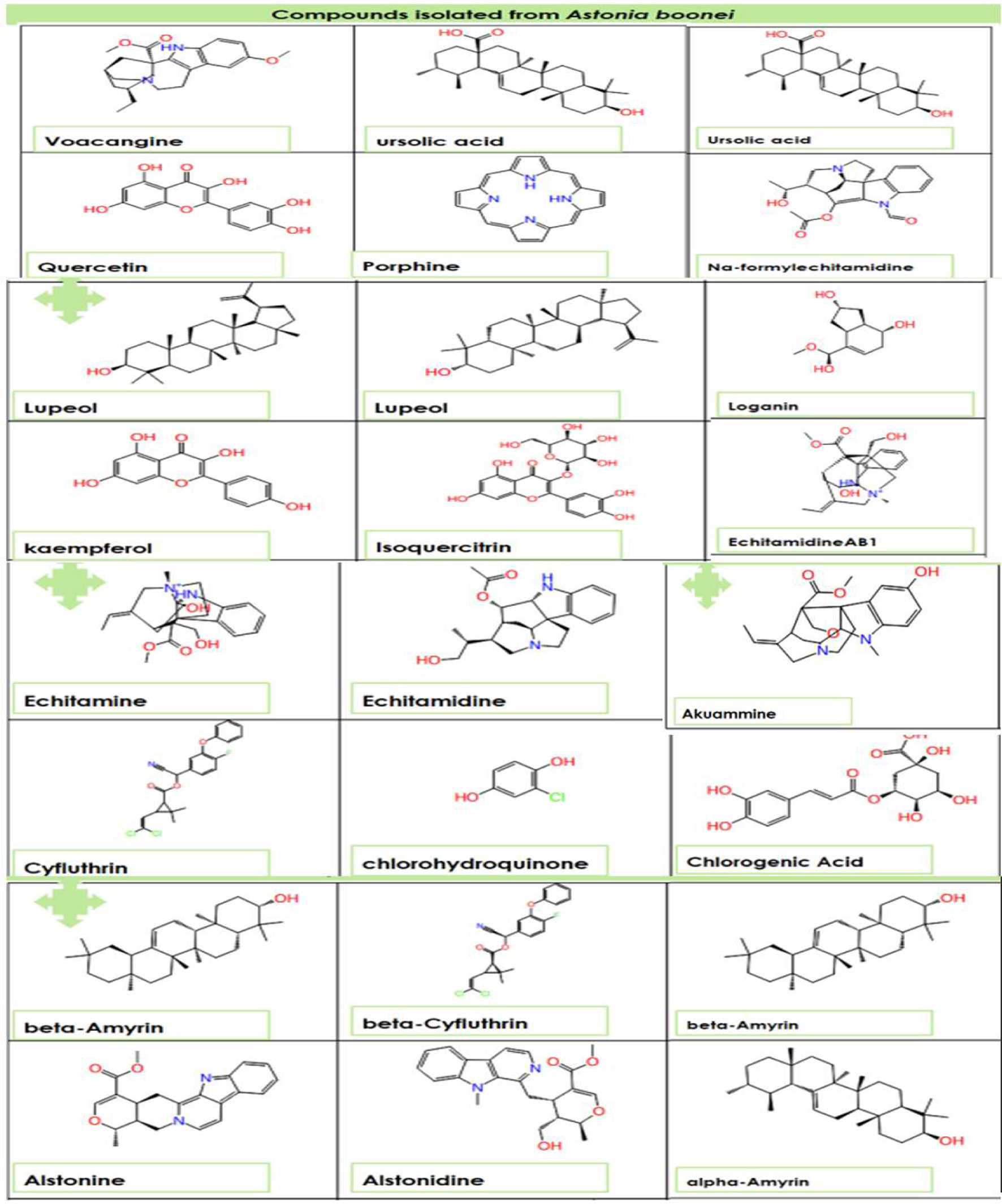
Figure 1. The two-dimensional structure of Alstonia boonei compounds.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 26, June 2024, pages 61-71
Exploring the therapeutic mechanisms of Astonia boonei in diabetes mellitus ligand-based virtual screening with TGR5 Receptor
Figures

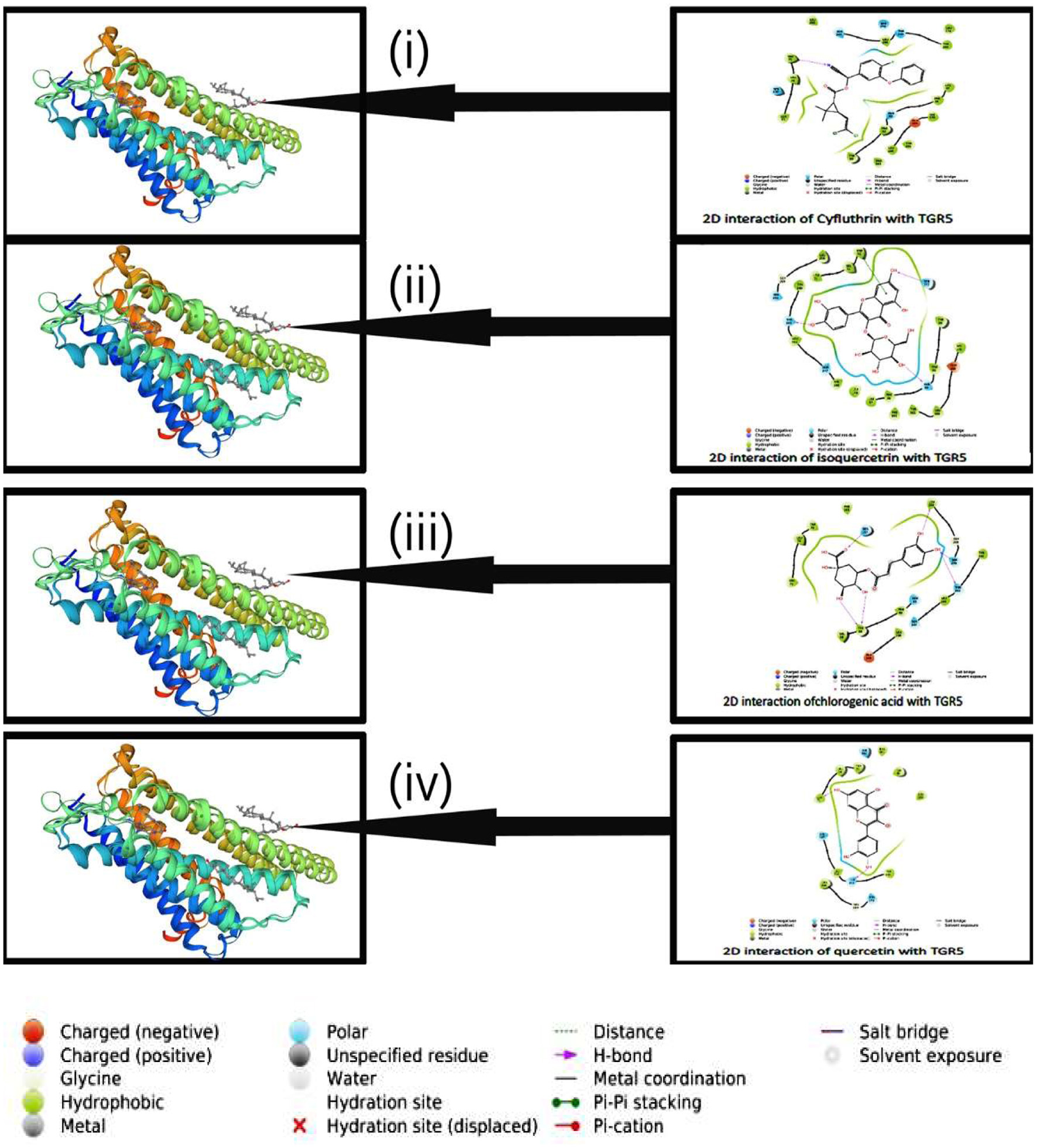
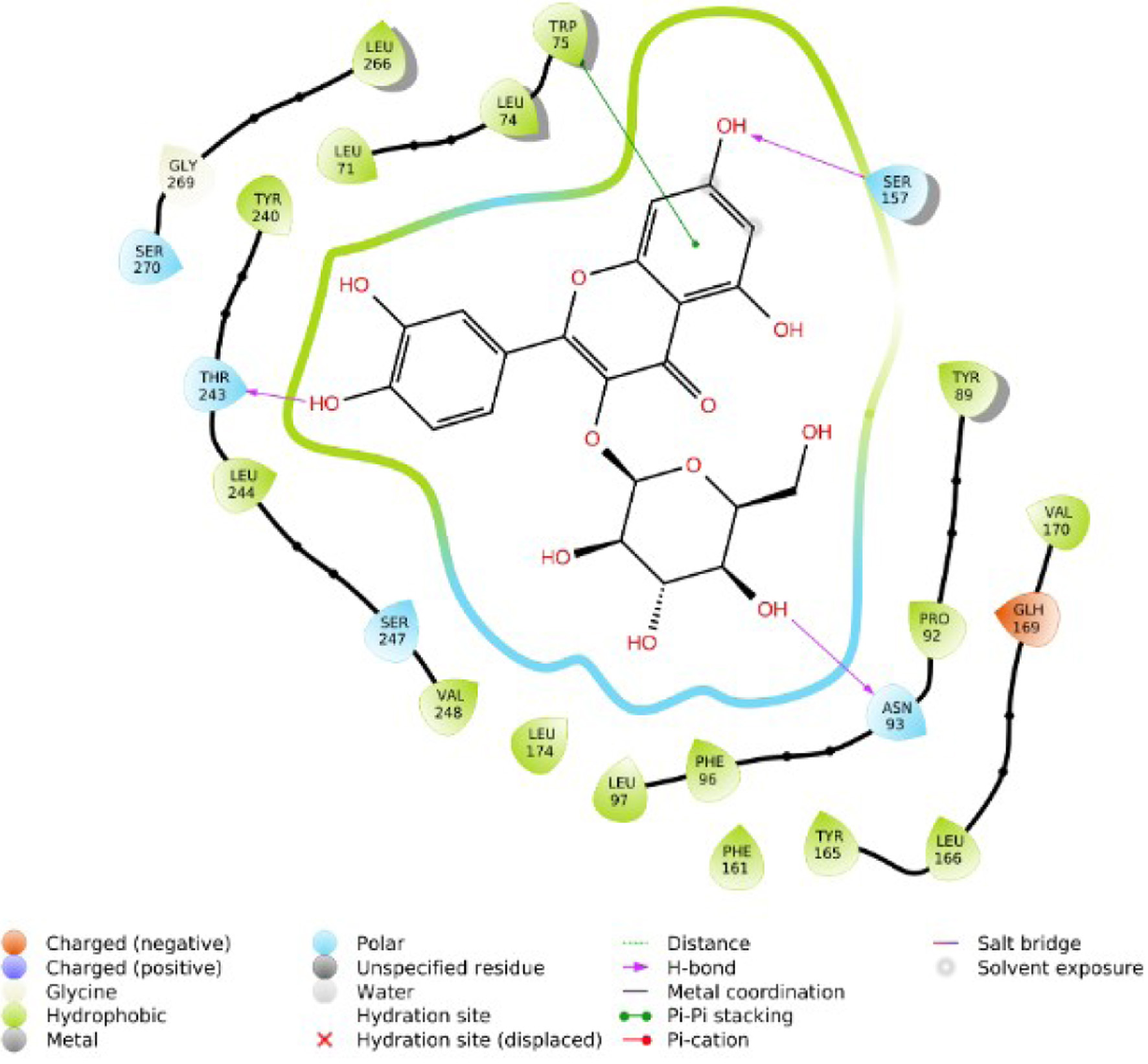
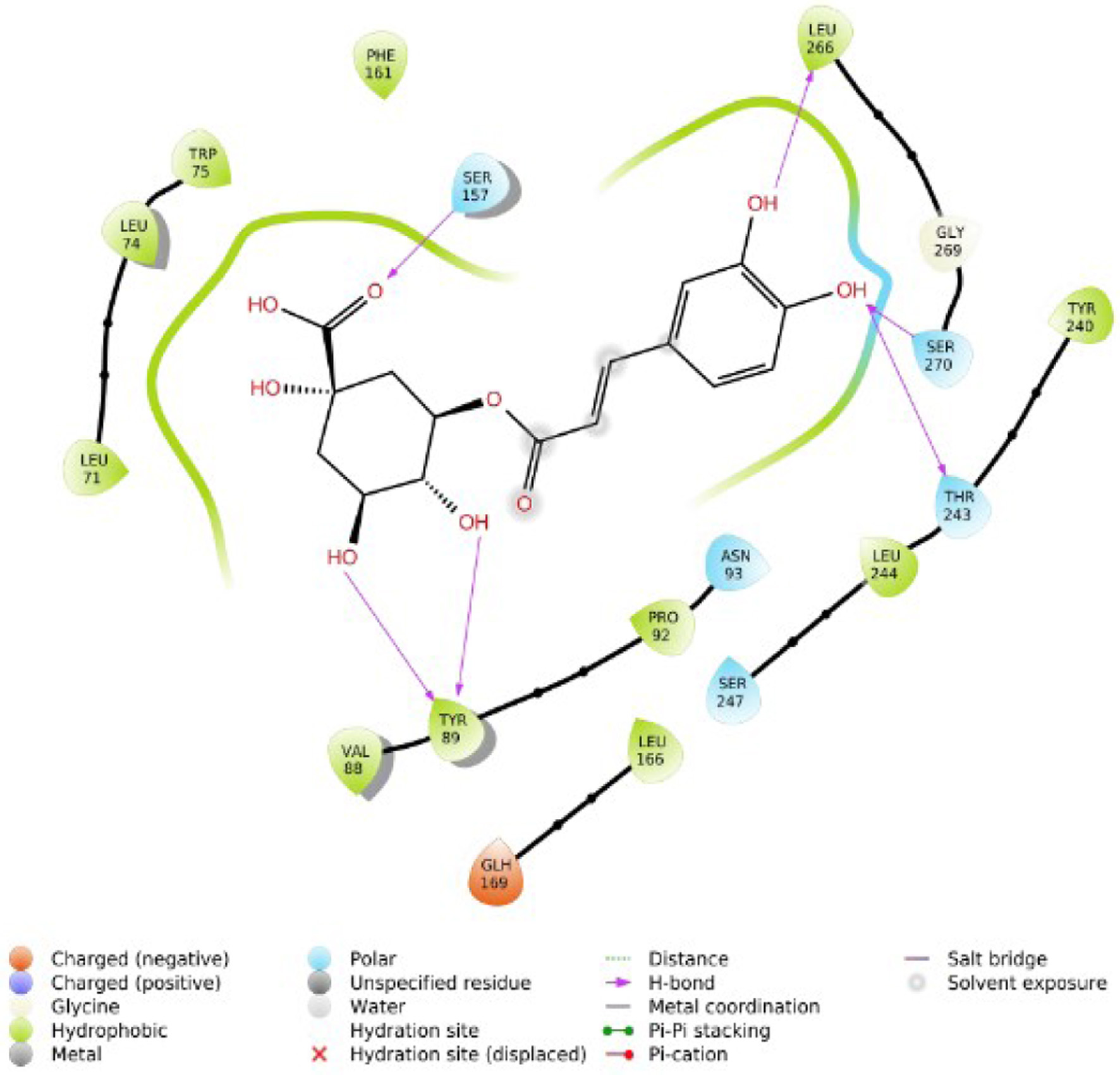
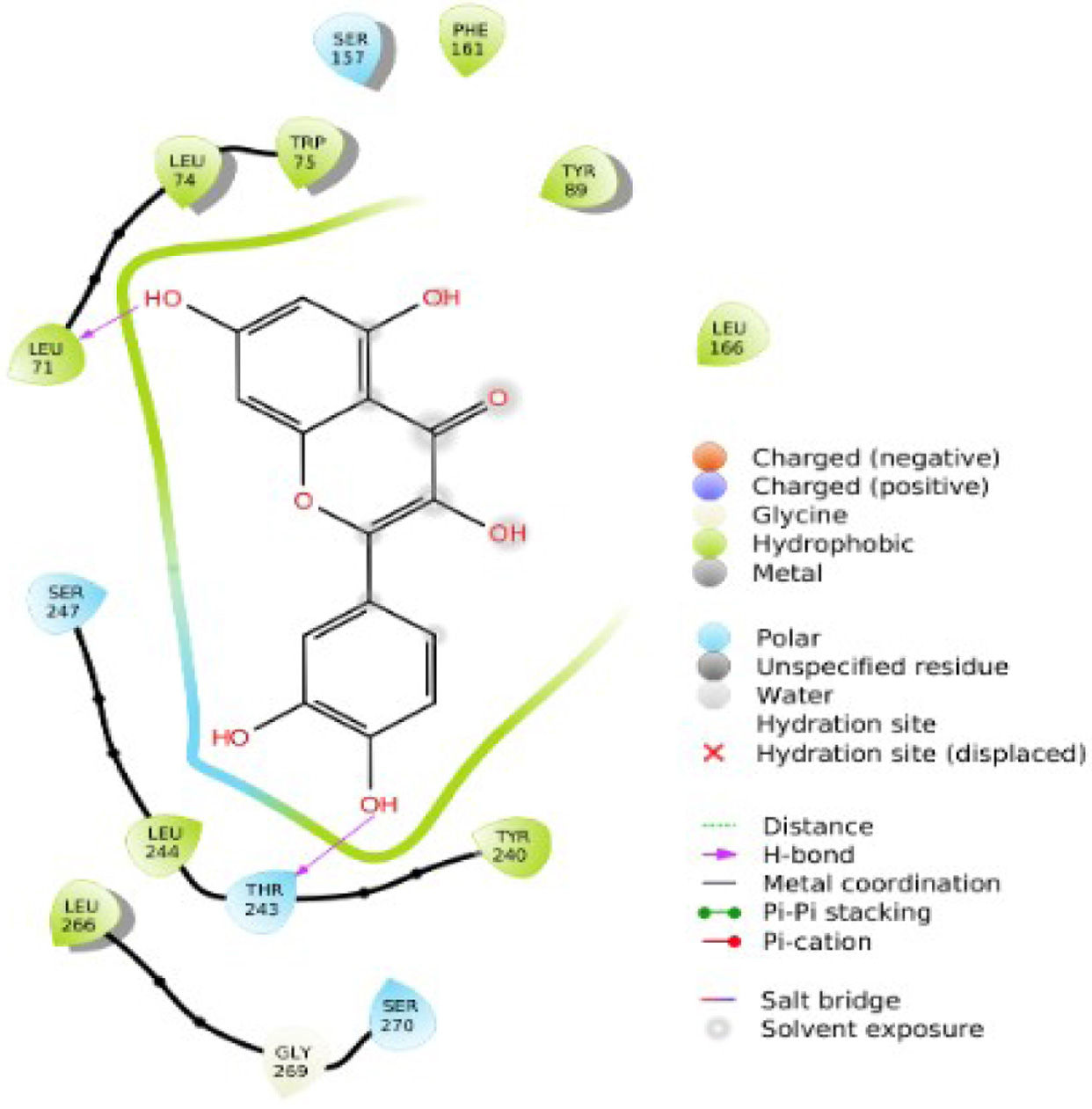
