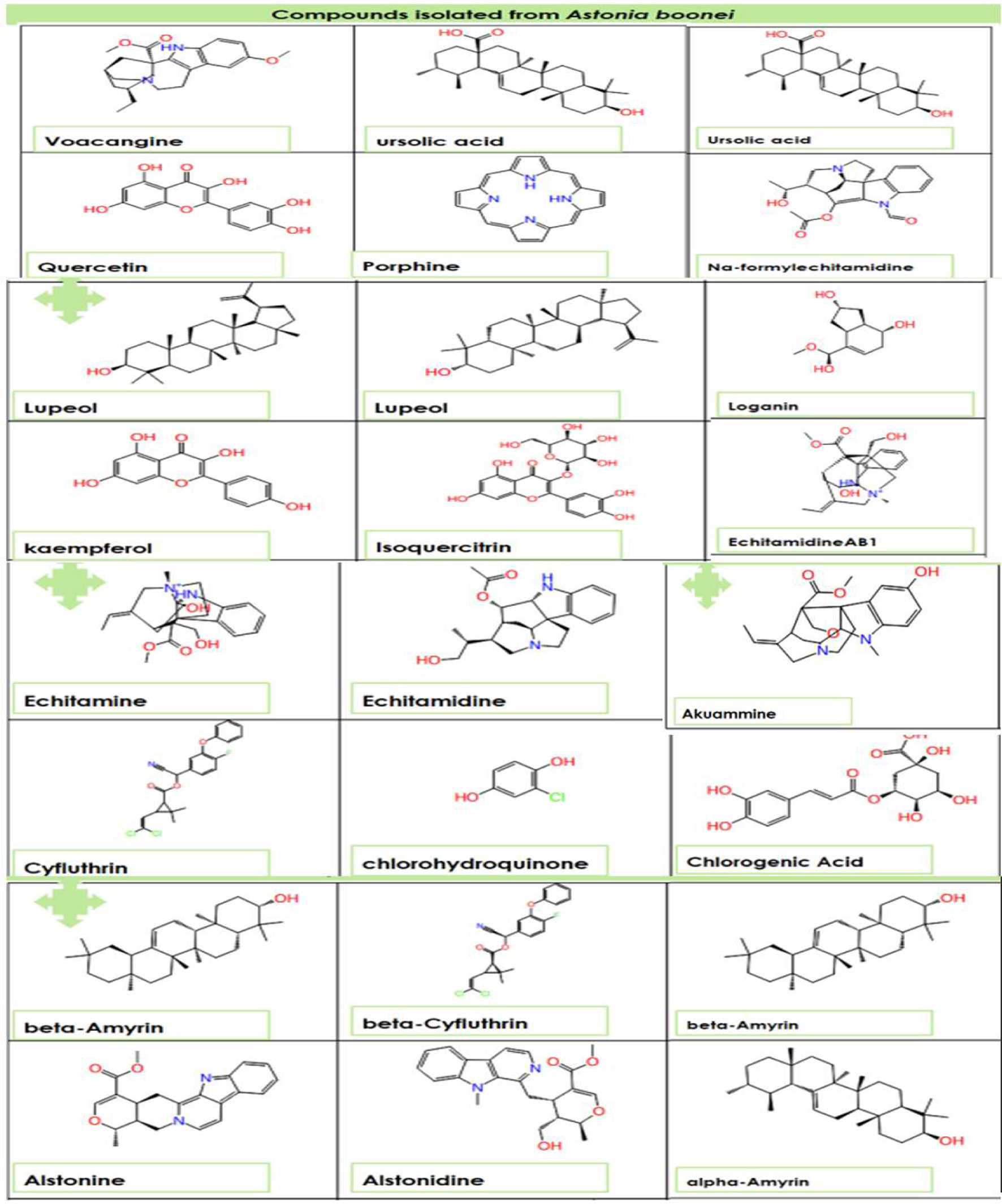 Click for large image | Figure 1. The two-dimensional structure of Alstonia boonei compounds. |
2.5. ADMET evaluation
The ADME features, which were established by Lipinski’s rule of five (Cheng, 2018; Hasan et al., 2022), show Alstonia boonei’s compound accessibility throughout the body. This rule’s standard parameter is provided below:
The QikProp module of Schrodinger maestro (v11.1) was used to ascertain the ADME (Absorption Distribution Metabolism Excretion) features of the chosen Alstonia boonei compounds, and an online server-based database called admetSAR was employed to ascertain the toxicity profile (Hasan et al., 2022). Figure 2 displays the proposed work’s graphical representation.
