| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 26, June 2024, pages 42-51
Ultrasound-assisted a food-grade double emulsion system for encapsulation of vitamin E and omega-3
Yuqing Zhanga, Minfang Luoa, B. Dave Oomahb, Farah Hosseiniana, c, *
aChemistry Department, Carleton University, Ottawa, ON, Canada
b(Retired) Formerly with Summerland Research and Development Centre, Agriculture and Agri-Food Canada, Summerland, BC
cInstitute of Biochemistry, Carleton University, Ottawa, ON, Canada
*Corresponding author: Farah Hosseinian, Chemistry Department, Carleton University, 1125 Colonel By Drive, 318 Steacie Building, Ottawa, ON, K1S 5B6, Canada. Tel: 1-613-520-2600 x 2840; E-mail: FarahHosseinian@cunet.carleton.ca
DOI: 10.31665/JFB.2024.18379
Received: March 28, 2024
Revised received & accepted: May 31, 2024
| Abstract | ▴Top |
Double emulsions are widely employed to encapsulate oxygen sensitive compounds. Low-frequency ultrasound (20 kHz) was used to design a novel food-grade O/W/O double emulsion delivery system with TFE (tannins-free extracts) and 1% psyllium husk. Three and nine distinct formulations were created to form single and double emulsions, respectively. The optimal formulation was 81% sunflower oil and 19% psyllium husk (1% concentration) for double emulsion. The additional TFE delayed lipid oxidation in the emulsion, as indicated by the oxygen radical absorbance capacity (ORAC) values. Microstructural evaluation of the TEF double emulsion used optical microscopy and scanning electron microscopy (SEM). The TEF double emulsion showed a zeta-potential of 23.25 (mV) ± 3.465. The feasibility of TFE double emulsion delivery system was investigated by encapsulating vitamin E (α-tocopherol) and omega-3 fatty acids. The ORAC values of the TFE double emulsions containing omega-3 or vitamin E were 371.06 ± 15.31 and 378.32 ± 11.23 μM Trolox/g, respectively, and remained stable after 30 days of storage.
Keywords: Double emulsion; Ultrasound; Encapsulation; Antioxidation; Food supplements
| 1. Introduction | ▴Top |
Double emulsions, also known as multiple emulsions, are special type of emulsions wherein one emulsion is dispersed within another (Iqbal et al., 2015). They have gained significant attention across the food, pharmaceutical, and cosmetic industries due to their unique capacity to encapsulate and protect sensitive or active ingredients within their innermost layer (Iqbal et al., 2015; Luo and Wei, 2023). For example, in the food industry, double emulsions have been used to encapsulate bioactive substances such as vitamins, thereby enhancing their protection (Xia and Yao, 2011). The key of double emulsion production lies in the selection of both the appropriate emulsifier and the optimal emulsification technique. Psyllium husk is a natural source of dietary fiber and polysaccharides (Jalanka et al., 2019), with arabinoxylan as the predominant polysaccharide, comprising up to 70% of the total polysaccharide content (Qaisrani et al., 2016). Arabinoxylan is a hydrophilic polysaccharide with high water affinity enabling it to stabilize oil-in-water emulsions and thus an excellent natural emulsifier (Fu et al., 2022). Low-frequency ultrasound is a widely used technique for emulsification (Cabrera-Trujillo et al., 2016). The process involves the generation of acoustic cavitation, which creates series of high- and low-pressure waves in the liquid, resulting in the formation and collapse of tiny bubbles. The rapid collapse of these bubbles generates intense shear forces that can atomize the dispersed phase of the emulsion into smaller droplets, leading to a reduction in particle size (Udepurkar et al., 2023). The reduction in droplet size during ultrasonication increases the surface area of the dispersed phase, which can enhance the emulsion stability (Wang et al., 2022).
In double emulsions, oxidation of the oil phase in the emulsion can affect its stability, and in this study, natural polyphenols extracted from grape pomace were added in order to address this problem. (Muschiolik and Dickinson, 2017). Grape pomace, originating as the solid residue in grape juice and wine production, is frequently relegated to the status of waste by-product, overlooking its significant polyphenolic content (Bordiga et al., 2019). This composite material primarily consists of grape skins, seeds, and stems, containing high polyphenolic compounds, such as flavanols, catechins, anthocyanins, and proanthocyanidins, all known for their antioxidative attributes (Hegedüs et al., 2022; Khan et al., 2020; Pintać et al., 2018). However, the full potential of this resource remains largely untapped (Gemede and Ratta, 2014). One significant challenge in polyphenols extraction from grape pomace is the presence of tannins, which have negative sensory attributes, such as bitterness and astringency (Soares et al., 2020). It has been reported that excessive tannin intake may be associated with esophageal cancer. Moreover, tannins can bind to proteins and minerals reducing their solubility and bioavailability and limiting their absorption in the body (Fekadu Gemede, 2014). Therefore, tannins removal is crucial for the use of grape pomace extracts in the food industry.
Omega-3 rich oils are susceptible to lipid oxidation, which can result in off-odors and off-flavors (Li et al., 2020). Double emulsion (O1/W/O2) method can be used to protect omega-3 within the inner oil phase to prevent lipid oxidation. In this study, omega-3 and vitamin E were added to the sunflower oil inner phase (O1), with the TFE water continuous phase of primary emulsion (O1/W). After, the primary emulsion (O1/W) was added in the sunflower oil continuous phase of double emulsion (O1/W/O2) which is less sensitive to oxidation compared with primary emulsion (O1/W). This results in good oxidative stability while preserving the omega-3 functional properties (Dwyer et al., 2012).
In this study, nine different O/W/O double emulsions were prepared through ultrasound technology and optimized according to the microscopic morphology, diameter and stability of the double emulsion. Furthermore, lipid oxidation between emulsion surfaces is prevented by adding TFE to the aqueous phase. The lipid oxidation sensitive food supplements were encapsulated in the inner oil phase of the novel double emulsion system. Finally, the double emulsion system was investigated for storage (4 °C, 30 days) stability.
| 2. Materials and methods | ▴Top |
2.1. Materials and instruments
Sunflower oil was from Saporito foods (Montreal, Quebec, Canada). Psyllium husk was purchased from a local grocery store (Bulk Barn Foods Ltd Co., Ontario, Canada). Omega-3 was bought from Jamieson. Analytical grade Fluorescein, Trolox (6- hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH), rutin, and HPLC grade Vitamin E (α-tocopherol) were obtained from Sigma-Aldrich (Oakville, ON, Canada). Water was purified by Milli-Q Integral Water Purification System (EMD Millipore Corp.).