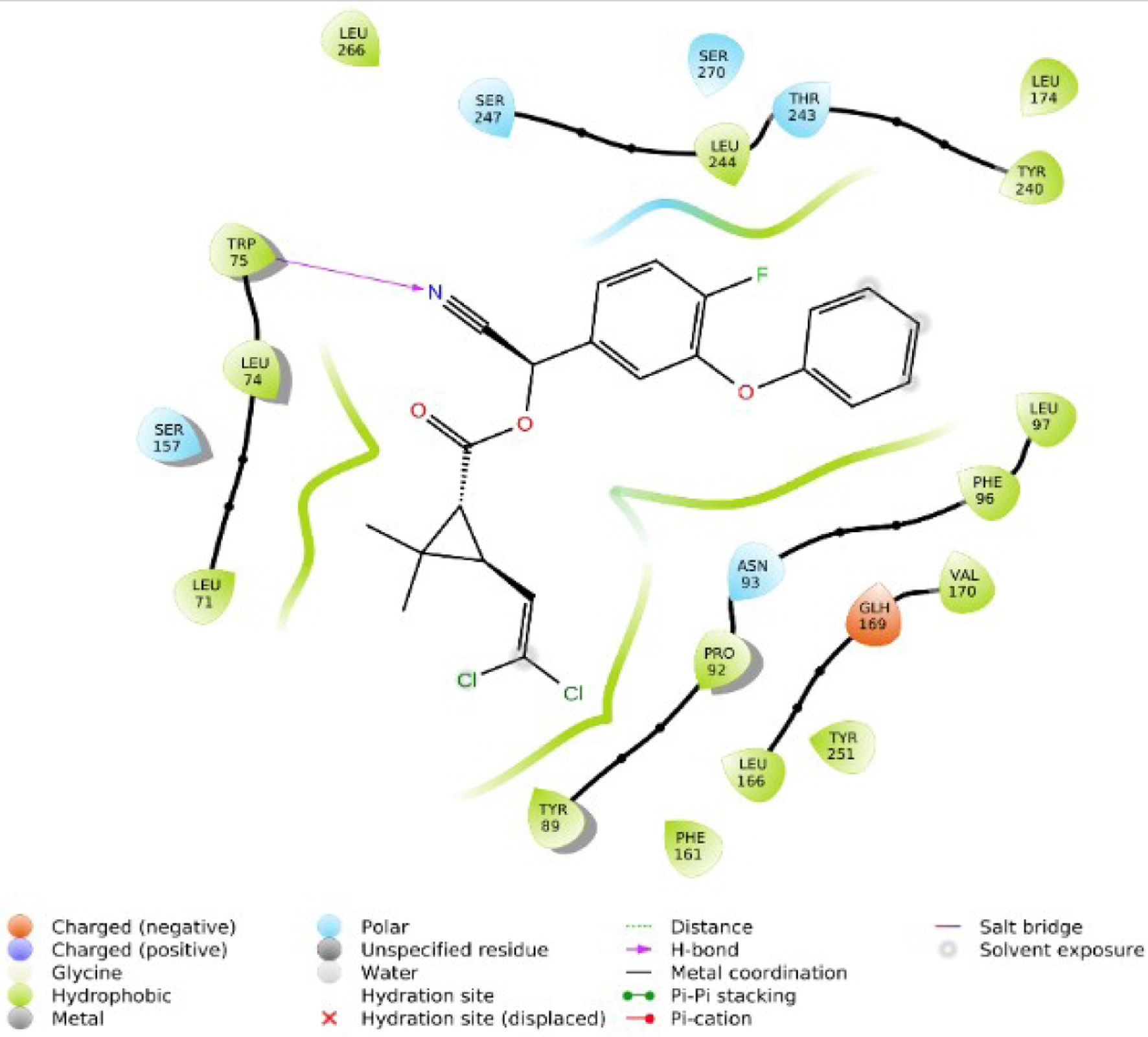
Tables
| Compounds | docking score (kcal/mol) |
|---|---|
| Isoquercitrin | −11.466 |
| Chlorogenic acid | −10.661 |
| Alstonidine | −9.982 |
| Quercetin | −9.661 |
| Cyfluthrin | −9.647 |
| Beta_cyfluthrin | −9.647 |
| Nα formylechitamidineAB3 | −8.773 |
| Echitamidine | −8.739 |
| Kaempferol | −8.643 |
| Porphyrin | −8.509 |
| Loganin | −6.544 |
| Chlorohydroquinone | −4.767 |
| Compounds | IFDScore |
|---|---|
