| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 26, June 2024, pages 7-23
The influence of food matrix and processing methods on the bioaccessibility of lutein: A review
Jiangfeng Songa, b, c, *, Yan Zhangb, Hongjuan Wangc, Caie Wub, Ying Lia
aInstitute of Agro-product Processing, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, China
bCollege of Light Industry and Food Engineering, Nanjing Forestry University, Nanjing 210037, China
cCollege of Food Science and Technology, Nanjing Agricultural University, Nanjing 210014, China
*Corresponding author: Jiangfeng Song, Institute of Agro-product Processing, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, China. E-mail: songjiangfeng102@163.com
DOI: 10.31665/JFB.2024.18376
Received: May 22, 2024
Revised received & accepted: June 20, 2024
| Abstract | ▴Top |
Lutein belongs to oxygen-containing carotenoids and has various functional activity potentials such as antioxidant, preventing age-related macular degeneration (AMD), anti-inflammation, protecting cardio-cerebrovascular, anti-diabetes, and anti-cancer. However, it is typically consumed along with the food matrix, and its bioaccessibility is relatively low. Some food processing techniques have been developed to promote the release of lutein in plant-based foods and/or accelerate the decomposition of food matrix, which may damage the activity of lutein or reduce its bioaccessibility. By appropriately changing the food matrix and food processing methods, the functional characteristics of lutein in the diet can be enhanced. Therefore, this paper detailedly reviews the influence and action mechanism of lutein existence form and amount, food matrix structure and components, and food processing methods on the bioaccessibility and chemical interactions of lutein. Meanwhile, it also focuses on improving the bioaccessibility of lutein through the design of food matrix systems such as emulsions, hydrogels, molecular complexes, and liposomes, etc. This information will assist in selecting the suitable encapsulation system for commercial applications and provide a reference for increasing the bioaccessibility of lutein in food matrix.
Keywords: Lutein; Bioaccessibility; Food Matrix; Food processing
| 1. Introduction | ▴Top |
Lutein is oxygen-containing carotenoid two cyclic end groups, namely α- and β-ionone ring, and has the basic structure of C40 isoprenoids (Mares, 2016) . Although the polyene double bonds in lutein can exist in cis or trans form, forming a large number of mono cis and multi cis isomers, the vast majority of carotenoids are in an all-trans configuration. The two rings are hydroxylated at positions 3 and 3′, respectively referred to as β (the C5=C6 double bond) and ε (the C4=C5 double bond). The position of a single double bond is the sole difference between lutein and zeaxanthin (Figure 1) (Makuch et al., 2021). Lutein and zeaxanthin are not stereoisomers, but rather naturally exist as all-trans geometric isomers (Mares, 2016; Demmig-Adams et al., 2022). meso-Zeaxanthin is an intermediate isomer of lutein, yet its structure is slightly different from lutein and zeaxanthin (Xu et al., 2013; Li et al., 2023).
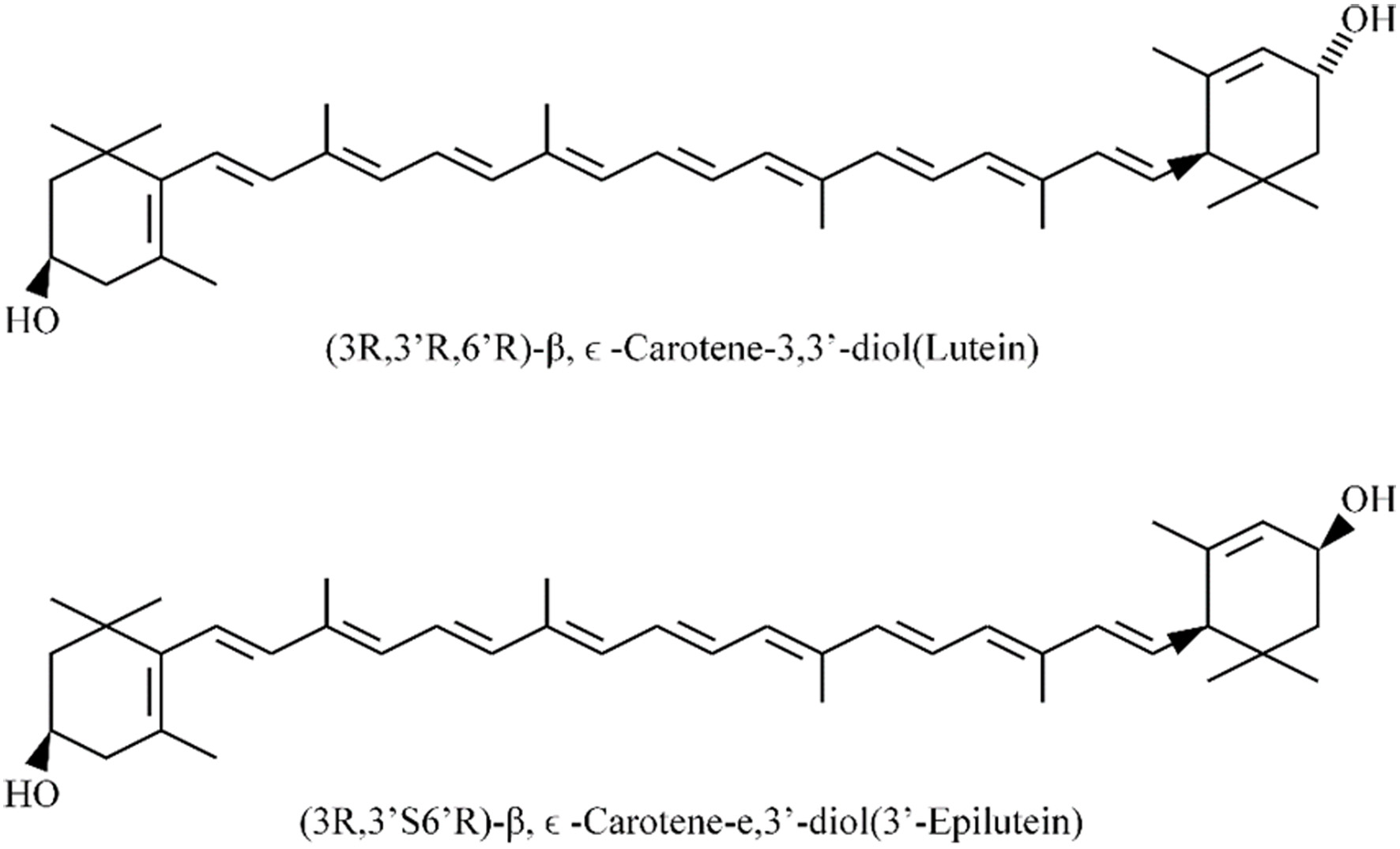 Click for large image | Figure 1. The molecular structures of lutein and zeaxanthin. |
Lutein is one of the most important carotenoids in the human body. There are over 20 types of carotenoids in plasma, with only lutein and zeaxanthin selectively accumulating in the human retina (Grudzinski et al., 2023). These carotenoids in the retina have higher concentrations in the central sulcus (0.1–1 mM) and can serve as antioxidants or blue light filters to combat oxidative stress (Widomska et al., 2023). Moreover, lutein also exists in many human tissues such as serum (0.1–1.23 μM), liver (0.1–3.0 μM), kidneys (0.037–2.1 μM), lungs (0.1–2.3 μM), etc. (Grudzinski et al., 2023).
Due to its polarity and the number of conjugated double bonds, lutein serves as an effective antioxidant (Mordi et al., 2020). These macular carotenoids play a decisive role in preventing ophthalmic diseases such as age-related macular degeneration (AMD), retinitis pigmentosa, and cataracts (Li et al., 2020). Besides being particularly important for eye health, lutein is the most abundant carotenoid found in the brains of infants and children, playing a role in their cognitive development (Gazzolo et al., 2021). It is speculated that lutein has a protective effect on all active regions of the brain, such as the prefrontal cortex, cerebellum, striatum, and hippocampus, to prevent docosanoic acid oxidation and overcome age-related cognitive decline and oxidative stress in Alzheimer’s disease (Nazari et al., 2022). Furthermore, in patients with diabetes, lutein inhibits the transcription of redox-sensitive factors in the immune system caused by high glucose levels (Pan et al., 2022). Some observational studies indicate that lutein has beneficial impacts on breast cancer, lung cancer, atherosclerosis and other cardiovascular diseases (Li et al., 2018).