2.2. Optimization of psyllium husk concentration in primary emulsions
Psyllium husk was used as a stabilizer for emulsion formulations. Emulsions were created using psyllium husk concentrations ranging from 0.5% to 4% (w/w) to determine the optimal level. During the preparation, psyllium husk at 0.5% to 4% (w/w) concentrations was fully dissolved in 89.5% to 86% (w/w) of water at 85 °C, respectively. Then, 10% sunflower oil was added to the water phase, followed with mechanical stirring using a Tissuemiser (Fisher Scientific, Ontario, CA). Ultrasound (10 W, amplitude 30%, 40–60 seconds) was applied to prepare emulsion with above formulations.
2.3. Visual examination of emulsion stability
The fresh primary emulsion samples were poured into 5 mL glass tubes and sealed with a sealing film to assess their visual appearance and stability. The emulsions were subsequently stored at 4 °C. The visual observation of the emulsions and the extent of emulsification were then imaged after 3 weeks.
2.4. Optimization of primary and double emulsions
Double emulsion was performed by a two-step emulsification process. To optimize the morphology and characteristic of primary emulsions and double emulsions, different formulation was prepared according to Leong et al., (2017) with some modifications. Three different primary emulsions and nine different double emulsions were formulated (Table 1) and prepared using ultrasound. All primary emulsions were prepared under the ultrasound power 10 W, amplitude 30% for 40–60 seconds. All double emulsions were prepared under the ultrasound power 2 W, amplitude 10% for 10 seconds.
 Click to view | Table 1. Different formulation of primary and double emulsions |
2.5. Preparation of tannins-free grape pomace extracts (TFE)
Tannis-free grape pomace extracts were prepared according to previously described methods (Downey et al., 2007; Hosseinian and Beta, 2007) with some modifications. Briefly, freeze dried grape pomace (1 g) was mixed with 20 mL of acidified (1% acetic acid) 95% ethanol solution. The mixture was stirred for 6 hours at room temperature (23 °C) and centrifuged (4,000 RPM, 15 min, 23 °C). The collected supernatant was stored at −20 °C in the freezer. Each sample extraction was performed in triplicates.
Electrochemical treatment was used to remove tannins to prepare TFE. Electrochemical experiments were carried out using two parallel flat rectangular aluminum electrodes (30 × 6 cm) as anode and cathode according to the method of Hassoune et al. (2017). The active surface area was 21 cm2 (3.5 × 6 cm). The aluminum plates consisted of 99% Al. A DC power supply (Sky Toppower STP3010, Shenzhen Sky Toppower Technology Co., Ltd, China) was used to regulate electric current to the electrochemical cell. Electrodes were connected to the positive and negative terminals of the DC power supply.
The solution pH was 5.0 and its composition was 25 mL grape pomace extract and 25 mL distilled water. A magnetic stirrer (200 rpm) was used to maintain homogeneous mixing of the solution in the reactor. All experiments were carried out at 0 °C in an ice bath for 30 mins. At the end of the treatment, suspensions were allowed to stand for approximately 30 min to reach spontaneous separation of formed flocs. The liquid phase was then centrifuged at 10,000 rpm for 15 min and the supernatant kept for further use.
2.6. Preparation of double emulsions encapsulating grape pomace extracts, vitamin E (α-tocopherol) and omega-3
After obtaining the best emulsion formulation (section 2.4), the primary emulsion was prepared by dissolving vitamin E and omega-3 (1 g each) in the inner oil phase and the TFE in the aqueous phase. Then the same method was used to perform the double emulsion (Table 2). All emulsions were stored in a refrigerator at 4 °C for future experiments.
 Click to view | Table 2. Formulation of double emulsion after encapsulation |
2.7. Particle size analysis
Particle size of all single emulsions and double emulsions were determined by polarized light microscope (Axioplan 2 imaging and Zeiss Axiophot 2 universal microscope, Carl Zeiss Inc., Jena, Germany). The images were taken with a Retiga 1300 camera linked to Northern eclipse software. The dispersed particle size from the images was analyzed via Image J software.
2.8. Polarized light microscope (PLM) of emulsion
All emulsions were examined under PLM. The images were taken with a Retiga 1300 camera linked to the Northern eclipse software.
2.9. Cryo-scanning electron microscope of emulsion
The surface distribution and structure of the fresh single and double emulsions were performed with cold field emission scanning electron microscopy (Nano Imaging Facility Laboratory of Carleton University, Ottawa, ON). The fresh emulsion was diluted 10 times with distilled water, and emulsions (100 μL) were transferred to a plate holder and immediately frozen in liquid nitrogen. Then the emulsion was sublimated for 20 min, and finally, the micro-morphology images were captured by SEM at 5 kV accelerating voltage.
2.10. Measurement of emulsion droplet ζ-potential
The ζ-potential values of the droplets in the emulsions were measured using a zeta-potential analyzer (Malvern Nano-ZS Zetasizer) at 20 °C. The emulsions were diluted 1:100 using distilled water in an electrophoresis cell that had electrodes at both ends. The measurements were done one night after emulsion preparation.
2.11. Oxygen radical antioxidant capacity (ORAC) assay
In the modified ORAC assay (Gunenc et al., 2013), all reagents were prepared with ORAC working buffer (potassium phosphate buffer pH 7.4), including different concentrations of Trolox standard solutions (100, 50, 25, 12.5 and 6.25 mΜ), fluorescein working solution (0.068 μM), rutin control solution (10 μM), and 153 mM AAPH. Phenolic extracts (5 mL) were dried, dissolved and diluted by ORAC working buffer. Trolox standards 20 μL, rutin control, fluorescein working solution (120 μL) and diluted sample were added into wells of a 96 micro-well plate respectively, and the plate was inserted into the fluorescence reader. After incubation (30 min, 37 °C), 60 μL of AAPH (153 mM) was added to each well, and the total volume of each well was 200 μL. ORAC working buffer 200 μL was used as blank. The experimental results of samples were recorded as μM TE (Trolox equivalent)/g of sample, since they were calculated from the Trolox standard curve.
2.12. Statistical analysis
All results were analyzed using IBM SPSS Statistics software (IBM corp, Armonk, New York, USA). Significant difference between triplicate values (3 individual runs) was determined using statistical analysis of variance (ANOVA) with one-way ANOVA. A P-value inferior to 0.05 indicated a significant difference among triplicate values. The mean values were then compared using Duncan’s Multiple Range test with a = 0.05.
| 3. Results and discussion | ▴Top |
3.1. Psyllium husk concentrate optimization
Previous research indicated that the stability of primary emulsions significantly affects the stability of double emulsions (Ding et al., 2019). To maintain the stability of the emulsion, psyllium husk was used as the stabilizer in this study (Gharibzahedi et al., 2013). Therefore, it is important to determine the optimal concentration of psyllium husk that can sustain and prolong the stability of the primary emulsion to achieve the successful formation of double emulsions. Different concentrations of psyllium husk in primary emulsions (O/W) were formulated to find the optimal psyllium husk concentration. Figure 1a shows the visual observation of the primary emulsion formed by different concentrations of psyllium husk.
