| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 26, June 2024, pages 24-28
Polyphenol ingredients and health effects of Green Oats: A brief update
Huilin Fang, Hui Zhao, Miao Li*
Tianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China
*Corresponding author: Miao Li, Tianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China. E-mail: limiao_hl@tjcu.edu.cn
DOI: 10.31665/JFB.2024.18377
Received: June 18, 2024
Revised received & accepted: June 30, 2024
| Abstract | ▴Top |
Green Oats (Avena sativa L.), also known as “Avena Sativa”, are a type of cereal mainly grown in North America and Europe. Green Oats are highly effective for targeting a broad spectrum of diseases. The beneficial effects are largely due to various bioactive phytochemicals, such as β-glucan, dietary fiber, and phenolic compounds, especially avenanthramides. These nutrients help reduce oxidative damage, inflammation, and tumor progression, and promote cognitive health. This review highlights the nutraceuticals and health-promoting values of green oat and its extracts, focusing on their compositions, biological effects, and underlying molecular mechanisms.
Keywords: Green oat; Antioxidant; Avenanthramides; Anti-inflammation; Antineoplastic
| 1. Introduction | ▴Top |
Green oat (Avena sativa L.), also known as Wild Green Oat, is a species of wild and domesticated annual grasses in Gramineae (Poaceae) family (Leggett, 1992). The genus Avena L. includes 29 to 31 species, with Avena sativa being the primary species cultivated worldwide, particularly in the Western regions. As an annual crop, oats have been cultivated for over 2000 years and are consumed in countries such as China, the USA, Switzerland, Denmark, Finland, Norway, and Sweden (Sang and Chu, 2017). Green oat originated in Switzerland and has been included in the German pharmacopoeia for over 200 years. As the fifth most economically important cereal, green oat is grown in temperate regions, particularly in North America and Europe (Peterson and Murphy, 2000).
Beyond its use as a cereal, green oat has a long history of medicinal use. People often eat the seed of green oats, the leaves and stem (oat straw), and the oat bran (the outer layer of whole oats). (Abascal and Yarnell, 2004; Reynertson et al., 2015). In recent decades, accumulating evidence indicates that green Oats are highly effective for targeting a broad spectrum of diseases. The beneficial effects are largely due to various bioactive phytochemicals, such as β-glucan, dietary fiber, and phenolic compounds, especially avenanthramides (AVAs). These phytochemicals have been shown to be effective in oxidative stress, inflammation, tumor progression, hyperlipidemia, and cognitive health.
This brief review will be focused on our current understanding of the main ingredients of green oats and the mechanisms of these ingredients contributing to the health benefits of green oat.
| 2. Composition and effects of the polyphenol compounds in Green Oats | ▴Top |
Green Oats possess various bioactive ingredients including carbohydrates, protein, lipids, dietary fiber, vitamins, minerals, and other bioactive substances, offer numerous health benefits by reducing the risk of diseases such as hyperlipidemia, cardiovascular diseases, and cancers. Among these bioactive ingredients, polyphenols and their derivatives are not only abundant but also represent beneficial activities of green oats.
2.1. Phenolic compounds
Phenolic compounds, ranging from simple polyphenols to polymerized compounds, are a major class of secondary metabolites in plants. In green oat, phenolic compounds include phenolic acids, flavonoids, and others such as stilbenes and lignans (Figure 1). Oat products are a rich source of polyphenols, with significant amounts of phenolic acids. (Soycan et al., 2019). Major phenolic acids in oat grains are hydroxybenzoic acids (e.g., protocatechuic, vanillic, p-hydroxybenzoic, gallic, and syringic acids) and hydroxycinnamic acids (e.g., ferulic, p-coumaric, o-coumaric, caffeic, and sinapic acids) (Li et al., 2008).