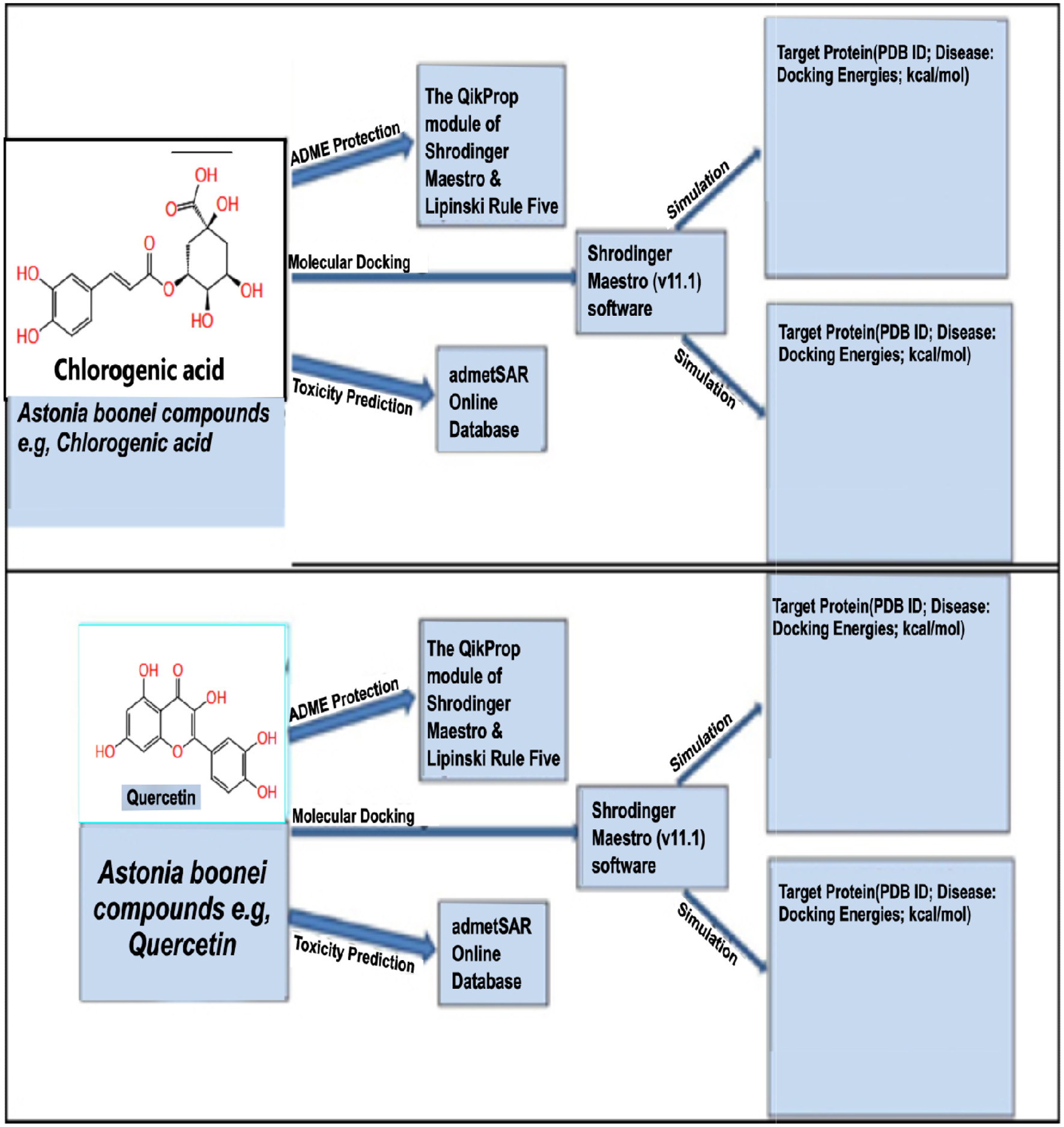 Click for large image | Figure 2. Broad-spectrum therapeutic potential against diabetes as assessed by in silico molecular docking and ADME/T analysis of certain Alstonia boonei compounds, utilizing quercetin and chlorogenic acid as examples. |
| 3. Results | ▴Top |
3.1. Molecular Docking, Induced-fit docking and MMGBSA
Figure 1 shows the two-dimensional structures of the Alstonia boonei compounds: Nα formylechitamidine, echitamidine Kaempferol, Porphyrin, Loganin, Chlorohydroquinone, Isoquercitrin, Chlorogenic acid, Alstonidine, Quercetin, Cyfluthrin, Beta Cyfluthrin, etc. The selected Alstonia boonei compounds’ used in docking simulation for TGR5 are displayed in Figure 1. Table 1 shows the molecular docking scores or binding energy (kcal/mol) between specific Astonia boonei compounds and the TGR5 receptor. Chlorogenic acid, alstonidine and quercetin have a binding energy of −10.661, −9.982 and −9.661 kcal/mol respectively with TGR5 binding site. The selected compounds produced promising results of high binding energy. Figure 2 shows the flowchart of the computational procedures. Figure 3 shows the 3D structure of TGR5. Figures 4–7 reveal the binding interaction (hydrogen and hydrophobic) between specific Alstonia boonei compounds (Cyfluthrin, Nα formylechitamidine, echitamidine, Kaempferol, Porphyrin, Loganin, and Chlorohydroquinone) and TGR5 revealing they are potent ligands