Nevertheless, lutein cannot be synthesized by the human body and can only be absorbed through external sources. Generally, fruits (mangoes, papaya, citrus, kiwi, and avocados), vegetables (chili peppers, zucchini, ivy gourds, broccoli, pumpkin, chives, and carrots), marigold, and other green leafy vegetables (kale, spinach, and lettuce) are acceptable sources of lutein. Additionally, egg yolk has been recognized as an excellent source of lutein. The concentration of lutein in egg yolk is 1,622 μg/100g egg yolk, and due to its high fat content, the bioavailability of lutein is extremely high (Ochoa Becerra et al., 2020). Green leafy vegetables rich in lutein can maintain their levels in retinal tissue, serum, and adipose tissue. The concentration of carotenoids in serum often reflects their recent intake, while adipose tissue serves as a good indicator of long-term intake. Although many foods naturally contain high levels of lutein, its low water solubility limits its absorption in the gastrointestinal tract, thereby reducing its bioaccessibility (Table 1). Bioaccessibility is considered to be the actual amount of lutein released from food, incorporated into mixed micelle phases, and absorbed from the gastrointestinal tract. A study has shown that a considerable amount of lutein (approximately 60%) is not absorbed due to its low water solubility and difficulty in blending into mixed micelles in the gastrointestinal tract (Steiner et al., 2018). Over the past decade, research activities in the field of carotenoid chemistry have touched upon both internal and external factors that affect the bioaccessibility of lutein. We utilized databases including Web of Science, Scopus, and PubMed to undertake a search for pertinent literature within the last ten years, with lutein, bioaccessibility, food matrix, and processing methods regarded as the primary keywords. This paper mainly reviews the influence and action mechanism of lutein existence form and amount, food matrix structure and components, and food processing methods on the bioaccessibility and chemical interactions of lutein. Meanwhile, it also focuses on improving the bioaccessibility of lutein through the design of food matrix systems such as emulsions, hydrogels, molecular complexes, and liposomes, etc. This information will help to select the suitable encapsulation system for commercial applications, and provide a reference for increasing the bioaccessibility of lutein in the food matrix.
 Click to view | Table 1. Common lutein-rich foods along with their concentrations and bioaccessibility |
| 2. Absorption and transportation of lutein | ▴Top |
Lutein is mainly released from the food matrix under the influence of mechanical damage and gastrointestinal fluids. As lutein released is unstable in aqueous solution, it is rapidly dispersed into lipid as emulsion, and then emulsified into fat drops in stomach and duodenum. The pancreatic lipase present in the small intestine promotes the binding of lutein to bile salt micelles, which are generated by the interaction between triglyceride lyases, phospholipids, and cholesterol esters. Ultimately, they form micelles composed of fatty acids, oleic acid, monoglycerides, cholesterol, and phosphatidylcholine. These micelles embedded with intact lutein are absorbed into the intestinal mucosal cells of the duodenum through SR-B1 and CD36 transmembrane proteins. Additionally, the transmembrane protein NPC1L1 is considered to be associated with lutein absorption (Figure 2) (Chen et al., 2021). Lutein is not metabolized within intestinal mucosal cells. Due to the selectivity of transport proteins, there are absorption competitions among carotenoids, other fat-soluble micronutrients, and lipid digestion products.
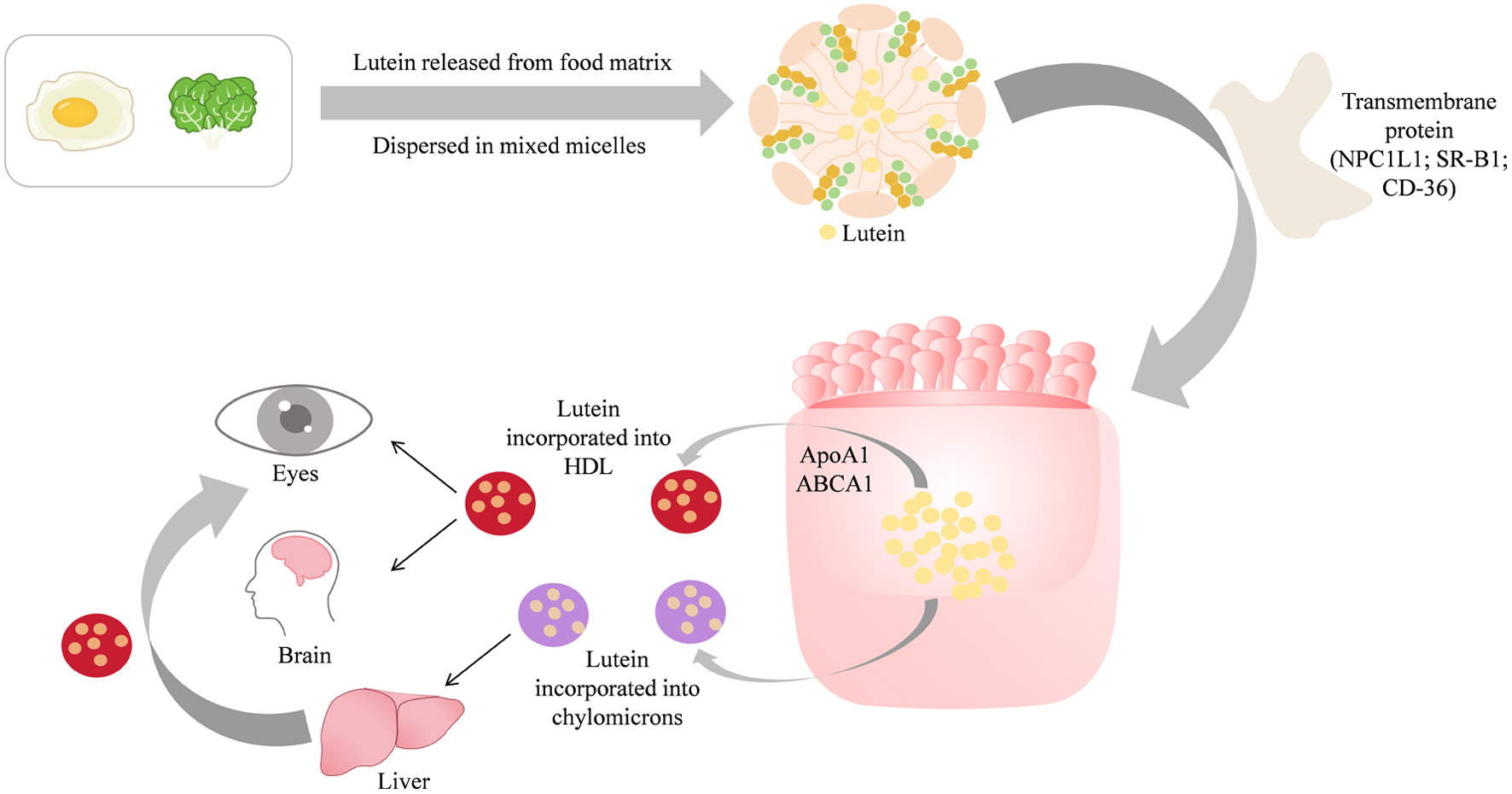 Click for large image | Figure 2. Absorption of lutein in intestinal mucosal cells. Adapted from Bhat and Mamatha (2021). |
Lutein is incorporated into the chylomicrons of the Golgi apparatus and transported to the liver through the portal vein circulation. It is partially absorbed and stored for biological activity, or further secreted into other specific human tissues or excreted from the body. Lutein can also be directly incorporated into high density lipoprotein (HDL) through the ATP binding cassette transporter A1 (ABCA1) and enter target tissues such as the eyes and brain through blood circulation. Among the lipoproteins present in the human body, lutein is mainly dispersed in HDL (53%), followed by low-density lipoprotein (LDL) (31%) and extremely low LDL (16%) (Bhat and Mamatha, 2021). Lutein has been confirmed to be highly correlated with HDL, but its mechanism remains unclear.
| 3. Lutein bioaccessibility | ▴Top |
The effective concentration of lutein at the target site depends not only on the host’s consumption, but also on its bioaccessibility and bioavailability. Lutein is released from food matrix, dissolved and diffused into dietary lipids, and then dispersed in digestive juice in the form of emulsion. The digestion of dietary lipids in emulsion is accomplished with the assistance of lipolytic enzymes and bile, and finally lutein is dissolved in the mixed micelles. Lutein dissolved in mixed micelles is believed to be uptake by intestinal epithelial cells (Figure 3). Therefore, the bioaccessibility is defined as the ratio of lutein dissolved in mixed micelles to total lutein ingested.