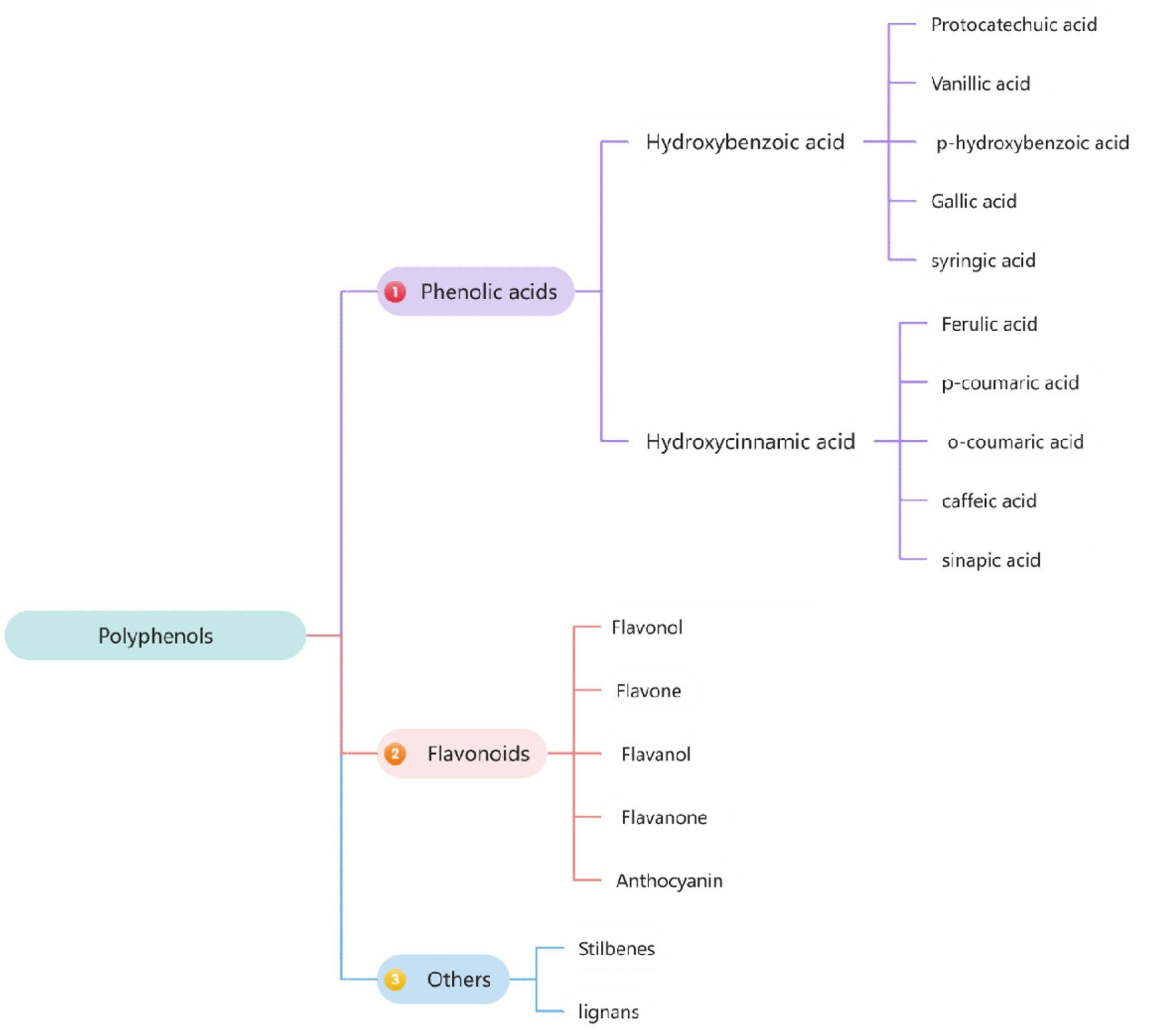 Click for large image | Figure 1. Composition and classification of polyphenols in Oat. |
The phenolic acids in green oats display broad health-promoting effects. For example, ferulic acid inhibited tumor activity by targeting fibroblast growth factor receptor 1-mediated angiogenesis (Yang et al., 2015). p-Coumaric acid has antibacterial, anti-inflammatory, antioxidant, and antitumor effects by promoting apoptosis and inhibiting cell proliferation. (Hu et al., 2020) (Lou et al., 2012; Rafiee et al., 2020; Sharma et al., 2017). Gallic acid, another phenolic compound, acts as a free radical scavenger and inhibits cyclooxygenase-2 (COX-2) (Amaravani et al., 2012).