 Click to view | Table 1. Molecular docking scores between selected Astonia boonei compounds and TGR5 |
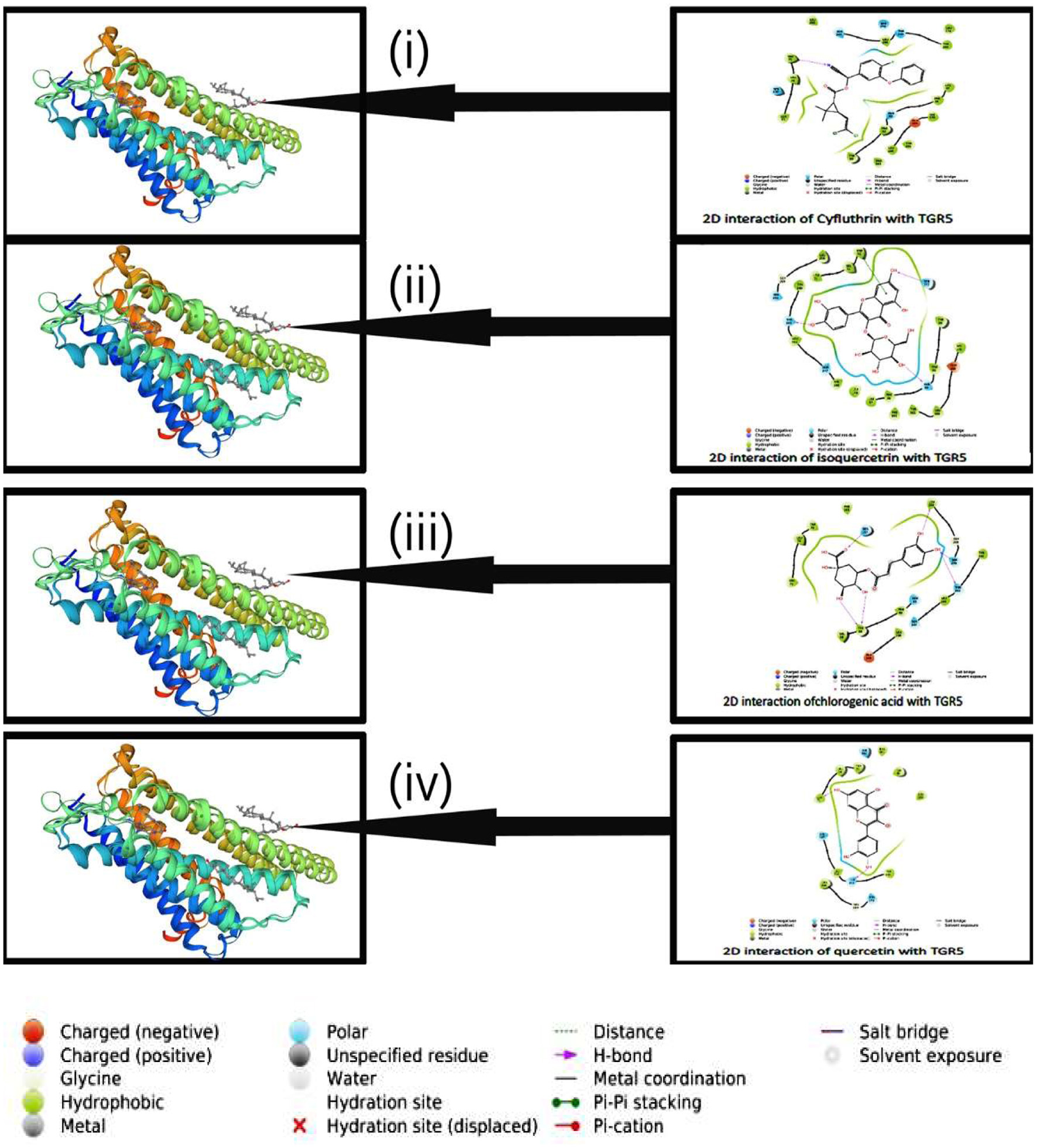 Click for large image | Figure 3. Analysis of molecular docking simulation, the following interactions have been observed. (i) Interaction of Cyfluthrin with TGR5 (PDB id: 7BW0), (ii) Interaction of Isoquercitrin with TGR5 (PD id: 7BW0), (iii) Interaction of Chlorogenic acid with TGR5 (PDB id: 7BW0), (iv) Interaction of Quercetin with TGR5 (PDB id: 7BW0). |
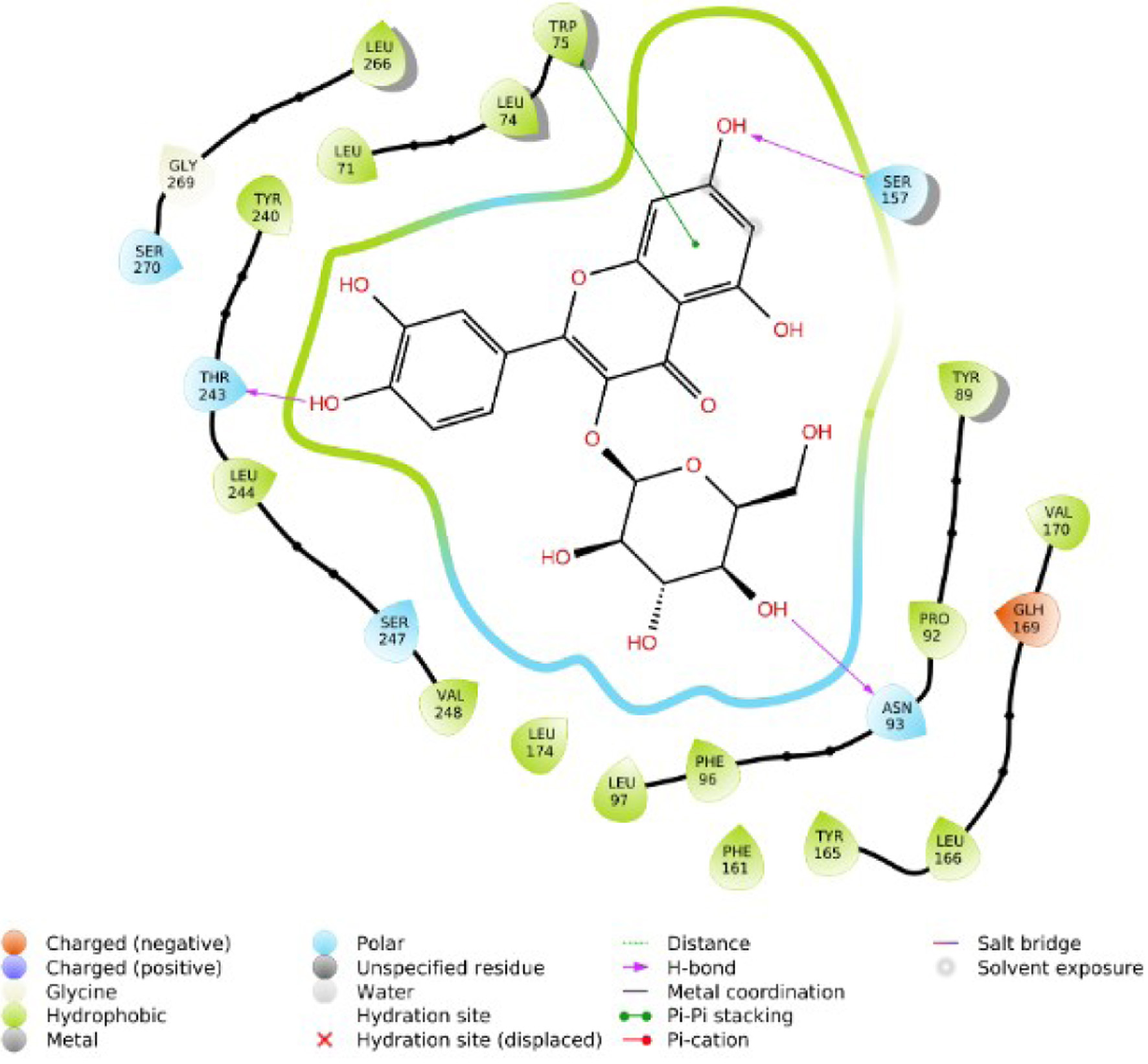 Click for large image | Figure 4. 2D interaction of isoquercetrin with TGR5. |
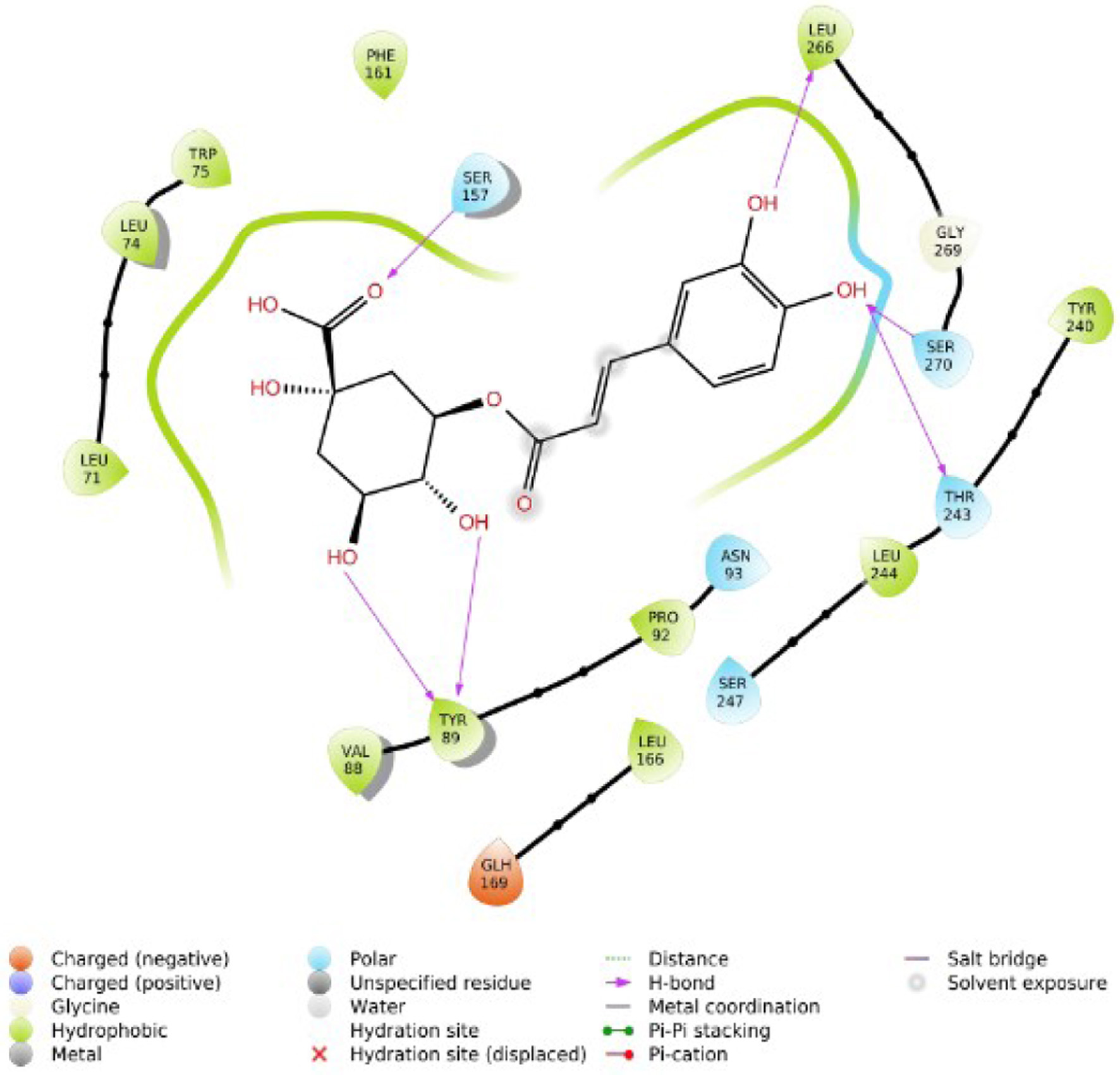 Click for large image | Figure 5. 2D interaction of chlorogenic acid with TGR5. |
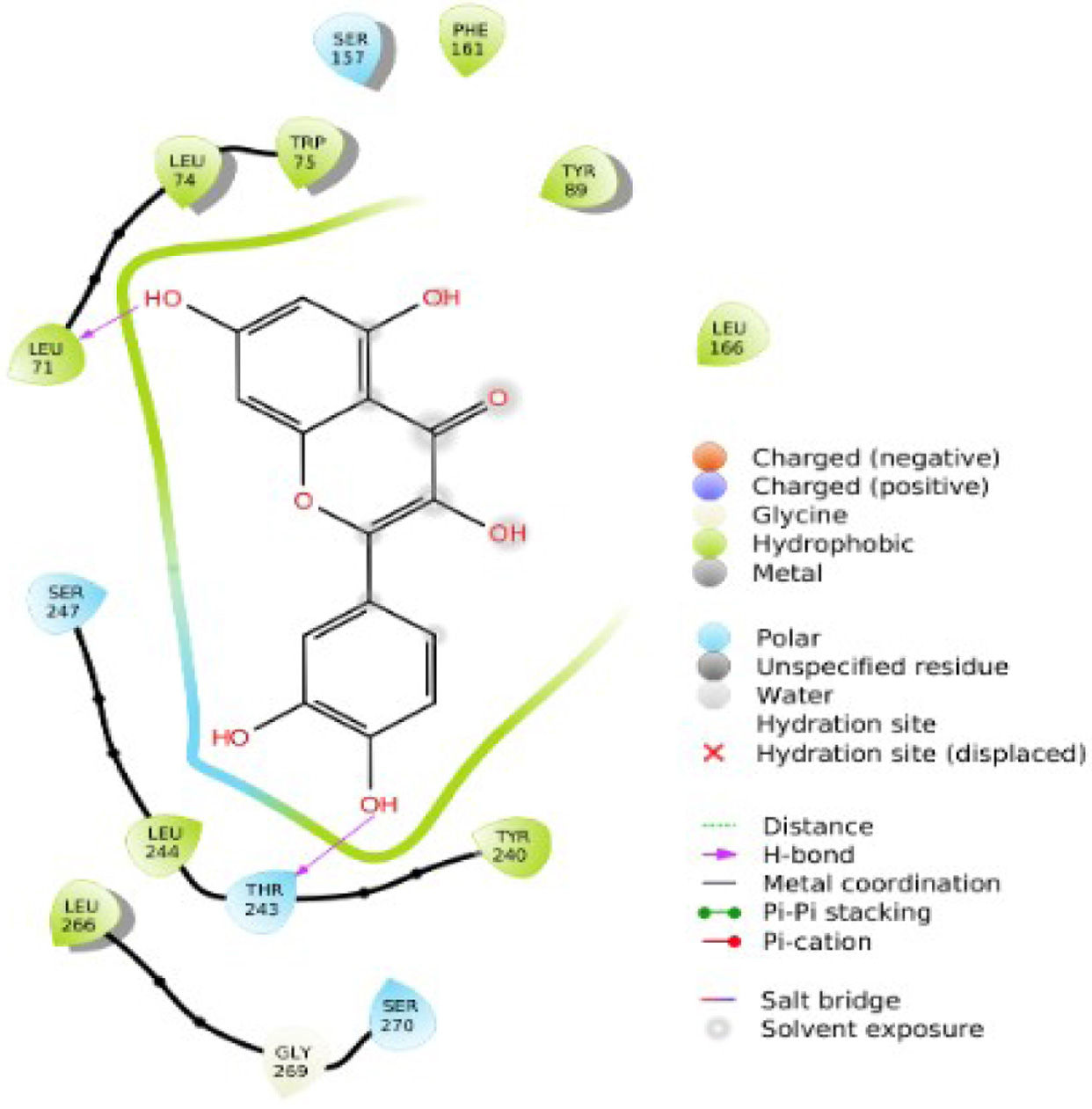 Click for large image | Figure 6. 2D interaction of quercetin with TGR5. |
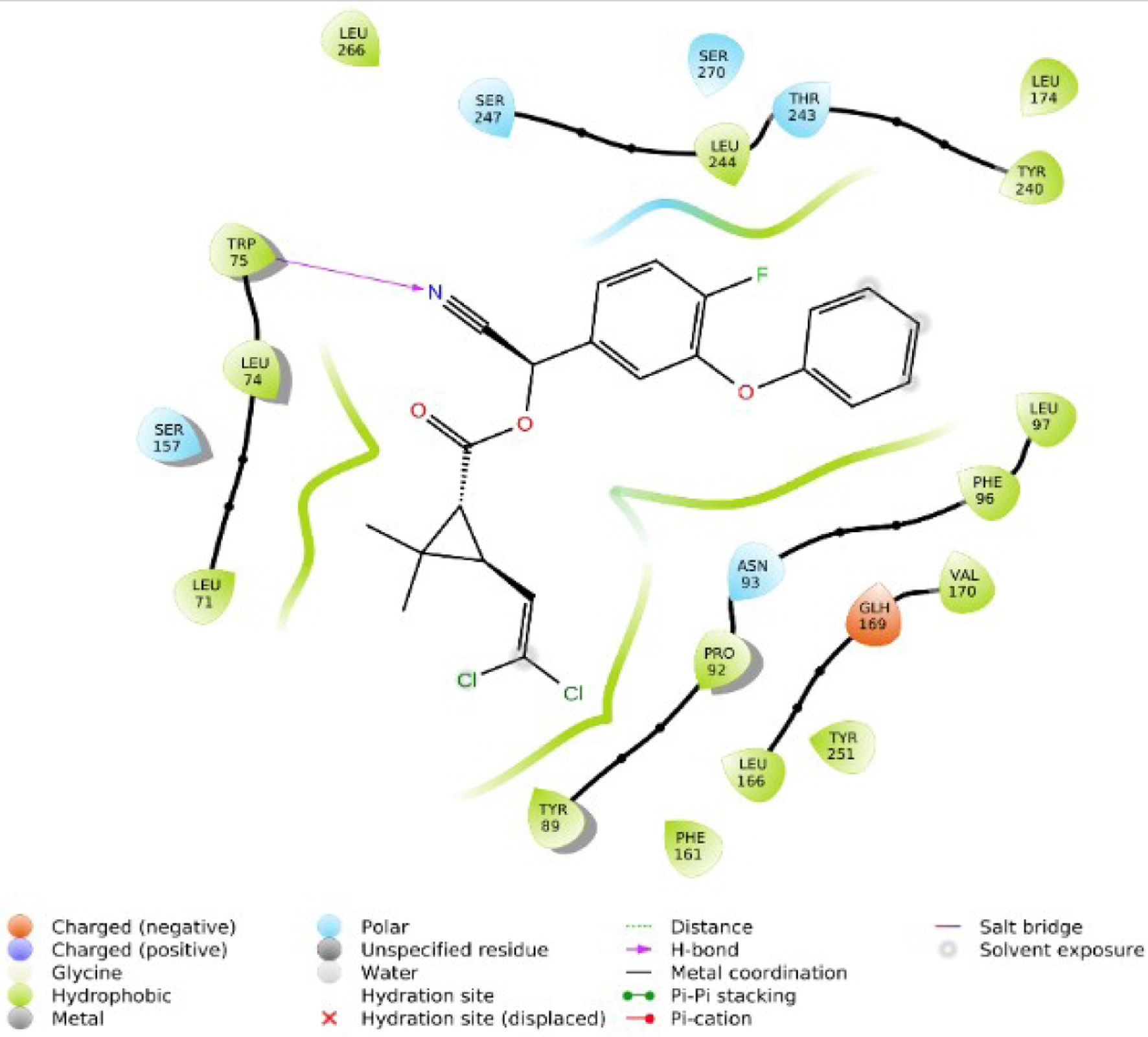 Click for large image | Figure 7. 2D interaction of Cyfluthrin with TGR5. |
Table 2 revealed chlorogenic acid, alstonidine and quercetin have induced-fit scores of −533.89, −528.94 and −528.66 kcal/mol respectively with TGR5.
 Click to view | Table 2. Induced fit docking between selected Astonia boonei compounds with TGR5 Receptor |
Table 3 shows the molecular docking binding free Energy MMGBSA scores between specific Astonia boonei compounds and TGR5 receptor The MMGBSA free binding energy of chlorogenic acid, alstonidine and quercetin with TGR5 is −71.28, −88.62 and −55.21 as seen in Table 3.