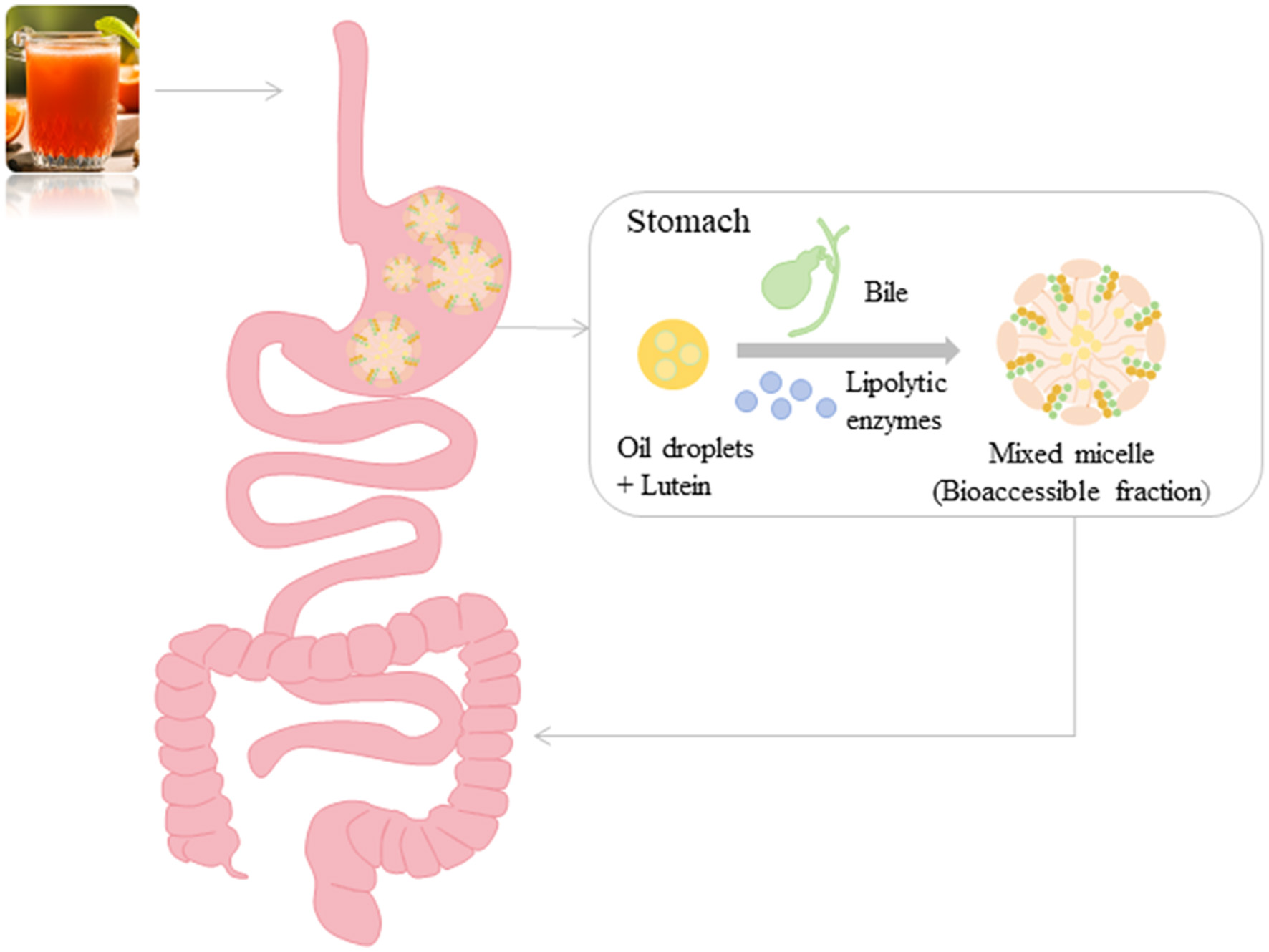 Click for large image | Figure 3. Digestion process of lutein in the human gastrointestinal tract. |
| 4. The effect of food matrix on the bioaccessibility of lutein | ▴Top |
Micellarization is a crucial initial stage necessary for lutein to become bioaccessible (Viera et al., 2018). Although it is just the first step in determining the bioavailability post-release from the matrix, some studies have shown a good correlation between carotenoid bioaccessibility and bioavailability in humans, highlighting the crucial role of carotenoid bioaccessibility (Molteni et al., 2022). Therefore, any factors influencing the bioaccessibility of lutein can potentially impact its bioavailability. These factors typically involve the molecular and chemical properties of lutein, its quantity, the dietary matrix, processing methods, and biological encapsulation.
4.1. The existence form and amount of lutein
4.1.1. The existence form of lutein
The solubility, hydrophobicity, molecular weight, and isomer configuration of bioactive compounds are one of the factors that affect their bioaccessibility and bioavailability. The bioaccessibility of carotenoids also varies due to their structure, and lutein is usually more bioaccessible than carotenes because of their greater polarity, which facilitates transfer to mixed micelles. In nature, lutein is often esterified with fatty acids to form carotenoid esters with lower polarity than free lutein. Therefore, esterification affects the bioaccessibility of carotenoids. For example, free lutein in murici fruits exhibited higher bioaccessibility than lutein monoesters and diesters (Rodrigues et al., 2016). All free xanthophylls were more bioaccessible than carotenes and xanthophyll esters in cajá frozen pulp-based beverages. The most bioaccessible carotenoid was lutein, ranging from 11.8 to 34.6%, followed by zeaxanthin from 7.4 to 15.2%. The bioaccessibility of xanthophyll esters were in the range of 2.9–8.9% (da Costa and Mercadante, 2018). Perhaps due to the fact that carotene is located within the core of micelles and lutein is located near the surface, it promotes the transfer of free lutein to micelles.
Due to its lower water solubility and higher aggregation tendency, lutein exists in crystalline form in the matrix. Compared to more loosely packed lutein J-aggregates (such as when there are no hydrogen bonds between molecules at lower pH or esterification), densely packed H-aggregates have lower bioaccessibility.
Additionally, the cis isomers of carotenoids appear to possess higher bioaccessibility, which is likely due to their more curved configuration and shorter apparent molecular length that improves micellization. The bioaccessibility of 13′-cis-lutein and 9-cis-lutein were respectively 23.0% and 23.3%, significantly higher than that of all-trans lutein (13.6%) at the end of gastrointestinal digestion (Yang et al., 2018). Meanwhile, in vitro digestion models of other foods or supplements rich in lutein had been proved to have higher bioaccessibility of 13-cis-lutein (Rodrigues et al., 2016). The cis-carotenoids are more easily soluble in the intestinal stage and better form micelle together with bile salts and pancreatic enzymes (Yang et al., 2018). The compounds including 15-cis-lutein, 13-cis-lutein, 9-cis-lutein, 9-cis-zeaxanthin, and 9-cis-β-carotene showed greater bioaccessibility when compared to their all-trans isomers (2.00 to 6.70 times more) (Fernandes et al., 2021). The cis isomers of lutein and β-carotene presented higher bioaccessibility levels in vitro than their all-trans isomers (do Nascimento et al., 2021). The high bioaccessibility of cis-lutein isomers can also be explained by their potential high solubility in simulated digestive chyme.
4.1.2. The amount of lutein
Research has demonstrated that although the degree of micellization of carotenoids varies depending on their polarity, their degree of micellization is significantly correlated with their content in digestive fluids. The trends in micellization efficiency and correlation were observed in corn samples with different seed coat colors, indicating that increasing the content of carotenoids in corn led to an increase in micellization (Dube et al., 2018). Xavier et al. (2018) tested the bioaccessibility of lutein in cupcakes with different levels of reinforcement and found that there was no linear correlation between its bioaccessibility and reinforcement, indicating that increasing lutein content did not always lead to a net increase in lutein bioaccessibility. Read et al. (2015) confirmed that lutein bioaccessibility decreased with increasing product lutein content or the level of enrichment from wholegrain baked foods. Lutein bioaccessibility was inversely correlated with product lutein content. It might be attributed to the influence of different food types.