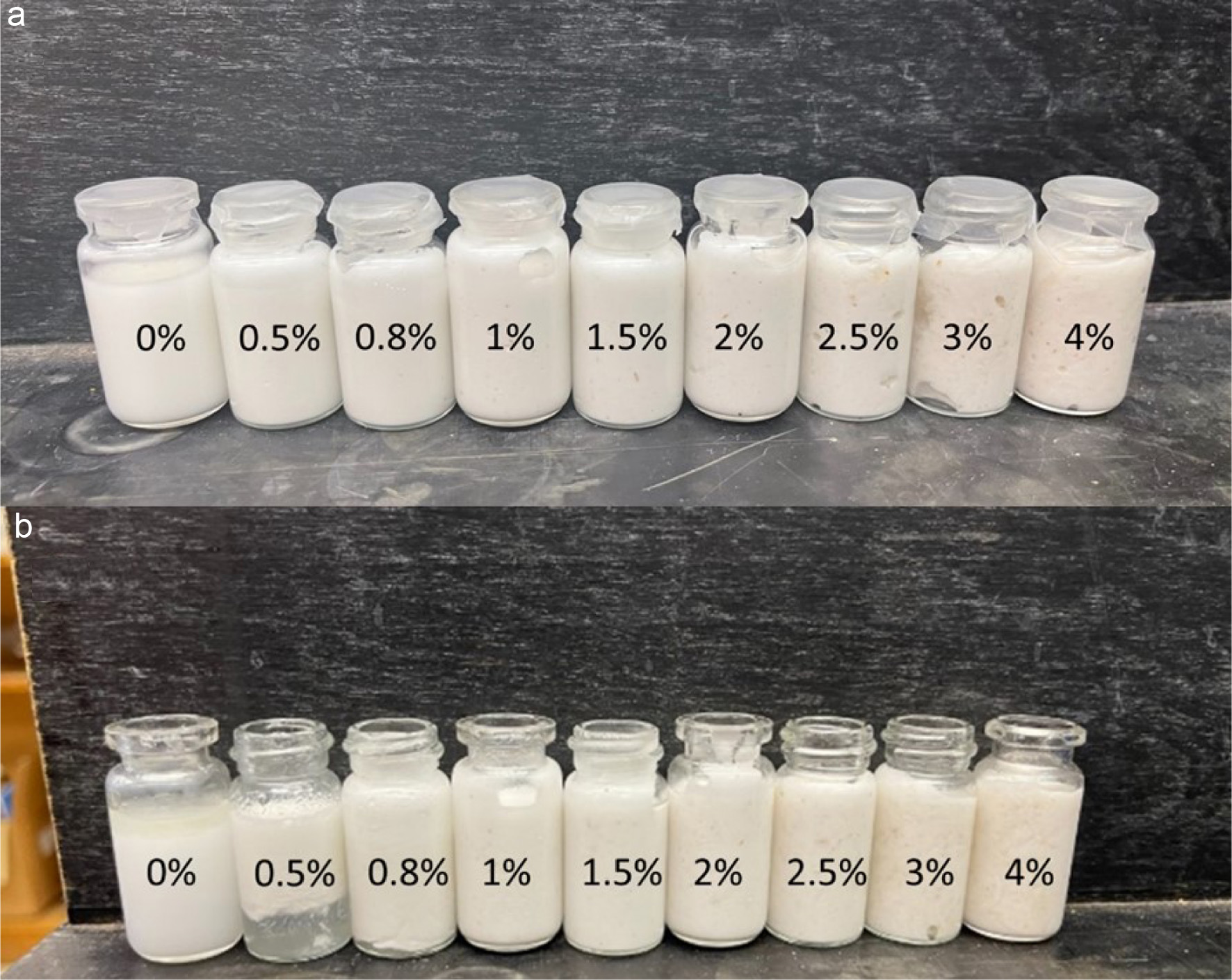 Click for large image | Figure 1. (a) Primary emulsions with 0%, 0.5%, 0.8%, 1%, 1.5%, 2%, 2.5%, 3% and 3.5% psyllium husk in sunflower oil. (b) Primary emulsions in Figure 1.A after 3 weeks storage in 4 °C refrigerator. |
Emulsions prepared with psyllium husk (0% to 1%) were in a liquid and flowing state, while those prepared at higher concentrations (1.5% to 4%) became increasingly thicker, and eventually reaching a highly viscous state with no flow at 4% psyllium husk.
Figure 1b shows the primary emulsions after three-week refrigerated storage (4 °C). The emulsion without psyllium husk showed clear oil separation on the upper layer of the emulsion, while the emulsions containing 0.5% and 0.8% psyllium husk concentrations showed slight separation phase. Increasing psyllium husk concentration to 1% stabilized the emulsion without significant separation after three-week storage. These results demonstrated that increasing the psyllium husk concentration improved the storage stability of the emulsions. This improvement can be attributed to the presence of arabinoxylan from psyllium husk in the aqueous phase, which increased the viscosity of the continuous phase and promoted network structure formation in the emulsion (Zhou et al., 2022). This network structure delayed the mobility of the oil droplets and eventually stabilized their coalescence, thus contributing to the overall emulsion stability.
Fu et al. (2022) reported increased viscosity with increase in psyllium husk concentration in emulsions. However, primary emulsions with high viscosity are challenging to manipulate during the subsequent formation of double emulsions due to the difficulty in homogeneously mixing the oil and water phases (Liu et al., 2021). Therefore, the concentration of 1.5–4% psyllium husk was not considered the optimal concentration; instead, 1% psyllium husk was selected to form the primary emulsion.
3.2. Polarized light microscopy of emulsions
The microstructure of primary emulsions is one of the key factors affecting the stability of primary and double emulsions. Optical microscopy allows direct observation of the morphology, droplet size, and distribution, which are important parameters to evaluate emulsion stability.
Figure 2a and b show the morphology of primary emulsion droplets formed by psyllium husk and sunflower oil, with and without ultrasonic homogenization. Both emulsion with and without ultrasonic homogenization exhibited spherical-shaped droplets. However, the emulsion droplets formed without sonication (Figure 2a) were large and non-homogeneous. In contrast, droplets formed with sonication (Figure 2b) were significantly smaller in diameter and more homogeneous, with a greater number of droplets.