 Click to view | Table 3. MMGBSA between selected Astonia boonei compounds and TGR5 Receptor |
3.2. ADME/T evaluation
The ADME property assesses a chemical agent’s capacity to function as a drug. Table 3 shows the pharmacokinetic profile of a few compounds from Alstonia boonei (Chlorogenic acid, Alstonidine, Quercetin, Cyfluthrin, Beta cyfluthrin, Nα formylechitamidine, echitamidine, Kaempferol, Porphyrin, Loganin, Chlorohydroquinone), showing that they do not violate more than one of the Lipinski’s rule of five for druggability. Table 4 reveals the results of the ADMET analysis between specific Astonia boonei compounds and the TGR5 receptor. Table 5 shows the molecular weight of these bioactive compounds
 Click to view | Table 4. ADME between selected Astonia boonei compounds and TGR5 |
 Click to view | Table 5. Some Astonia boonei compound’s molecular weight |
| 4. Discussion | ▴Top |
Understanding the pharmacological activity of bioactive compounds in curative conditions can be accomplished by the utilization of the findings and understanding derived from in silico molecular docking studies (Cheng, 2018; Hasan et al., 2022). Recent research has demonstrated the potential therapeutic benefits of compounds isolated from Alstonia boonei, such as the inhibition of Enoyl Acyl Carrier Protein Reductase in Plasmodium falciparum (PfENR) (Johnson et al., 2021). We employed molecular docking and ADME/T studies to