4.2. Components of the food matrix
4.2.1. Matrix structure
The bioaccessibility of carotenoids is largely influenced by the physicochemical properties of the food matrix. In green leafy vegetables, carotenoids are mainly present in the thylakoid membrane of chloroplast subcellular organelles. In fruits and roots, carotenoids appear in a semi crystalline structure within the membrane. This difference has a significant impact on the required extraction and analysis methods, as well as the cellular uptake of carotenoids. A study had verified that non green vegetables (tomato sauce, chili peppers, carrots) generally had higher bioaccessibility of lutein, while green vegetables (broccoli, spinach) had the lowest bioaccessibility, despite being the most abundant source of lutein (Phan et al., 2019). This might be related to the cellular localization of lutein, and therefore to its extractability. This was similar to the results of Burgos et al. (2013), which demonstrated that the bioaccessibility of lutein in yellow meat potatoes was higher than that in green leafy vegetables (63–71%). Another study (Jeffery et al., 2012) identified that cell walls and chromatids were the most important structural barriers in the process of carotenoid digestion and release. The higher encapsulation level of carotenoids, the lower their bioaccessibility. Therefore, the bioaccessibility of carotenoids in non-plant-based foods is higher than that in plant-based foods because they are not encapsulated by cell walls and dietary fibers. For example, lutein in eggs had higher bioavailability than lutein in spinach (Chacón-Ordóñez et al., 2019). Margier et al. (2018) suggested that when the positive effects of spinach substrate on the bioaccessibility of lutein were combined with the negative effects of this matrix on the absorption of lutein by Caco-2 cells, spinach food matrix had no significant effect on the overall bioaccessibility of lutein. Overall, the food matrix had no effect on the absorption of lutein due to its opposite effect in the digestion and absorption steps: the food matrix seemed to protect lutein from degradation and facilitate its transfer to mixed micelles, while also damaging the absorption of lutein by intestinal cells.
The physical state of the matrix in which carotenoids are incorporated may affect their release during digestion, thereby affecting their bioaccessibility. The fiber content in spinach limited the micellization of carotenoids, and most of the carotenoids in spinach were present in chloroplasts, while carotenoids appeared as solid crystalline deposits in tomatoes and carrots (Phan et al., 2019). Although the original position of carotenoids might have changed due to more intense heat treatment, some cells still remained intact even after juice processing. Compared to tomatoes with very large cells and thin cell walls, carrots had smaller cell sizes, paired with thicker, very fibrous, and denser cell walls, which was why tomato juice exhibited higher overall bioaccessibility than carrot juice (Mapelli-Brahm et al., 2017). Lutein and zeaxanthin from liquid matrix had better bioaccessibility compared to their homologous compounds in spinach, following the order of tomato juice>carrot juice>spinach (Iddir et al., 2021).
4.2.2. Fat
An important factor affecting the bioaccessibility of lutein is the presence of fat in the diet. The micellization of carotenoids requires the presence of lipids. They promote the dissolution of carotenoids into the fat droplets of gastric emulsions, stimulate the secretion of bile acids and lipase, and accelerate the formation of mixed micelles. Additionally, the intake of fat may delay gastric emptying, produce more lipid digestion products, and thus form more micelles. Therefore, the presence of fat in the diet helps with the absorption of lutein.
Granado-Lorencio et al. (2010) observed that fat enhanced the in vitro bioaccessibility of lutein, but this effect seemsed to be disproportionate. Actually, a seven-fold increase in final fat content resulted in a two-fold increase in the relative bioaccessibility of lutein. Furthermore, even in the case of very low-fat content in the final product, it was possible to achieve relatively high bioaccessibility of lutein.
There was a significant difference in the bioaccessibility of lutein between muffins, crackers, and cookies prepared from high carotenoid whole wheat flour. Crackers (23.0%) had the lowest bioaccessibility, followed by muffins (37.9%) and cookies (56.0%). These samples contained 0.3%, 12%, and 22% fat, respectively, further indicating that high fat contributed to the bioaccessibility of lutein (Read et al., 2015).
Moreover, the fatty acid chain length and unsaturation of triglycerides (TAG) also have a significant impact on the bioaccessibility of carotenoids. For example, when consumed with long-chain TAG, β-carotene exhibited higher bioaccessibility, while lutein consumed with medium chain TAG exhibited higher bioaccessibility. There was evidence to suggest that β-carotene was more easily bioavailable in lipids rich in unsaturated fatty acids (UFAs), while lutein was more easily bioavailable in the presence of saturated fatty acids (SFAs) (Yuan et al., 2018). Studies testified a marked inhibition of lutein, lycopene and astaxanthin bioaccessibility/absorption suspended with palm, fish, corn and coconut oils, whereas enhanced absorption was observed with olive oil, beef tallow and sunflower oil in lutein-sufficient rat model or in vitro. 91.6% of lutein in olive oil was micellized due to its high oleic acid content (Nidhi et al., 2014). Compared to oil composed of higher monounsaturated fatty acids (MUFAs) (88.1–92.7%) and polyunsaturated fatty acids (PUFAs) (86.9–90.8%), oil composed of higher SFAs had a higher amount of lutein micelles (92.4–95.7%) (Bhat et al., 2022). The unsaturation of dietary lipids might affect the absorption of carotenoids and the size of micelles. Compared with MUFAs and PUFAs, oils with higher SFAs exhibited significantly higher degree of micellization (Bhat et al., 2022). The impact of the properties of TAG on the bioaccessibility of carotenoids is not directly related, but rather the result of the interaction between concentration, TAG chain length and unsaturation, and the hydrophobicity of carotenoid molecules.
Fernandes et al. (2021) suggested that the lipid concentration of the matrix (14.2% of Scenedesmus bijuga and 8.9% of Chlorella sorokiniana) was not directly related to the bioaccessibility of total carotenoids. However, lipid components directly interfered with the bioaccessibility of carotene and lutein. The study indicated that the concentration of medium chain fatty acids was related to the extended bioaccessibility of 15-cis-violaxanthin, 9-cis-lutein, all-trans zeaxanthin, and 9-cis-zeaxanthin in Scenedesmus bijuga. Meanwhile, unsaturated fatty acids (UFAs) and PUFAs promoted the bioaccessibility of 9-cis-β-carotene. Conversely, the higher bioaccessibility of total carotenoids and total lutein in Chlorella sorokiniana might be associated with the levels of long-chain MUFAs and SFAs, respectively.
However, there were also reports that PUFAs were not conducive to improving the bioaccessibility of lutein and other hydrophobic compounds, as they could lead to compound oxidation during digestion (Wolf-Schnurrbusch et al., 2015). Oleic acid was identified as one of the main fatty acids that affected the absorption of lutein, and the decrease in oleic acid content in these oils might hinder the permeation of lutein. Futhermore, the hydrophobicity of fatty acids could also affect the bioavailability of carotenoids (Courtot et al., 2022).
Combining dietary fat with lutein in the diet was found to have a beneficial impact on the absorption of lutein, as indicated by several studies (Kobayashi et al., 2019; Marriage et al., 2017; Tso et al., 2018). These findings not only support the idea that consuming dietary fat is crucial for lutein absorption, but also propose the development of a lipid-based emulsifying medium to enhance the delivery of lutein.
4.2.3. Protein
Proteins may be hydrolyzed to produce peptides with emulsifying properties during digestion, which helps carotenoids transition from lipid droplets to mixed micelles. Their emulsifying properties have been applied to encapsulate carotenoids to improve shelf-life stability and bioavailability. Proteins can alter the bioavailability of carotenoids by altering their fate during digestion, due to the emulsifying properties of the obtained peptides or affecting the pathway of digestive enzymes entering lipid droplets. In a survey by Iddir et al. (2020), it was discovered that adding proteins had a positive impact on the bioaccessibility of β-carotene, yet it caused an overall reduction in the bioaccessibility of lutein. Nevertheless, the effect significantly depended on the type of protein and its concentration. Although in the presence of sodium caseinate (SC), as compared to whey protein isolate (WPI) and gelatin (GEL), the bioaccessibility of β-carotene was greatly improved, but the presence of soy protein isolate (SPI) greatly reduced the bioaccessibility of carotenoids. Higher protein concentration positively correlated with carotenoid bioaccessibility, smaller micelle size, decreased repulsive forces, and higher surface tension. In summary, proteins have diverse effects on the bioaccessibility of carotenoids during the digestion process, depending on the type of carotenoids and proteins involved, resulting in both positive and negative interactions. However, there have been no reports regarding human experiments that investigate the effect of proteins on carotenoid absorption.