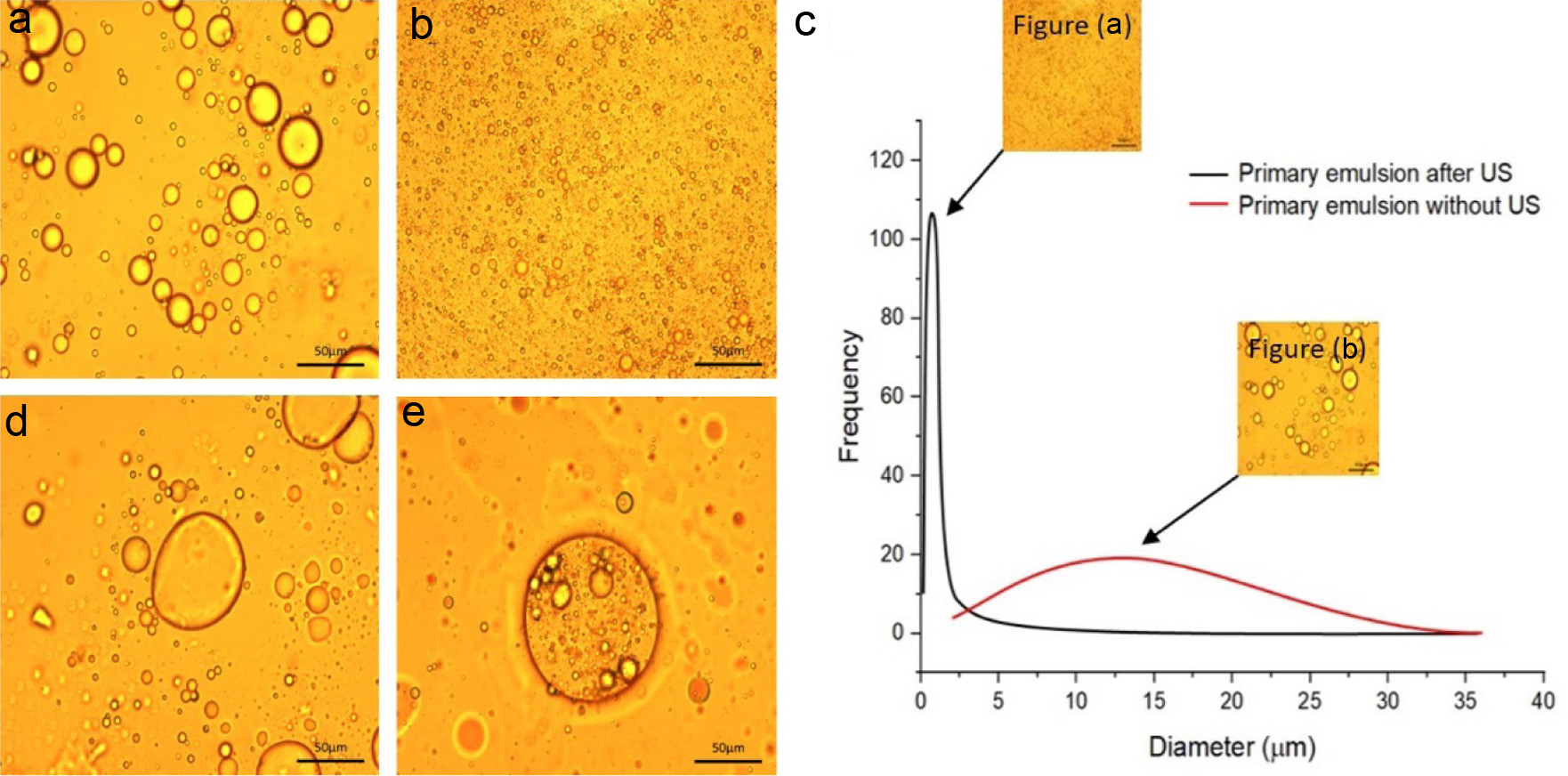 Click for large image | Figure 2. Optical microscope images of emulsions. (a) Primary emulsion without ultrasound treatment. (b) Primary emulsion with ultrasound treatment. (c) Particle size analysis of primary emulsions. (d) Double emulsion without ultrasound treatment. (e) Double emulsion with ultrasound treatment. |
Figure 2c shows the droplet diameter and distribution frequency of the primary emulsion both before and after ultrasonic homogenization. Notably, the particle size distribution of the emulsion system exhibits a single peak, which becomes sharper and shifts progressively towards smaller droplet sizes after ultrasonic homogenization. The average particle size of primary emulsion before ultrasound treatment was 15.85 ± 8.29 μm and it decreased to 3.28 ± 0.95 μm after ultrasound treatment. These results confirm that ultrasonic homogenization effectively reduces the diameter of the emulsion droplets and leads to a uniform distribution.
Particle size and distribution are important factors that can influence the stability and other functional properties of emulsions, such as potential biological fate of delivery system (Salvia-Trujillo et al., 2013). A small particle size and uniform particle size distribution, in particular, can significantly improve emulsion stability. Because small droplets have large interfacial area, they have stronger intermolecular interactions and are more resistant to coalescence (Canselier et al., 2002; Costa et al., 2020). Furthermore, uniform particle size distribution can reduce the presence of large droplets prone to sediment, resulting in a stable emulsion (Hwangbo et al., 2022). Therefore, controlling particle size and distribution is important to optimize emulsion properties for industrial applications.
The effect of ultrasonic treatment on the formation of double emulsion was also investigated (Figure 2d and e). The high-speed mixer failed to produce a double emulsion (Figure 2d). However, the formation of double emulsion droplets encapsulating multiple primary emulsions (Figure 2e) is clear after ultrasonic treatment, indicating that ultrasound is effective in facilitating the formation of double emulsions. Therefore, the primary emulsions can be protected by the double emulsion in the oil continuous phase.
As shown in Table 1, different formulations of the primary and double emulsions were prepared to optimize the morphology observed under the microscope. Figure 3b shows the best primary emulsion under microscope with 10% sunflower oil, 90% of psyllium husk (1% concentration), and ultrasonication (US) conditions (40–60 s, 10 W and 30% amplitude). The primary emulsion with 10% loaded oil had small and uniform droplet diameter, large and dense number of droplets, and spherical droplet morphology. However, the microscopic morphology (Figure 3a) of the primary emulsion with 5% oil loading formed few primary emulsion droplets due to low oil content.