2.2. Avenanthramides
AVAs are exclusively found in oats and are the most abundant phenolic alkaloids with significant physiological effects. Chemically, AVAs are amide conjugates of anthranilic acid and hydroxycinnamic acids, with the three major types in oats being AVA 2p (Avenanthramide A, Figure 2), AVA 2f (Avenanthramide B), and AVA 2c (Avenanthramide C) (Figure 2). AVA 2c has the highest total antioxidant capacity (Yang et al., 2014). AVAs are present in all milling fractions and commercial oat products, being more abundant in oat bran than other tissues (Xie et al., 2024). Furthermore, comparison of the AVA content from five oat samples indicated that AVAs vary in different, species, growth stages, growth conditions, and locations and levels of AVAs (2p, 2f, 2c in Figure 2) are significantly higher in groats than those in oat hulls (Xie et al., 2024). In addition, Bratt et al suggested that the amount of the specific AVA is not correlated with its antioxidant activity, as AVA 2c is not the most abundant while it is considered to have the highest antioxidant capacity (Figure 3) (Bratt et al., 2003). AVAs exhibit strong antioxidant activity against rancidification and oxidation of polyunsaturated fatty acids in addition to bioactivities such as anti-inflammation, and anti-neoplasm (Molteberg et al., 1996) (Xue et al., 2021).
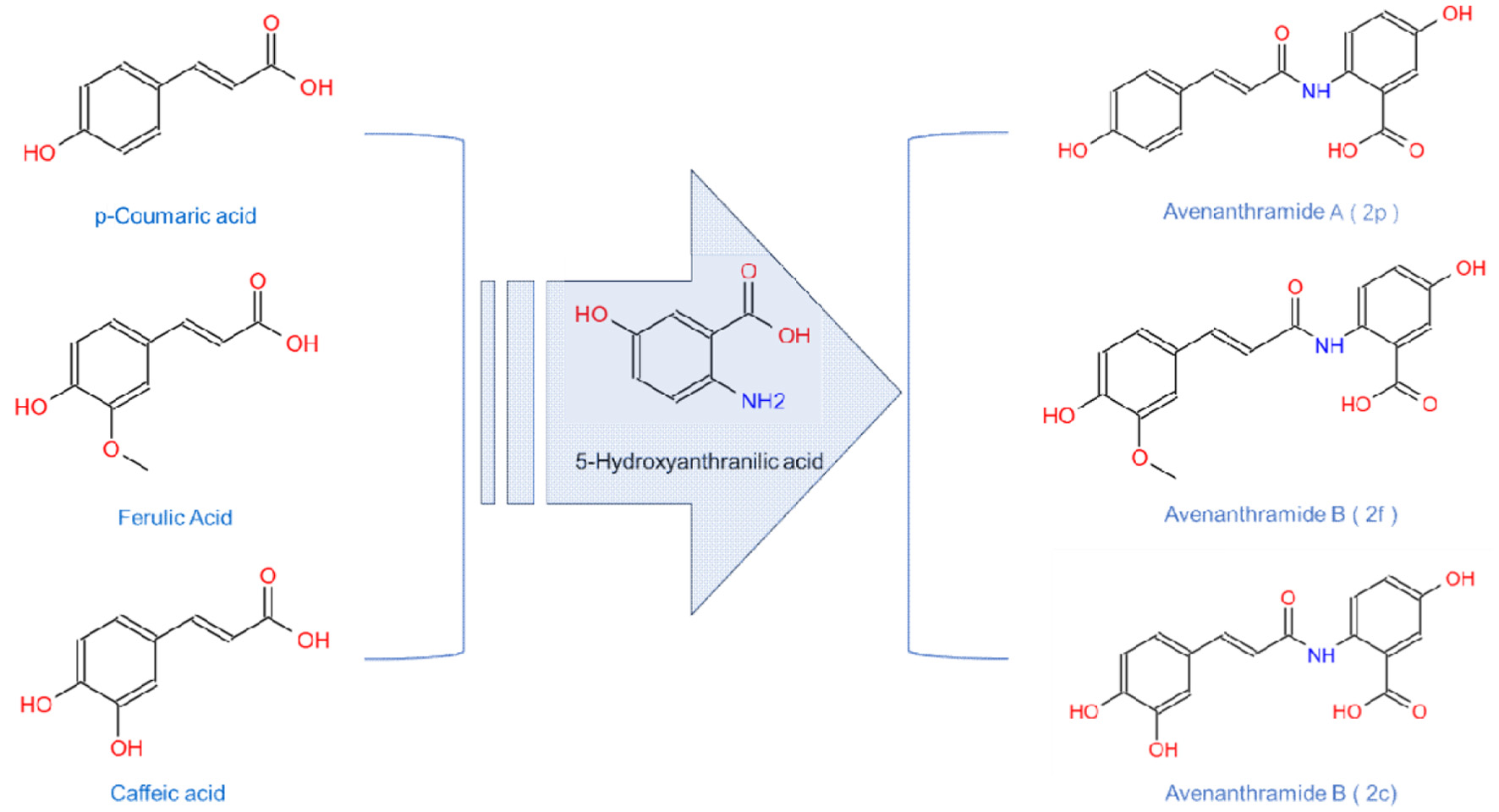 Click for large image | Figure 2. Molecular structure of the primary components of Avenanthramides. |
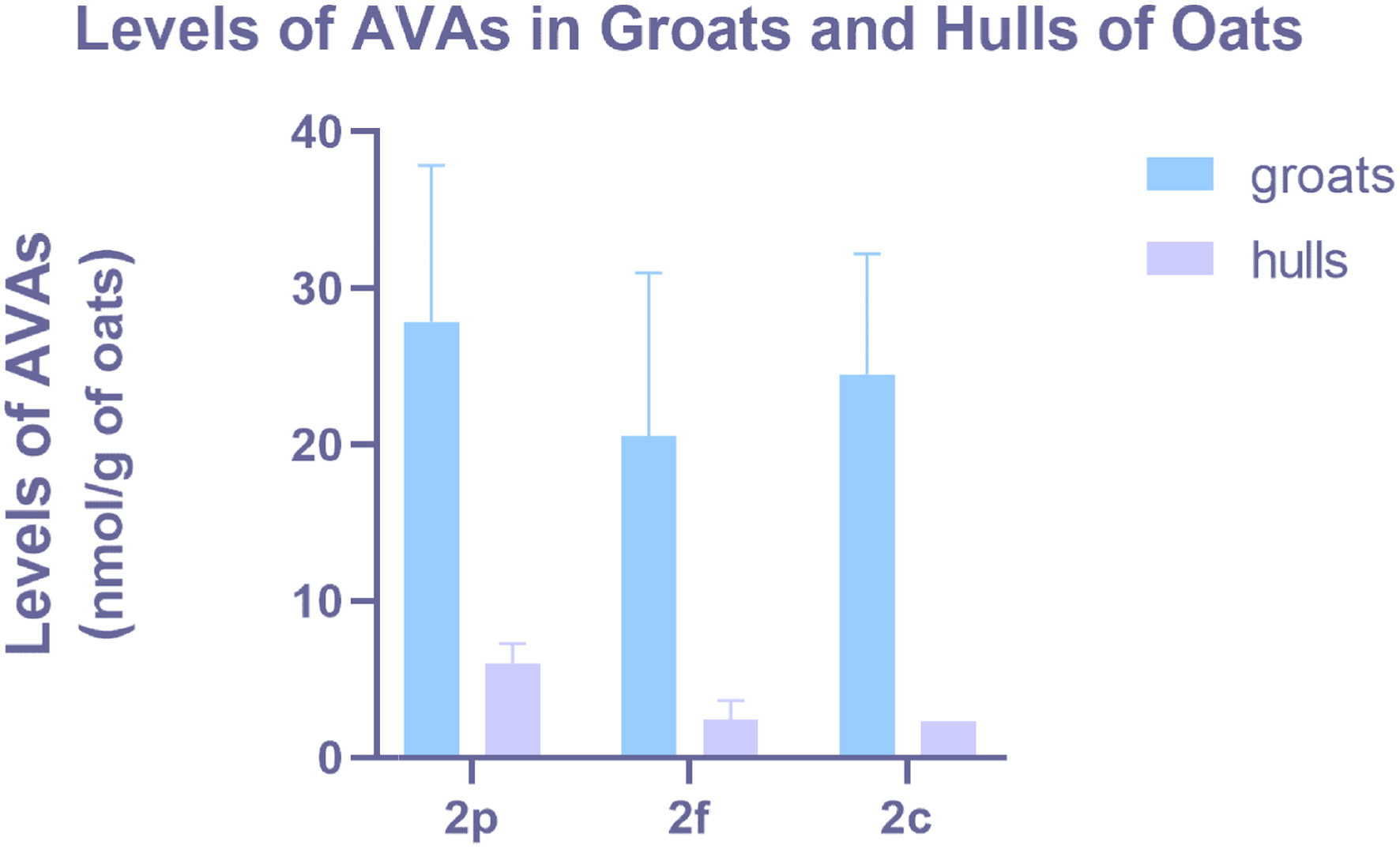 Click for large image | Figure 3. Levels of AVAs in groats and hulls of oats. |
| 3. Effects and mechanisms of AVAs | ▴Top |
3.1. Anti-oxidative stress
Oats are recognized as an excellent source of antioxidants (Peters, 1937). As the main bioactives of oats, AVAs exhibit highly potent antioxidant properties both in vitro and in vivo. As for the mechanisms of anti-oxidative stress, AVAs enhance the activity or expression of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), thereby defending against reactive oxygen species (ROS)-mediated injuries (Figure 4) (Liu et al., 2011). In particular, AVA 2c reduces H2O2-induced oxidative stress in human dermal fibroblasts by regulating the activity of antioxidant enzymes (Wang and Eskiw, 2019). Clinical studies showed that AVA-enriched oat extracts significantly increase plasma concentrations of reductive glutathione, a key antioxidant (Chen et al., 2007). Long-term AVA supplementation benefits health by reducing ROS and inflammatory cytokines (Koenig et al., 2016).