Proteins are predominantly adsorbed at the oil/water (o/w) interface through their hydrophobic segments dissolved in the oil phase. Around fat drops, protein assists in emulsifying fat-soluble dietary ingredients and converts non-polar ingredients into an emulsion during digestion. In the study of Iddir et al. (2021), the existence of proteins increased the bioaccessibility of total carotenoids in tomato juice, while reducing that of spinach and carrot juice. Even though the co-digested protein increased the bioaccessibility of β-carotene by up to 50% compared to the protein-free control, it hindered the bioavailability of lutein. Due to the significant differences in the content and distribution of carotenoids in the digested matrix, this might determine their distribution in lipid droplets and the degree of micellization. Polar zeaxanthin preferentially dissolved on the surface of lipid droplets, while non-polar β-caortene were almost completely dissolved at the core of lipid droplets. This led to negative interactions between adsorbed proteins and lutein at the o/w interface, especially considering that proteins frequently rearranged their molecular conformation to promote the adsorption of hydrophobic groups at the interface in order to obtain energetically favorable conformations. Therefore, on one hand, the accessibility of lutein to high-altitude organisms was hindered by the presence of adsorbed proteins, which limited their integration into mixed micelles. On the other hand, proteins enhanced the initial low degree of micellization of carotenoids by increasing their solubility and conversion to mixed micelles through their emulsifying properties (Iddir et al., 2021).
4.2.4. Dietary fiber
Dietary fiber is also believed to regulate the micellization of carotenoids. Studies had demonstrated that medium to high viscosity alginates and pectin inhibited lutein micellization in vitro (Yonekura and Nagao, 2009). A clinical trial confirmed that different types of dietary fiber significantly reduced the postprandial response of lutein (Riedl et al., 1999). However, in human experiments that adding soluble fiber and pectin (10 g/kg wet weight) to enzymatic hydrolysis of spinach did not affect the lutein response to dietary consumption within 3 weeks, indicating that the fiber effect might be moderate (Castenmiller et al., 1999). Pectin had a profound impact on the bioaccessibility of carotenoids through various mechanisms such as binding calcium ions and bile acids, increasing the viscosity of gastrointestinal media, altering the surface properties of oil droplets, and inhibiting lipase activity (Gence et al., 2018). The degree of this interaction was mainly related to the concentration of pectins, molecular weight (MW), and degree of methoxylation. High concentrations of pectin significantly reduced the micellization of carotenoids, while low concentrations might even be beneficial for the bioaccessibility of carotenoids; However, the influence of methoxylation degree and molecular weight remained controversial. Other components of dietary fibers, such as cellulose, guar gum, and alginate, might also reduce the bioaccessibility of carotenoids by increasing the viscosity of intestinal contents, but further studies should be conducted (Mun et al., 2016; Ubeyitogullari et al., 2022).
4.2.5. Minerals
During the digestion process, high concentrations of divalent minerals (DMs) may lead to the formation of insoluble lipid-soap complexes, thereby hindering the bioaccessibility of carotenoids. In vitro results indicated that the presence of dietary DMs impaired the bioaccessibility of carotenoids by binding to bile acids and interacting with fatty acids during digestion, leading to reduced micelle formation (Corte-Real and Bohn, 2018). This effect was concentration dependent, as the bioaccessibility of carotenoids was the lowest within the range of calcium and magnesium concentrations ranging from half of the recommended dietary intake to the entire range. On the contrary, compared to the absence of divalent cations, low concentrations of DMs enhanced the solubilization of carotenoids (Biehler et al., 2011). Although the reasons for conflicting results obtained from in vivo experiments were not yet clear, they might be related to differences in the structure and matrix composition of carotenoids.
High concentrations of DMs such as calcium, magnesium, iron, and zinc impaired the transfer of carotenoids to mixed micelles to varying degrees, thereby reducing up to 90% micellization, possibly by forming soap with fatty acids. Early study results had shown a correlation between triglyceride digestion and dietary minerals (Król et al., 2019). Although it was demonstrated that these minerals reduced the digestibility of triglycerides, a diet rich in difficult to absorb fats greatly reduced the absorption of calcium and magnesium. Due to the fact that carotenoids required a certain amount of fat in the diet to be effectively dissolved and emulsified, DM might impair the bioaccessibility of carotenoids by limiting the presence of available triglycerides and free fatty acids (FFAs). DMs also had a tendency to bind and precipitate bile acids, which reduced the bioaccessibility of carotenoids. On the contrary, the presence of salt might affect the surface tension, viscosity, and electrostatic properties of digested substances, thereby potentially altering the bioaccessibility of lipophilic components (Corte-Real et al., 2016).
4.2.6. Other carotenoids
The absoprption of lutein is less influenced by food matrix compared to lycopene and β-carotene. The food matrix plays a role in the transfer and absorption of lutein into mixed micelles. Carotenoids compete for transfer to mixed micelles, with carotene mainly affected by this competition from fat drops of emulsion to micelles. Conversely, some other factors such as antioxidants, may enhance the bioaccessibility of lutein by protecting it from degradation in micelles, potentially increasing the lutein content in the final micelles. A study (Iddir et al., 2021) revealed a particularly significant interaction between lutein and β-carotene, where the bioaccessibility of carotene increased threefold while the bioaccessibility of lutein decreased by over 50%. The competition for absorption between lutein and other carotenoids or fat-soluble vitamins has been observed both in vitro and in vivo.
4.2.7. Phenolics
The impact of phenolic compounds on the critical stage of cellular absorption of carotenoids is significant. Polyphenols may affect the activity of digestive enzymes and alter their secondary structure by binding within the active site. They may also reduce the emulsifying capacity of bile salts. These interferences will reduce the extraction of carotenoids and other lipids from the matrix, consequently decreasing the formation of mixed micelles (Marques et al., 2021).
The effects of some phenolics on the uptake of lutein by Caco-2 cells have been previously reported. (+)-catechin, gallic acid, and caffeic acid do not affect the absorption of lutein by cells, while naringin can cause obstacles in lutein absorption (Reboul et al., 2007). The latter was believed to be the result of the interaction between naringenin and membrane lipids, which affected the invagination of lipid rafts containing lutein receptors. Anthocyanins can integrate into the polar interface of the outer monolayer, leading to an increase in polarization area, which may result in a mismatch between the area of the polar head and the area of the hydrophobic tail. Therefore, the space between the two lipid layers can be increased, allowing for more freedom in the hydrocarbon chain. This effect is called membrane fluidization, which may affect the appearance and development of lipid rafts (so-called raft breaking effect), leading to reduced diffusion of some lipid molecules. On the other hand, membrane fluidization reduces the melting temperature of lipids, which may lead to the increase of lipid diffusion. The contradictory effects of polar flavonoids on the diffusion of lipophilic molecules can be seen in anthocyanins that affect the absorption of carotenoids. As previously reported that some anthocyanins (7.5 µM) increased the absorption of β-carotene (2.5 µM). However, these anthocyanin compounds reduced the absorption of lycopene and did not affect the absorption of lutein, Phan et al. (2018) speculated that the interaction between anthocyanins and cellular lipid membranes did not affect the lipid raft domain containing lutein receptors.
Regarding the stability of the digestive system, flavonoids may interact with the substrate to promote the protective effect of carotenoids on the oxidation process. Although hesperidin and hesperidin have improved micellization, naringin and naringin have reduced this process. Flavonoids exhibit different effects before micellization, which may be related to their chemical structure (Figure 4). The distance length of ligands may facilitate adsorption to the surface through hydrogen bonding, or facilitate droplet incorporation through non-polar interactions (Marques et al., 2021). The addition of turmeric extract during micellization resulted in a 5-fold increase in lutein incorporation rate, which was related to the interaction of phenolic compounds within the extract (Blank-Landeshammer et al., 2022).