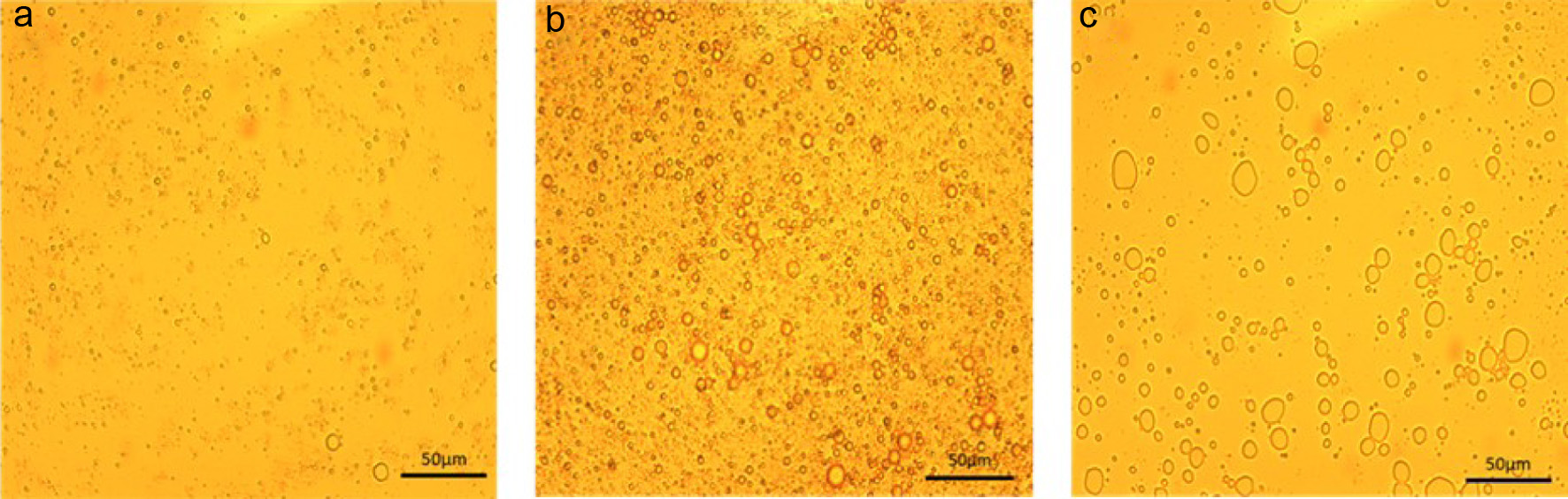 Click for large image | Figure 3. Microscopic observation (500×) of primary emulsion with different loaded oil content after ultrasound treatment. (a) 5% oil in primary emulsion (b) 10% oil in primary emulsion (c) 20% oil in primary emulsion. Dark line indicates length of 50µm. |
The primary emulsion with 20% oil content (Figure 3c) formed an irregular shape and larger diameter emulsion compared with Figure 3a and b, which was not conducive to the formation of double emulsions. The PLM images of double emulsions with varying primary emulsion contents were compared and analyzed in Figure 4. All DEs (double emulsions) with 20% primary emulsions displayed double emulsion morphology, as evidenced by Figure 4c, f, and i. Furthermore, DEs formed using different quantities of primary emulsions, showed that Figure 4f and 4i exhibited more primary emulsion droplets encapsulation than Figure 4c, making them more suitable for use as a delivery system. Notably, Figure 4f yielded greater overall number of double emulsions than Figure 4i, suggesting that it was more suitable as the optimal ratio. These findings support the conclusions (Figure 4), which recommended selecting primary emulsions with 10% oil content and also confirmed that the formation of smaller and more homogeneous primary emulsions was essential for double emulsion preparation. In conclusion, Figure 4f was chosen as the best formulation for double emulsion system which contains 81% oil and 19% of a 1% psyllium husk solution.
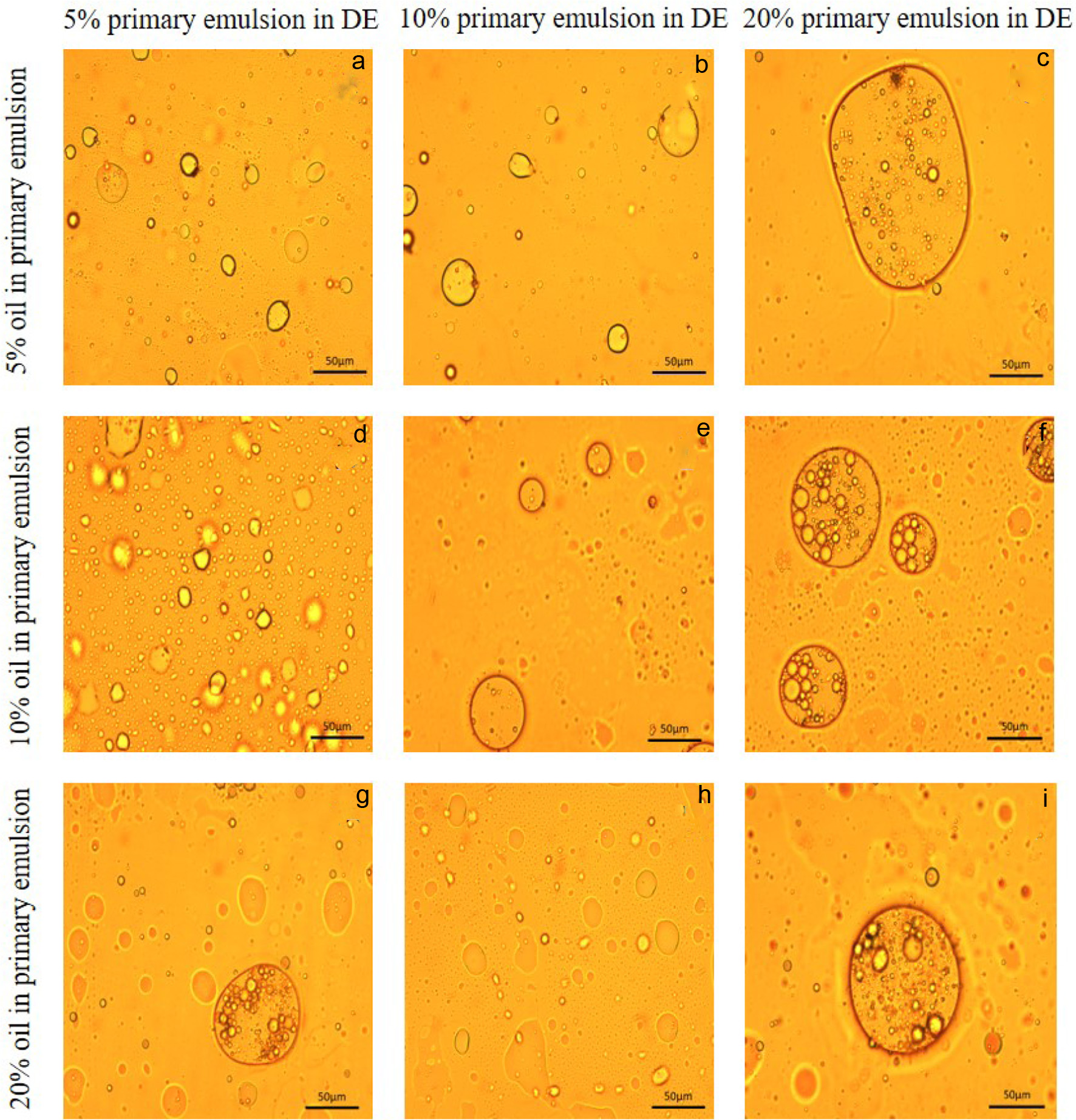 Click for large image | Figure 4. Microscopic observation (500×) of 9 different formulations double emulsion after ultrasound treatment. Dark line indicates length of 50 µm. |
The effect of the grape extract on the morphology of the O/W/O double emulsion was evaluated by adding it to the aqueous phase. Figure 5a showed that the morphology of the double emulsion was unaffected by TFE addition, as it remained unchanged under the microscope. Additionally, the double emulsion still demonstrated the ability to encapsulate multiple droplets of the primary emulsion after incorporating the extract into the aqueous phase. To confirm that the double emulsion could act as a delivery system, oxygen-sensitive oil-soluble substances (vitamin E and omega-3) were added to the inner oil phase. Figure 5b shows the morphology of the double emulsion encapsulated with vitamin E and Figure 5c shows the morphology of the double emulsion encapsulated with omega-3 under the microscope. Encapsulation of sensitive substances in the inner oil phase did not have a significant impact on the overall morphology of the double emulsion, and a considerable amount of primary emulsion droplets were still encapsulated within the double emulsion.