investigate the mode of action of specific compounds from Alstonia boonei as an agonist for the TGR5 pathways in diabetes. This study examined some specific Alstonia boonei bioactive compounds, including codonamycin, isoquercitrin, alstonidine, quercetin, cyfluthrin, beta cyfluthrin, Nα formylechitamidine, echitamidine, kaempferol, porphyrin, loginin, and chlorohydroquinone interactions with TGR5. Our study confirms previous research showing that drugs with higher docking scores are more effective against disease. Specifically, the compounds from Alstonia boonei, such as isoquercitrin, chlorogenic acid, alstonidine, quercetin, beta-cyfluthrin, Nα formylechitamidine, etc. have high docking scores (−11.466, −10.661, −9.982, −9.661, −9.647, −9.647, etc.) kcal/mol, and binding free energy (MMGBSA score) for isoquercitrin, chlorogenic acid, alstonidine, and quercetin (−71.52, −71.28, −88.62 and −55.21 respectively) as well as an agonist to the TGR5 receptor protein (7BW0) (Tables 1–3). Based on the information provided, we can infer that isoquercitrin has the greatest MMGBSA and docking score compared to other compounds (Table 3). Based on docking scores, it can be observed that isoquercitrin is potentially, a very effective compound against diabetes, with docking scores following suit. Other compounds that interact with proteins through the formation of covalent and non-covalent chemical bonds include echitamidine, Nα