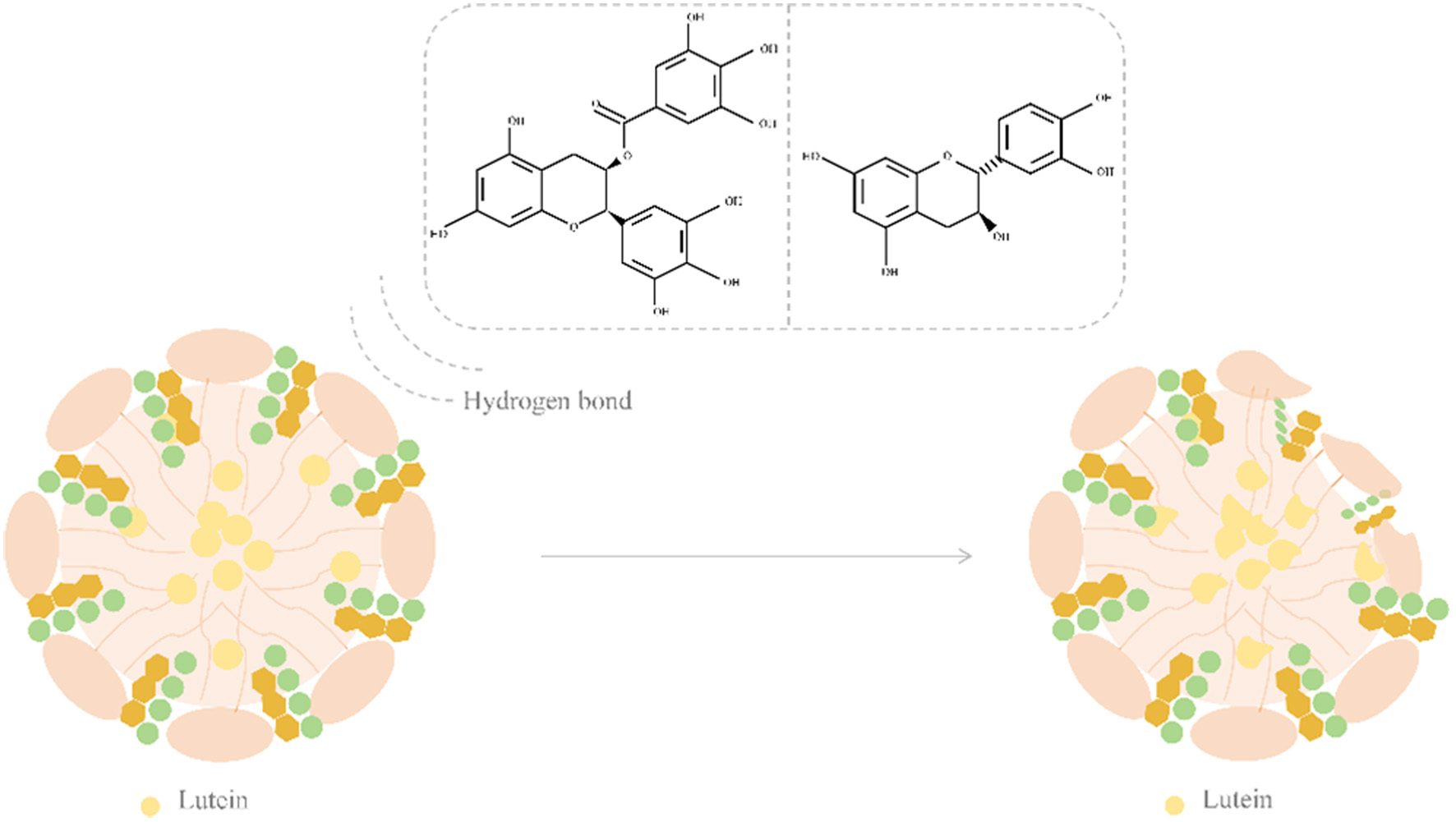 Click for large image | Figure 4. The effect of flavonoids on the structure of mixed micelles and the incorporation of lutein. |
Furthermore, the presence of epigallocatechin gallate (EGCG) increased the release of lutein in E+(with EGCG)/nanoemulsion-filled hydrogel (NE-FH), E+/ nanostructured lipid carrier-filled hydrogel (NLC-FH), and E+/ solid lipid nanoparticle-filled hydrogel (SLN-FH) to 82.6%, 75.0%, and 63.8%, respectively. The bioaccessibility of lutein in E+/NE-FH, E+/NLC-FH and E+/SLN-FH also raised to 29.7%, 28.2%, and 23.4%. The significant increase of lutein release and bioaccessibility was in accordance with the former study as it has been suggested that polyphenols and bile salts could collectively accelerate the adsorption of lipase on lipid surface, which further promoted the lipolysis and the release of lutein from lipid matrix to form water-soluble micelles (Shu et al., 2024). Additionally, EGCG may act as the interfacial barrier to improve the stability and prevent the flocculation of emulsions (Paximada et al., 2017; Tang and Huang, 2022). This might also be conducive to increasing the relative surface area of lipid particles during digestion and promoting the release of bioactive compounds from the matrix (McClements and McClements, 2023; Shu et al., 2023). As a result, the structural stability of filled composite hydrogel, the lipid composition of filled emulsified lipids, and the presence of EGCG jointly led to the overall differences in the release profile and bioaccessibility of lutein.
4.2.8. Others
Fat soluble vitamins (D, E, and K) are capable of reducing the bioaccessibility of carotenoids by participating in the process of micellization and cellular uptake through various transporters. There could potentially be a similar absorption competition between carotenoids and phytosterols. A meta-analysis showed that the intake of dietary plant sterols reduced the levels of α- and β-carotenes, lycopene, β-cryptoxanthin, and zeaxanthin in plasma, but did not affect lutein content (Baumgartner et al., 2017). However, the bioaccessibility of β-cryptoxanthin in concentrated beverages was not influenced by plant sterols, whether measured in vitro or in vivo (Garcia-Llatas et al., 2015). Further studies are requisite to comprehend the behavior of lipophilic phytochemicals and micronutrients during the simultaneous digestion of carotenoids.
| 5. The effect of food processing on the bioaccessibility of lutein | ▴Top |
The bioaccessibility of lutein in food processing can be influenced by factors such as heating treatment, processing methods, and improper storage conditions. Studies had revealed that wheat variety and cooking time were important factors affecting the bioaccessibility of lutein in refined semolina (RS) pasta, while only variety affected the bioaccessibility of zeaxanthin and lutein in whole wheat flour samples. Regardless of the wheat variety, cooking to al dente significantly increased the bioaccessibility of lutein in RS samples. The effect of cooking time (al dente > fully cooked> overcooked) was inversely proportional to the concentration of lutein in undigested pasta (overcooked = fully cooked > al dente) (Oduro-Obeng et al., 2022).
A study was aimed at assessing the differences between industrially processed and hand-squeezed orange juices (OJs) in relation to their carotenoid bioaccessibility. The results showed that the industrial extraction of OJs reduced the particle size distribution, and accordingly, the relative bioaccessibility of carotenoids increased. Independently of the type of OJ, the bioaccessibility of carotenoids in decreasing order was as follows: α-carotene > β-cryptoxanthin > β-carotene > zeaxanthin > lutein. In comparison to hand-squeezed OJs, the higher specific surface area and reduced particle size in industrial juice increased the surface area that could be conducive for digestive enzyme attacks, thereby improving overall digestive efficiency and gastrointestinal absorption of nutrients (Stinco et al., 2012).
Another study assessed the effect of boiling, roasting and fermenting on bioaccessibility of carotenoids from orange maize. The findings were pointed out that some of the processed foods showed a higher carotenoids content compared to unprocessed foods. Heat treatment helped to improve the bioaccessibility and bioavailability of carotenoids. During the processing, the food matrix softened, cell integrity was lost, and protein-carotenoid complexes ruptured. These processes could increase the extractability of carotenoids during the digestion process (Song et al., 2021). After releasing from complexes, carotenoids were incorporated with lipid droplets, after which they entered micelles and were absorbed by the intestinal epithelium. An interesting study (Marchetti et al., 2018) on the use of nettle as a carotenoid rich ingredient in egg pasta reported that lutein and β-carotene were bioavailable through high temperature (i.e. boiling water), and suggested that this fact might be due to the degradation of protein-carotenoid complexes.