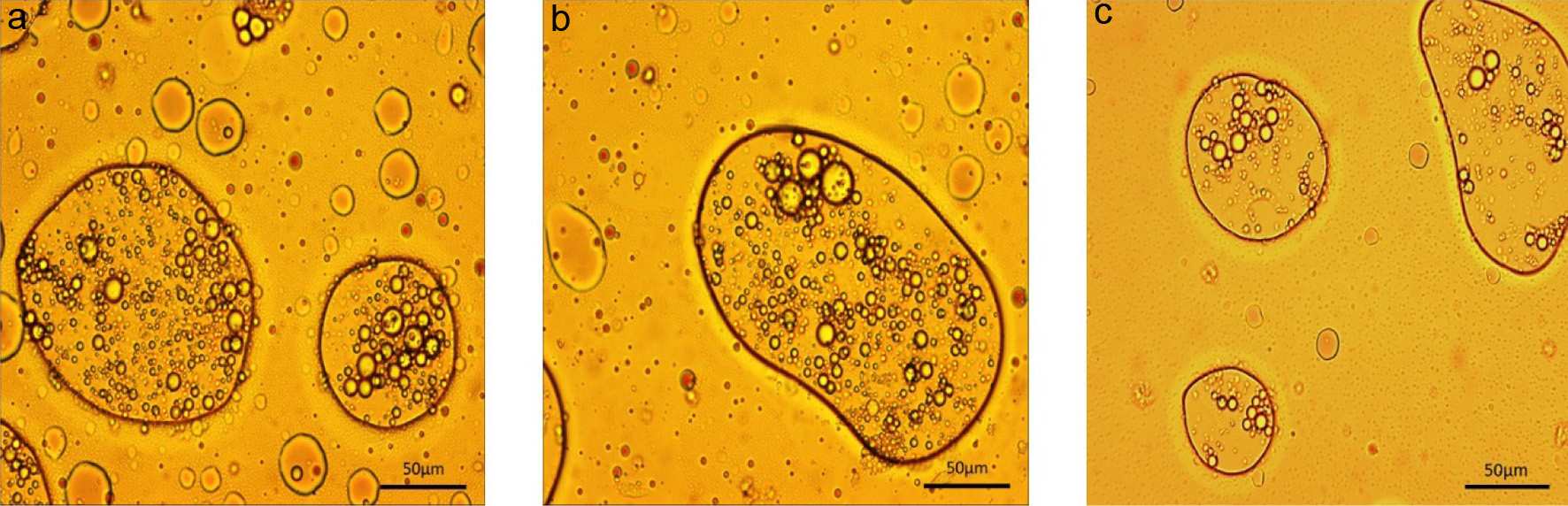 Click for large image | Figure 5. O/W/O double emulsion with TFE after ultrasound treatment with 81% oil, 1% psyllium husk and 18% water (a) encapsulating Vitamin E (b) and Omega-3 (c). |
3.3. Cryo-SEM observation
Cryo-SEM was used to examine the single and double emulsions after freezing in liquid nitrogen to investigate the surface morphology of double emulsions and the structural morphology of psyllium husk in single and double emulsions. The single emulsions (Figure 6a) exhibited a “network” structure composed of long-chain psyllium husk polymers. This property enables psyllium husk to function as an excellent stabilizer and emulsifier, leading to the formation of stable emulsions. Psyllium husk incorporation into the aqueous phase, increased the viscosity of the solution enabling psyllium husk to form a “network” structure that immobilizes the emulsion droplets (Fu et al., 2022). As a result, the single emulsions can maintain their stability over an extended period.
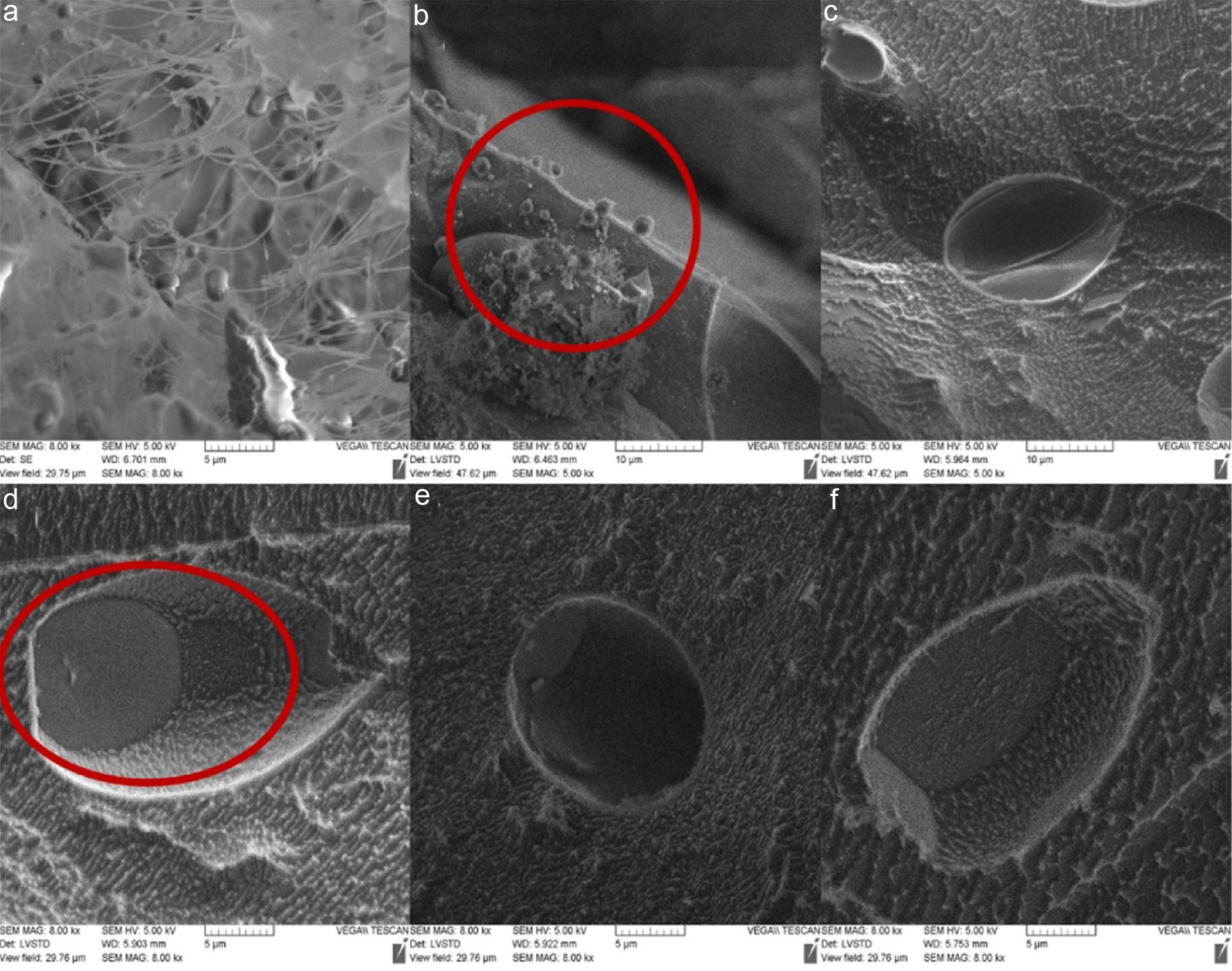 Click for large image | Figure 6. Cryo-SEM images of emulsions. (a) 1% concentration psyllium husk O/W single emulsions. (b) Single emulsion encapsulated inside double emulsion. (c) The overall shape of double emulsion under SEM. (d–f) The cross-sectional morphology of double emulsion under SEM. |
The sample was randomly cut after liquid nitrogen freezing to investigate the external shape and internal morphology (Figure 6); this also confirmed that double emulsion could protect single emulsions. Several tiny single emulsion droplets (Figure 6b, highlighted by red circle) were released from the larger double emulsion due to external force applied during the cutting process. The overall morphology of the double emulsion (Figure 6c) was similar to the morphology observed under PLM (Figure 4), with a larger size and an irregular, ellipsoidal shape. In contrast to PLM images, the size of the double emulsion droplets in the SEM images was slightly smaller. This may be due to the fact that larger size double emulsions are prone to splitting, whereas smaller size double emulsion droplets are better preserved during the cutting process. The overall size is smaller than that observed under PLM because the main observation area is in the cut and broken emulsion part, which necessitates a smaller size to enable observation of the internal structure of the double emulsion.
Figure 6d–f shows the internal morphology of a cross-sectional double emulsion at a larger magnification. It is evident in Figure 6d that a small single emulsion (highlighted by red circle) in the double emulsion, which again proves its viability as a delivery system.
3.4. Zeta potential values