formylechitamidine, alstonidine, quercetin, cyfluthrin, beta-cyfluthrin, Kaempferol, porphyrin, and Loganin (Cheng, 2018). When evaluating a novel drug’s or bioactive compounds efficacy, hydrophobic and hydrogen bond interactions are critical elements to take into consideration. The bioactive compounds binding affinity is also essential for the development of suitable pharmacologic and therapeutic qualities. Hydrogen bonds have a critical role in drug-receptor interactions as well as the structural stability of many bioactive compounds (Bekoe et al., 2020). Hydrogen bonds (H-bonds) are present between proteins and ligands and enhance a number of biological processes, as the hydrogen bond gets closer to faultless geometry, its strength increases (Johnson et al., 2021).
Hydrophobic interactions arise when proteins’ non-polar side chains come into direct contact with the lipophilic groups of the ligand. Research has demonstrated that hydrophobic interactions significantly boost the binding affinity of ligands with a high concentration of lipophilic groups (Hasan et al., 2022). Hydrophobicity affects a number of biological processes, such as the movement, dispersion, and metabolism of biological molecules. The bioavailability of bioactive compound molecules increases with an increase in the distance between hydrophobic interactions (Lucien et al., 2014). The selected compounds from Alstonia boonei exhibit lower bond distance (Å) in hydrogen bond interactions