In food matrices, carotenoids typically exist in the form of complexes. For example, carotenes form complexes with proteins in chromosomes. The bioaccessibility of carotenoids is influenced by the structure of the food substrate. Some food processing methods, such as pasteurization, microwave, or hot cooking, have the potential to soften cell walls, disrupt protein-carotenoid complexes, and liberate carotenoids, thus enabling digestive enzymes to function more efficiently (Cilla et al., 2018). Only those carotenoids released from food matrices could be absorbed and utilized. Forming complexes with emulsified gastrointestinal contents assists carotenoids in reaching intestinal cells and bestowing health benefits (Cervantes-Paz et al., 2016). After being absorbed by the intestinal epithelium, a portion of esterified lutein can be transported through the gastrointestinal tract via the lipid transport protein SR-BI. Lutein, zeaxanthin, and β-carotene can be absorbed by ARPE-19 cells and then transported to the human retina. Additionally, lutein and zeaxanthin accumulate in the retina and lens of the eye, which serves to filter blue light and prevent eye damage. Some researchers also reported that carotenoids possess the function of inhibiting the growth of human cancer cells located in organs such as the colon, liver, breasts, and skin.
Due to the fact that the bioactivity of carotenoids in the body depends on their bioaccessibility, the changes in the bioaccessibility of carotenoids during extrusion processing were investigated (Ortak et al., 2017). The in vitro bioaccessibility of β-carotene and lutein increased through extrusion, extrusion reduced antioxidant activity, total phenols, β-carotene, and lutein content. The results indicated that even with a decrease in the number of functional components, extrusion could enhance in vitro bioaccessibility. The production of smoothies was based on fruits and vegetables rich in carotenoids: carrot juice papaya mango (smoothie A) and carrot juice pumpkin mango (smoothie B). The study evaluated the effects of different thermal techniques (mild and intensive heat treatment) and unconventional techniques (ultrasound) on carotenoids (α-carotene, β-carotene, lutein, and β-cryptoxanthin) (Buniowska et al., 2019). The release rates of β-cryptoxanthin in mild and severe heat treatment, as well as ultrasound treatment, were between 3%, 10.3%, and 9%, respectively. The release rate of β-carotene was the highest after mild heat treatment (34.2%). The increase in carotenoid release after the technological process could be explained by the destruction of natural matrices. In terms of ultrasound therapy, the release of all carotenoids was between 9%. This might indicate that ultrasound during the cavitation process damaged the plant cell wall and led to the extraction of more bioactive compounds, thereby enhancing the release. Although heat treatment reduced the content of carotenoids in food, positive effects regarding the release, micellization, and bioavailability had been discovered.
The bioaccessibility of carotenoids involves two processes: (i) the release of carotenoids from food matrices, and (ii) their subsequent micellization. This is limited by various factors, such as the presence or addition of lipids, processing (cooking preparation, mechanical crushing), or the type of food matrix. The bioaccessibility of carotenoids in fruits and vegetables depends on the initial crystallization, liquid crystals, protein binding, or lipid dissolution deposition forms of carotenoids in chromosomes (Chacón-Ordóñez et al., 2019). Only micellar carotenoids are considered biologically obtainable. Compared with persimmons sterilized by pasteurization, persimmons under high hydrostatic pressure (HHP) had higher bioaccessibility. The carotenoids with the highest bioaccessibility in pressurized persimmons were all-trans-β-cryptoxanthin (54.2%), followed by all-trans-β-cryptoxanthin laurate (36.9%), all-trans-anthocyanin (30.1%), and lycopene (27.2%). The bioaccessibility of free all-trans-β-cryptoxanthin was 1.2–2.5 times higher than that of major esters. Among the pasteurized persimmons, the most bioavailable carotenoids were all-trans-β-cryptoxanthin laurate (23.9%), followed by all-trans-anthocyanin (18.4%) and lycopene (17.2%) and all-trans-β-cryptoxanthin (11.6%). This increase in bioaccessibility might be the result of changes in the composition and characteristics of polysaccharides (pectin) in persimmon tissue caused by processing. According to reports, the high pectin content in food matrices affected the bioaccessibility of carotenoids due to the difficulty in transferring them into micelles (Gence et al., 2018). The modification of pectin structure and its interaction with other plant tissue components produced by HPP might affect the micellization of carotenoids, thereby increasing their bioaccessibility.
The effects of pulsed electric field (PEF) and ohmic heating (OH) on the bioaccessibility of carotenoids and the uptake of Caco-2 cells in tomato juice, high-pressure processing (HPP), and PEF were measured (Zhong et al., 2019). Compared with raw (unprocessed) juice and conventional heat treated (TT) juice, kale juice had the same properties. PEF increased the bioaccessibility of lycopene by 150%, while reducing the bioaccessibility of carotene by 44% (relative to the original juice). All processing methods had increased the absorption of lutein. TT and PEF degraded carotenoids and lutein in fruit juice. No differences in bioaccessibility or cellular uptake were observed. The total delivery of lycopene, carotene, and lutein was independent of the type of processing. PEF and OH enhanced the overall delivery of lycopene and lutein from tomato juice to Caco-2 cells and TT.
The impact mechanism of processing methods on the bioaccessibility of lutein is mainly reflected in the following aspects: firstly, factors such as temperature and pressure during the processing may change its structure, leading to a decrease in activity. Secondly, solvents and additives used in processing may interact with lutein, affecting its solubility and stability. Moreover, processing may also lead to the binding of lutein with other food components, affecting its absorption. Meanwhile, the processing process may also cause chemical changes such as oxidation and degradation of lutein. In sum, different processing methods can affect the bioaccessibility of lutein through various pathways.
| 6. Construction of lutein nanodelivery system and development of excipient foods | ▴Top |
The composition of food matrix affects the bioaccessibility of lutein. Therefore, designing effective nutrition delivery systems (NDS) based on suitable food matrices to improve the bioaccessibility of lutein is of great significance. Many NDSs have been developed for the encapsulation, protection, and delivery of lutein, such as microemulsions, nanoemulsions, molecular complexes, liposomes, solid lipid nanoparticles, biopolymer particles and microgels as showed in Figure 5 and Table 2. These systems can change the bioaccessibility, absorption or transformation of lutein in the gastrointestinal tract.