Zeta-potential is the surface electrical characteristic of colloidal particles present in a liquid medium. This property indicates the extent of electrical attraction or repulsion that occurs at the particle surface, based on the potential values (Lunardi et al., 2021). A high zeta-potential value indicates great stability of particles without any agglomeration. Conversely, when particles coagulate due to Brownian motion, their stability is reduced resulting in low repulsive force between them and ultimately, a decrease in the zeta-potential value (Roldan-Cruz et al., 2016).
The zeta potential mean values of the primary and double emulsions were −28.3 (mV) ± 1.68 and 23.25 (mV) ± 3.465. According to Mirhosseini et al., 2008 an absolute value greater than 25 mV indicates deflocculated emulsions. Both primary and double emulsions had the absolute zeta-potential values higher than 25 mV, indicating that both emulsions were stable. Similar results were reported previously (Mudrić et al., 2019; Wang et al., 2010; Mirhosseini et al., 2008).
3.5. Antioxidant activity of emulsions (ORAC assay)
The antioxidant values of single and double emulsions encapsulating different oxygen-sensitive oil soluble substances (vitamin E and omega-3) were determined to investigate the potential of double emulsions as superior protective delivery systems compared to single emulsions. The antioxidant values of vitamin E, with and without emulsion system protection were compared. The results (Figure 7a) showed that without emulsion system protection, vitamin E had the highest fluorescence decay, indicating that it effectively scavenged the free radicals generated by AAPH. However, when vitamin E was encapsulated in single or double emulsions, the protective layer from the emulsion minimized the reaction between vitamin E and AAPH, resulting in significantly lower florescence decay. During ORAC test, fluorescence decayed because AAPH reacts with fluorescence generating non-fluorescent product (Prior, 2015). However, in vitamin E presence, the hydrogen atom from its functional group (OH) enables highly reactive AAPH free radicals, resulting in inhibited fluorescence decay (Litescu et al., 2014). Therefore, the lowest fluorescence decay was obtained after double emulsion encapsulation, indicating that double emulsions provided the strongest protection for the inner oxidation sensitive substance (vitamin E) against oxidation. Additionally, Martinez-Olivo et al. (2023) demonstrated that double emulsion can control antioxidant release exerting antioxidant capacity. A similar trend was observed with omega-3 (Figure 7b), where the highest fluorescence decay was obtained for omega-3 without emulsion system protection, while the lowest fluorescence decay was obtained for omega-3 encapsulated in the double emulsion system. This indicates that emulsions can protect the oxygen-sensitive oil-soluble components and can delay oxidation. Comparing single and double emulsion protection abilities, double emulsions have stronger protective effect.