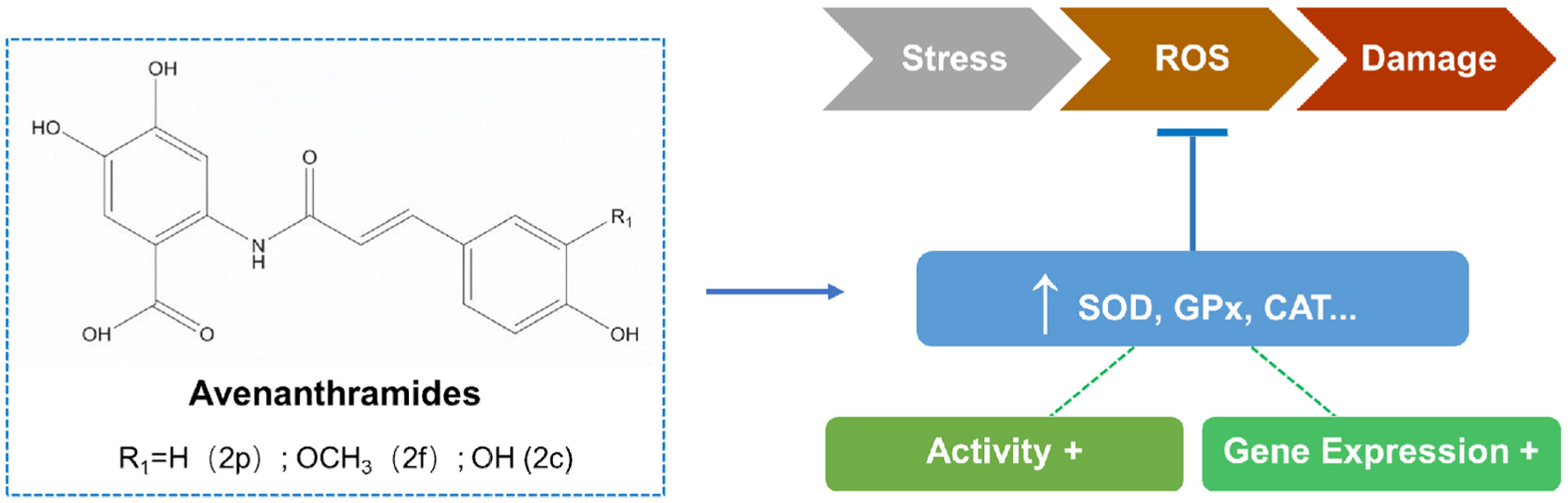 Click for large image | Figure 4. Possible mechanism of the antioxidant effects of Avenanthramides. |
3.2. Anti-inflammation
AVAs have been demonstrated to have anti-inflammatory properties in both histocytes (Kang et al., 2018) and immune cells (Dhakal et al., 2019), and play a role in conditions such as hypersensitivity (Sur et al., 2008) and neurodegenerative diseases (Wankhede et al., 2023). They inhibit NF-κB-mediated inflammatory responses by attenuating IKKβ phosphorylation, thus downregulating proinflammatory cytokines (Figure 5) (Guo et al., 2008; Kang et al., 2018). AVAs were able to modulate the Phosphoinositide 3-kinase (PI3K) signaling pathway, which is implicated in the inflammatory pathogenesis of neurodegenerative diseases like Parkinson’s and Alzheimer’s diseases (Wankhede et al., 2023).