| Chlorogenic acid | −533.89 |
| Isoquercitrin. | −531.15 |
| Alstonidine | −528.94 |
| Quercetin | −528.66 |
| Loganin | −527.87 |
| Porphyrin | −527.18 |
| Nα-formylechitamidine | −524.79 |
| Compounds | MMGBSA dG Bind | MMGBSA dG Bind Coulomb | MMGBSA dG Bind Covalent | MMGBSA dG Bind Hbond | MMGBSA dG Bind Lipo | MMGBSA dG Bind Solv GB | MMGBSA dG Bind vdW |
|---|---|---|---|---|---|---|---|
| Isoquercitrin | −71.52 | −37.34 | 8.22 | −1.32 | −37.01 | 31.9 | −35.37 |
| Chlorogenic acid | −71.28 | −21.33 | 2 | −1.24 | −33.54 | 23.9 | −41.06 |
| Alstonidine | −88.62 | −7.69 | 5.01 | −0.43 | −51.63 | 16.21 | −49.71 |
| Quercetin | −55.21 | −15.43 | 0.96 | −1.08 | −23.22 | 19.26 | −34.73 |
| Cyfluthrin | −90.97 | −8.41 | −0.63 | −0.32 | −47.02 | 15.6 | −50.1 |
| beta-cyfluthrin | −90.97 | −8.41 | −0.63 | −0.32 | −47.02 | 15.6 | −50.1 |
| Nα-formylechitamidin | −78.47 | −8.25 | 0.67 | −0.3 | −41.89 | 18.63 | −47.04 |
| Echitamidine | −74.72 | −1.22 | 2.4 | −0.03 | −48.1 | 18.95 | −46.43 |