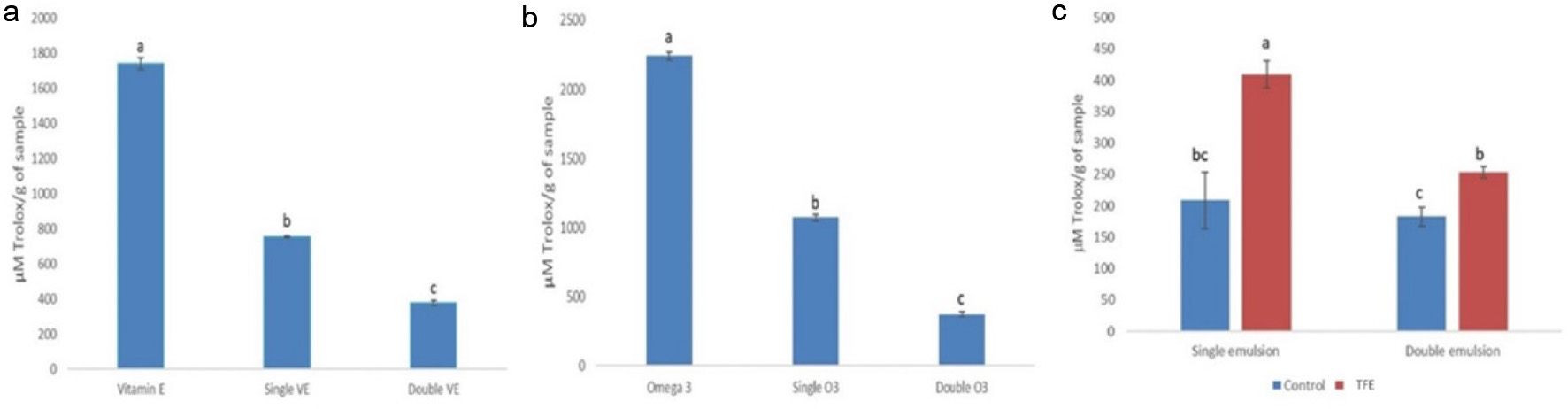 Click for large image | Figure 7. (a) Comparison of ORAC value of vitamin E, single emulsion encapsulating vitamin E and double emulsion encapsulation vitamin E. (b) Comparison of ORAC value of omega-3, single emulsion encapsulating omega-3 and double emulsion encapsulation omega-3. (c) Comparison of ORAC value of single and double emulsion before (blue bars) and after (red bars) loading TFE extracts. Different letters represent significant different at p < 0.05. |
Figure 7c shows the comparison of single and double emulsions antioxidant activities with and without TFE. The ORAC values of both single and double emulsions containing TFE were significantly (p < 0.05) higher than that of emulsions without TFE. The ORAC method shows the ability of an antioxidant to inhibit the peroxyl-radical-induced oxidation. Higher ORAC values indicate better antioxidant ability. Lipid oxidation mainly occurs at the droplet interface in emulsions (Jacobsen, 2016). TFE are rich in bioactive compounds such as anthocyanins, the antioxidant potential linked to phenolic compounds can be attributed to their ability to deactivate free radicals through hydrogen and electron transfer reactions (Mattos et al., 2017). The lipid oxidation reaction comprises three primary phases, initiation, propagation, and termination. These phases generate free radicals, hydroperoxides, and secondary volatile products such as aldehydes, ketones, and alcohols. These compounds are responsible for the development of off-flavors and the typical rancidity associated with lipid oxidation (Damodaran et al., 2007). TFE antioxidants have the ability to hinder the initiation phase and/or halt the propagation phase of lipid oxidation. Alternatively, antioxidants can also sequester free radicals from the system (Athukorala et al., 2003; Brewer, 2011).
DE encapsulating vitamin E and omega-3 emulsions were stored in the fridge (4 °C, 30 days) to test ORAC value and DE stability. Figure 8 compares antioxidant values between the double emulsion with vitamin E or omega-3 and double emulsion with free vitamin E or omega-3. It is clear that the antioxidant values fluctuated slightly from 1 to 7 days, which may be due to the partial rupture of DE to release the inner layer of vitamin E and omega-3. The overall antioxidant values did not change significantly from day 1 to day 30, which indicates that DE remained stable during the 30-day storage period. Similar results were reported previously (Jolayemi et al., 2021). Additionally, both DE encapsulated vitamin E and omega-3 had higher antioxidant value than the DE with free encapsulation. This indicates DE with vitamin E and omega-3 is valuable to use in food industry and supplement. Moreover, rheological and physical characteristics can be used for emulsion stability that are related to oxidative stability and shelf life of emulsions (Kargar et al., 2011). In this study, beside ORAC (chemical test), physical stability assessments including zeta-potential values (Section 3.4), diameter sizes (Figure 2) and morphology observations (Figures 5 and 6) were used to evaluate emulsion stability. The results were in agreements to those reported in literatures (Tong et al., 2021).
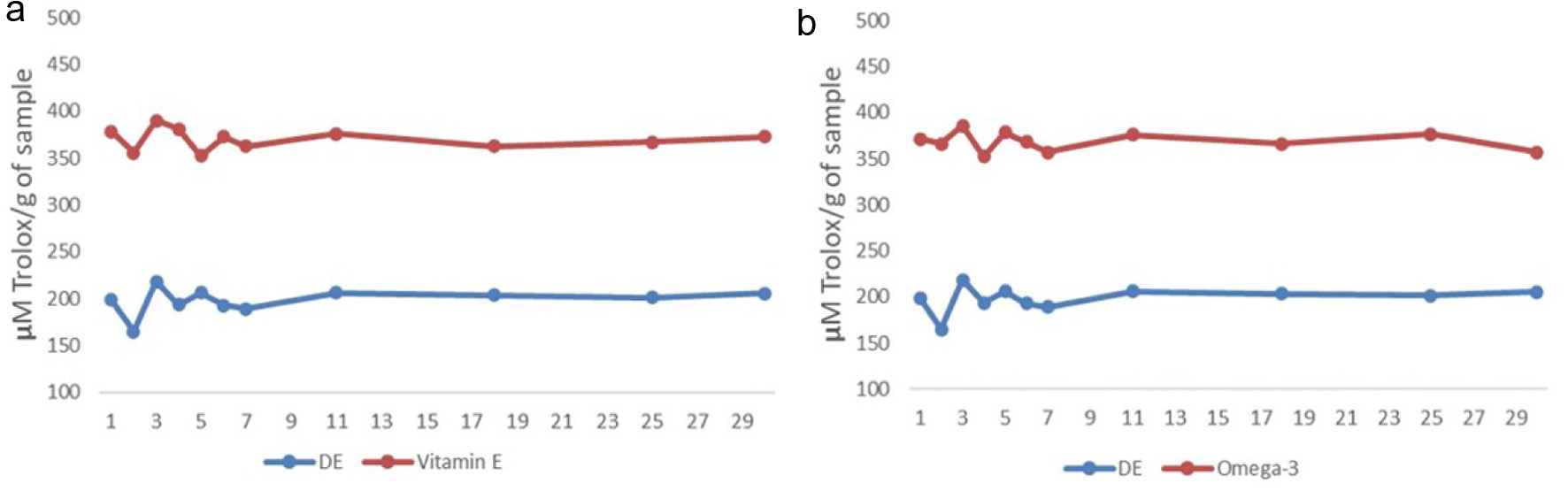 Click for large image | Figure 8. Comparison of antioxidant values between double emulsion loading food supplements (red line) and without loading (blue line) food supplements over 30 days in 4 °C, (a) Double emulsions loading vitamin E. (b) Double emulsions loading omega-3. |
| 4. Conclusion | ▴Top |
An optimized novel food grade ultrasound-assisted psyllium husk double emulsion was developed as a delivery system to encapsulate vitamin E and omega-3. Psyllium husk was chosen as an emulsifier, the optimal 1% concentration was applied in double emulsion formulation. Ultrasound homogenization can reduce the droplet size of primary emulsions and enhance the ability to form double emulsion. SEM images showed that psyllium husk can form “network” structure between emulsion droplets and also confirmed the viability of double emulsion as a delivery system. The zeta potential value and 30 days ORAC test both showed that double emulsion system was stable up to 30 days. TFE incorporation can increase the antioxidant value to delay lipid oxidation of the oil phase in the double emulsion system. This kind of double emulsion has a potential to reduce fat content in oil-based foods, further research is focused on the use of this double emulsion in food application.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
| References | ▴Top |