and higher distance values in hydrophobic interactions with the receptor at the active site (Figures 4–7). These values suggest that the compounds have a higher binding affinity for TGR5 receptor molecules and are also associated with relevant bioavailability properties. To make more precise assumptions, further preliminary research on ligand binding is needed. Toxicological analysis and pharmacokinetics studies are essential components of drug discovery (Johnson et al., 2021). Table 4 indicates that all the selected compounds, except isoquercetrin, have hydrogen-bond donor (donorHB) values not greater than seven and hydrogen-bond receptor (accptHB) values not greater than ten. This suggests that isoquercetrin has a higher binding affinity and better absorption, as demonstrated by its high lipophilicity, and a partition co-efficient of less than five (logP).
Drug-like molecules have molar refractivity between 40 and 130, molecular weight between 160 and 480 g/mol, and a logarithm of the partition coefficient (log P) between 0.4 and 5.6, which varies according to the molecule’s volume and molecular weight (Table 5) (Bekoe et al., 2020). In particular, compounds from Alstonia boonei, particularly isoquercitrin, chlorogenic acid, alstonidine, quercetin, beta-cyfluthrin, Nα formylechitamidine, echitamidine, Kaempferol, Porphyrin, Loganin, and chlorohydroquinone, docked with TGR5, fulfilled the requirements of Lipinski’s rules of five, including