| Porphyrin | −61.13 | 0.25 | 1.33 | 0 | −34.74 | 14.63 | −41.89 |
| Loganin | −49.2 | −14.7 | 1.3 | −0.49 | −29.36 | 15.21 | −21.16 |
| Chlorohydroquinone | −33.78 | −7.66 | 0.75 | −0.3 | −16.69 | 9.16 | −18.35 |
| Compounds | donorHB | accptHB | QPlogPo/w | RuleOfFive |
|---|---|---|---|---|
| Isoquercetrin | 7 | 13.75 | −1.308 | 2 |
| Chlorogenic Acid | 6 | 9.65 | −0.457 | 1 |
| Alstonidine | 1 | 6.4 | 3.771 | 0 |
| Quercetin | 4 | 5.25 | 0.355 | 0 |
| Cyfluthrin | 0 | 4 | 5.69 | 1 |
| beta-Cyfluthrin | 0 | 4 | 5.69 | 1 |
| Nα formylechitamidineAB3 | 1 | 9.2 | 1.397 | 0 |
| echitamidineAB1 | 2 | 6.7 | 1.822 | 0 |
| Kaempferol | 3 | 4.5 | 1.047 | 0 |
| Porphine | 2 | 3 | 4.261 | 0 |
| Loganin | 3 | 6.8 | 0.165 | 0 |
| Chlorohydroquinone | 2 | 1.5 | 0.883 | 0 |
| Compounds | Docking score (Da) |
|---|---|
| Isoquercitrin | 464.382 |
| Chlorogenic acid | 354.313 |
| Quercetin | 302.24 |
| Loganin | 214.261 |
| Chlorohydroquinone | 144.557 |
| Kaempferol | 286.24 |
| Voacangine | 368.475 |
| Cyfluthrin. | 434.293 |
| Akuammine | 382.458 |
| a-Amyrin palmitate | 454.778 |
| Kaempferol | 286.24 |
| echitamidineAB1 | 356.464 |
| Nα-formylechitamidineAB3 | 368.432 |
| Porphyrin | 310.357 |
| Echitamidin | 340.421 |
| Beta_cyfluthrin | 380.443 |
| Chlorohydroquinone | 144.557 |
| Lupeol | 426.724 |
| Beta_cyfluthrin | 380.443 |
| alpha-Amyrin | 426.724 |
| lupeolAB5 | 440.751 |
| b-Amyrin | 426.724 |