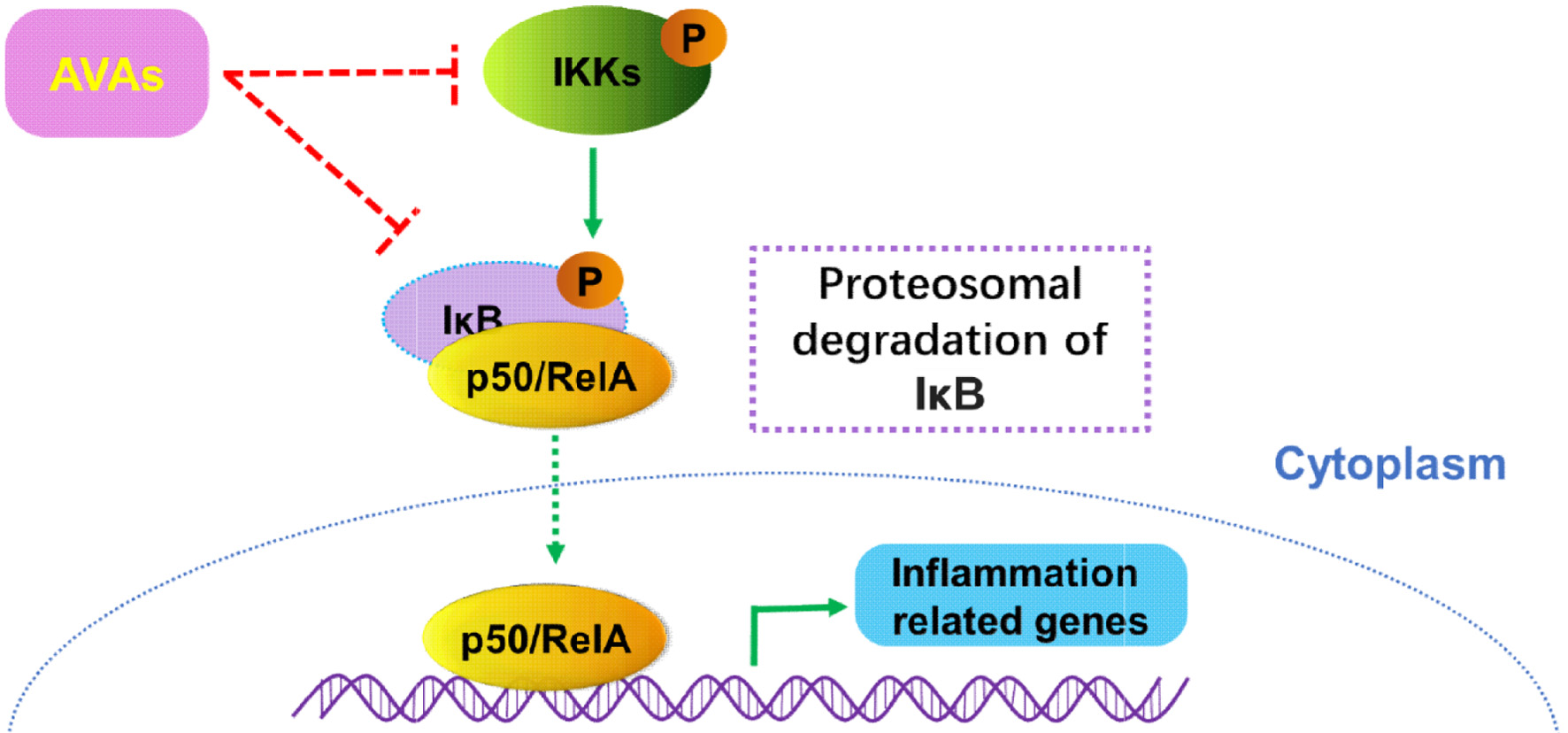 Click for large image | Figure 5. Possible mechanism of the anti-inflammatory effects of Avenanthramides. |
3.3. Antineoplastic activity
AVAs exhibit antineoplastic activity by inhibiting cell proliferation, promoting apoptosis, and reducing inflammation in tumorigenesis (Figure 6). They negatively regulate genes involved in survival and angiogenesis, such as BIRC5, HIF1A, and VEGFA (Scarpa et al., 2018). AVAs-enriched extracts were identified to inhibit the generation of COX-2/PGE2 in mouse peritoneal macrophages, which establishes the inflammatory microenvironments and angiogenesis in cancer cells (Guo et al., 2010; Hashemi Goradel et al., 2019). In addition, AVA 2c suppressed growth of colorectal cancer cells through promoting senescence by attenuating β-catenin-mediated transactivation (Fu et al., 2022).