absorption analysis, distribution, metabolism, excretion, and shows good oral bioavailability (Lipinski, 2004). Additionally, a computer simulation study and the ADME analysis demonstrated that the compounds are safe because they do not violate more than one rule of Lipinski for druggability (except isoquercitrin). It takes clinical research to ascertain overall toxicity. This work demonstrates that intestine glucagon-like peptide-1 (GLP-1) can be released when TGR5 signaling is activated, leading to an increase in pancreatic activity and insulin release. The results of this study demonstrate that TGR5 is activated by agonists found in Alstonia boonei compounds, such as isoquercitrin, chlorogenic acid, alstonidine, quercetin, cyfluthrin, beta cyfluthrin, Nα formylechitamidine, echitamidine, kaempferol, porphyrin, loginin, and chlorohydroquinone, among others.
| 5. Conclusion | ▴Top |
The study reveals that the TGR5 signaling pathway plays a significant role in controlling intestinal GLP-1 production and insulin secretion. It also suggests that pharmacologically targeting the TGR5 receptor may provide a potential approach for the treatment of diabetes. These findings highlight the potent compounds of Alstonia boonei with significant activity and offer insight into its potential that could be explored as therapeutics in diabetes treatment.
Acknowledgments
Not applicable.
This research received no external funding
| References | ▴Top |