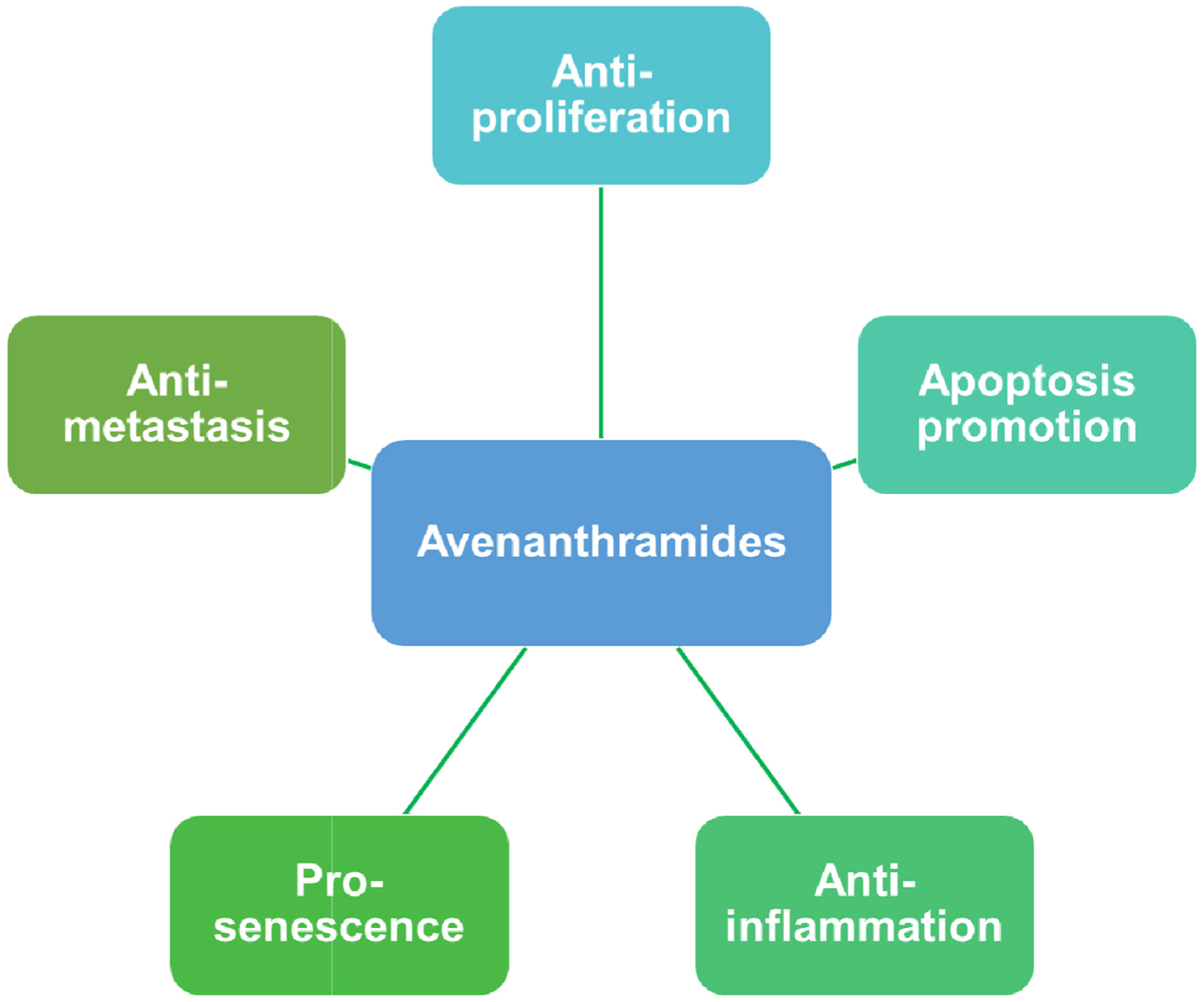 Click for large image | Figure 6. Possible mechanism of the antineoplastic activity of Avenanthramides. |
| 4. Conclusion | ▴Top |
Green oat is rich in bioactive nutrients which contribute to target oxidative damage, inflammation, tumor progression, and promote cognitive health. Oat extracts have shown significant biological activities, making them a preferred source for chronic disease prevention. Of its bioactive substances, polyphenols and AVAs are not only abundant and heat-stable but also act as the bioactive representatives of green oats. Thus, further research regarding the bioactive mechanisms and development of processing of green oat and its extracts, especially AVAs, will be essential to promote the health and clinical translation of green oat- and/or green oat extract-based products.
| References | ▴Top |