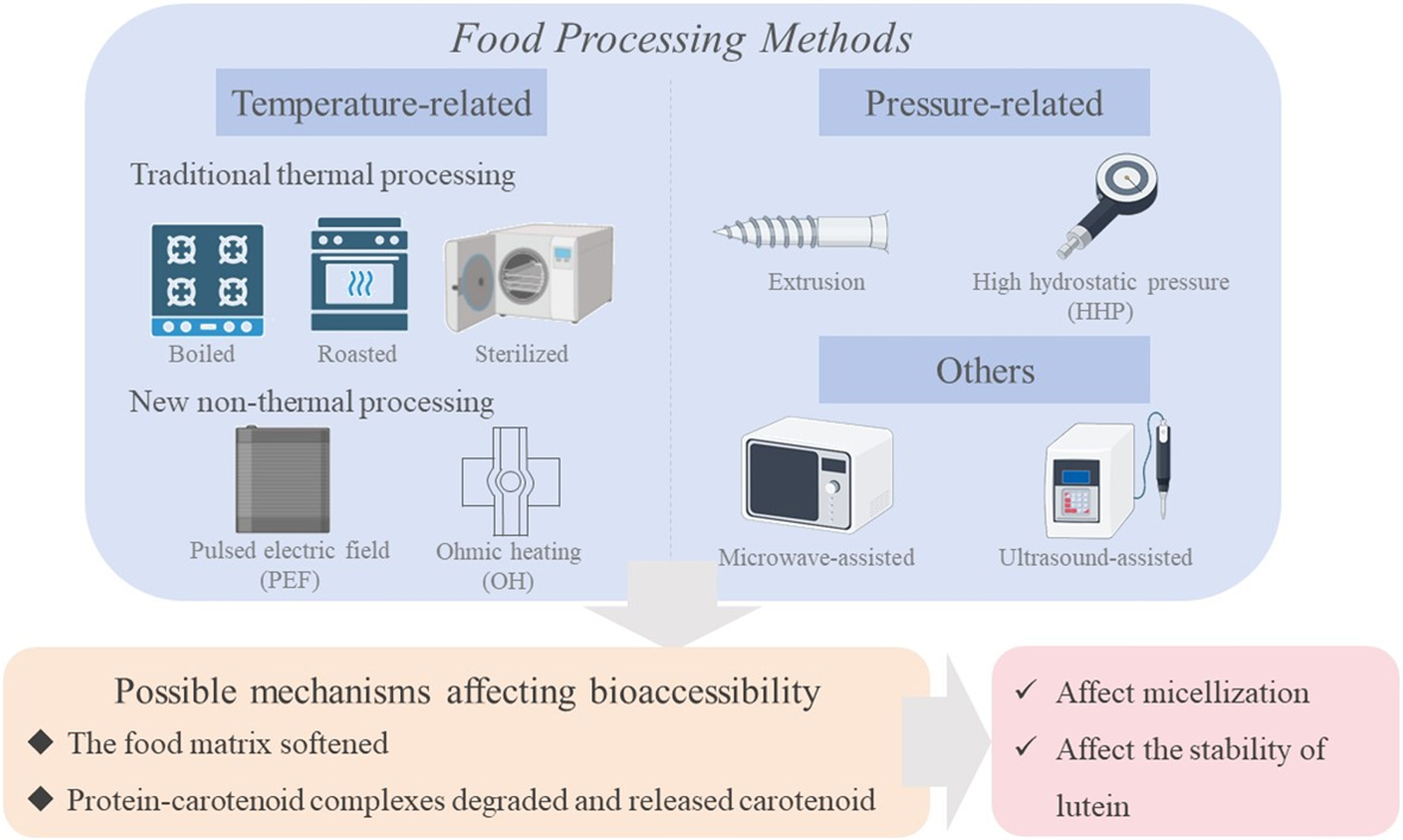 Click for large image | Figure 5. The effect of food processing on the bioaccessibility of lutein. |
 Click to view | Table 2. Changes in lutein bioaccessibility in nano-scale delivery system |
Lutein typically exhibits low bioaccessibility, with the bioaccessibility determined from fresh fruits and vegetables ranging from 9% to 59%. A previous study (González-Casado et al., 2018) formulated a milk beverage rich in carotenoids, and the bioaccessibility of lutein was also low or equal to 7%. In the study by Yao et al. (2021), the bioaccessibility of lutein fortified noodle-like food was approximately 3% to 4%. There are several reasons that may lead to this relatively low bioaccessibility: a) the degradation of lutein occurrs during the hot processing; b) lutein remains in lipid droplets and be trapped within the alginate layer; c) trapped and undigested lipids lead to insufficient fatty acids and monoacylglycerols to form mixed micelles, which in turn hinder the micellization of lutein; d) the presence of alginate increases viscosity and induce the aggregation and precipitation of lutein, making it difficult to dissolve in mixed micelles. The advantage of the manufactured multi-layer emulsion structure via microfluidics lies in the controlled release of the encapsulated compounds rather than improvement of the lutein bioaccessibility.
Dai et al. (2021) explored the effect of lutein-loaded nanoemulsions and excipient nanoemulsions mixed with lutein-based dietary supplements (capsules and soft gels) on the bioaccessibility of lutein. The co-administration of excipient nanoemulsions significantly increased the bioaccessibility of lutein in capsules (35.2%) and soft gels (28.7%). This was attributed to the quick digestion of small oil droplets in excipient nanoemulsions and the further formation of mixed micelles to dissolve all lutein released from the supplements. Xu et al. (2021) evaluated the physicochemical stability and potential gastrointestinal outcomes of the lutein-enriched high internal phase emulsions (LE-HIPE) stabilized by egg yolk-modified starch complex. The in vitro digestion results indicated that high levels of hydroxypropyl distarch phosphate (HPDSP) in LE-HIPE were advantageous in improving the bioaccessibility of lutein. The improved bioaccessibility of lutein was probably due to the small droplet size, which was instructive to the access of lipase, thus improving the release and micellization of lutein from oil dorplets (Feng et al., 2017; Yuan et al., 2019). Lutein emulsion was stabilized by biopolymers served as a colloidal delivery system to enhance the bioaccessibility of lutein. Compared with free lutein, the bioaccessibility of capsule TA® (CTA), HI-CAP®100 (HC) and TICAmulsion® 3020 (TM)-stabilized lutein emulsion was 2.3, 1.3 and 2.3 times higher, respectively. Moreover, the smaller drop size provided a larger surface area for lipase entry and hydrolysis, which increased the bioaccessibility of the emulsion (Zhang et al., 2024).
Most of the published data on the bioaccessibility of lutein in the emulsion system came from in vitro studies, which used simulated gastric and intestinal models and determined the ratio of lutein dissolved in the mixed micelles to lutein content in the initial chyme. The droplet size of nanoemulsions is crucial for increasing the bioaccessibility of lutein. The effect of particle size on the bioaccessibility of lutein is related to the increase in surface area of small droplets. This promotes the interaction between the droplet surface and digestive components (enzymes, bile salts, phospholipids, cholesterol), and leads to complete digestion under simulated gastrointestinal conditions, followed by the incorporation of lutein into the micelle portion. Another factor that may affect the bioaccessibility of lutein in emulsions is the type of oil used as the dispersion phase. Some possible mechanisms have been proposed to explain the low bioaccessibility of lutein in emulsion containing corn oil, which is mainly related to the high level of PUFAs. Firstly, polyunsaturated fatty acids are prone to oxidative reactions, which may promote the oxidation of lutein in the intestine. Secondly, the size of mixed micelles containing PUFAs may be larger, which in turn slows down their diffusion through the undisturbed water layer adjacent to intestinal cells and reduces their uptake at the intestinal level.
Recently, research has focused on the inclusion complexes of carotenoids with natural oligosaccharides/polysaccharides, suggesting that these complexes have protective properties against carotenoids and can reduce their hydrophobicity (Figure 6). For example, the supramolecular complexes formed by zeaxanthin and lutein with glycyrrhetinic acid and its disodium salt, as well as with arabinogalactan, significantly enhanced the solubility and stability of zeaxanthin and lutein (Zuo et al., 2023). The bioaccessibility of lutein encapsulated increased, due to the presence of lutein in the amorphous state. Moreover, the higher bioaccessibility of lutein encapsulated in nanoparticles was attributed to small particle size and that entrapment in particles prevent precipitation of lutein. Hao et al. (2022) manufactured biopolymer microparticles to encapsulate and transport lutein by electrostatically combining sodium caseinate (NaCas) and sodium alginate (ALG) to improve its physiochemical stability and bioaccessibility. Compared with emulsions (12.8%), microparticles exhibited higher FFAs release and lutein bioaccessibility (51.3%). Yu et al. (2022) constructed self-assembled composite nanoparticles composed of Stauntonia brachyanthera seed albumin (SBSA), arabic gum (GA), and carboxymethyl cellulose (CMC) for lutein encapsulation. After encapsulation into nanoparticles, the thermal stability and storage stability of lutein were significantly enhanced. Additionally, the bioaccessibility of lutein increased from 17.5% to 46.8%. The anionic polysaccharides (GA and CMC) had the ability to form a stable nanocolloidal structure with SBSA which could prevent SBSA from hydrolysis by pepsin and ensure the integrity of the nanoparticles. The selective release of lutein in the gastrointestinal environment enhanced its bioaccessibility after simulated intestinal fluid (SIF) digestion. More importantly, the SBSA could rapidly adsorb at the surface of oil droplets in the formation of a densely packed layer, and aggregate to form membranes at the oil/water surface, which promoted the formation of mixed micelles that could solubilize and protect the lutein. The release of lutein from calcium alginate hydrogels during simulated digestion in vitro was investigated, appropriate concentration of Ca2+ cross-linked hydrogel carrier improved lutein bioavailability while also extending the residence period (Luo et al., 2022). Ahmadzadeh and Ubeyitogullari (2023) reported an innovative lutein encapsulation method using a coaxial 3D printer, where starch paste was used as an outflow and ethyl cellulose lutein was used as an inflow. This method utilized 3D printing and porous biopolymers to improve the stability of lipophilic lutein. However, future research needed to address the issues of process amplification parameters and the release and bioavailability of encapsulated lutein in 3D printed samples.
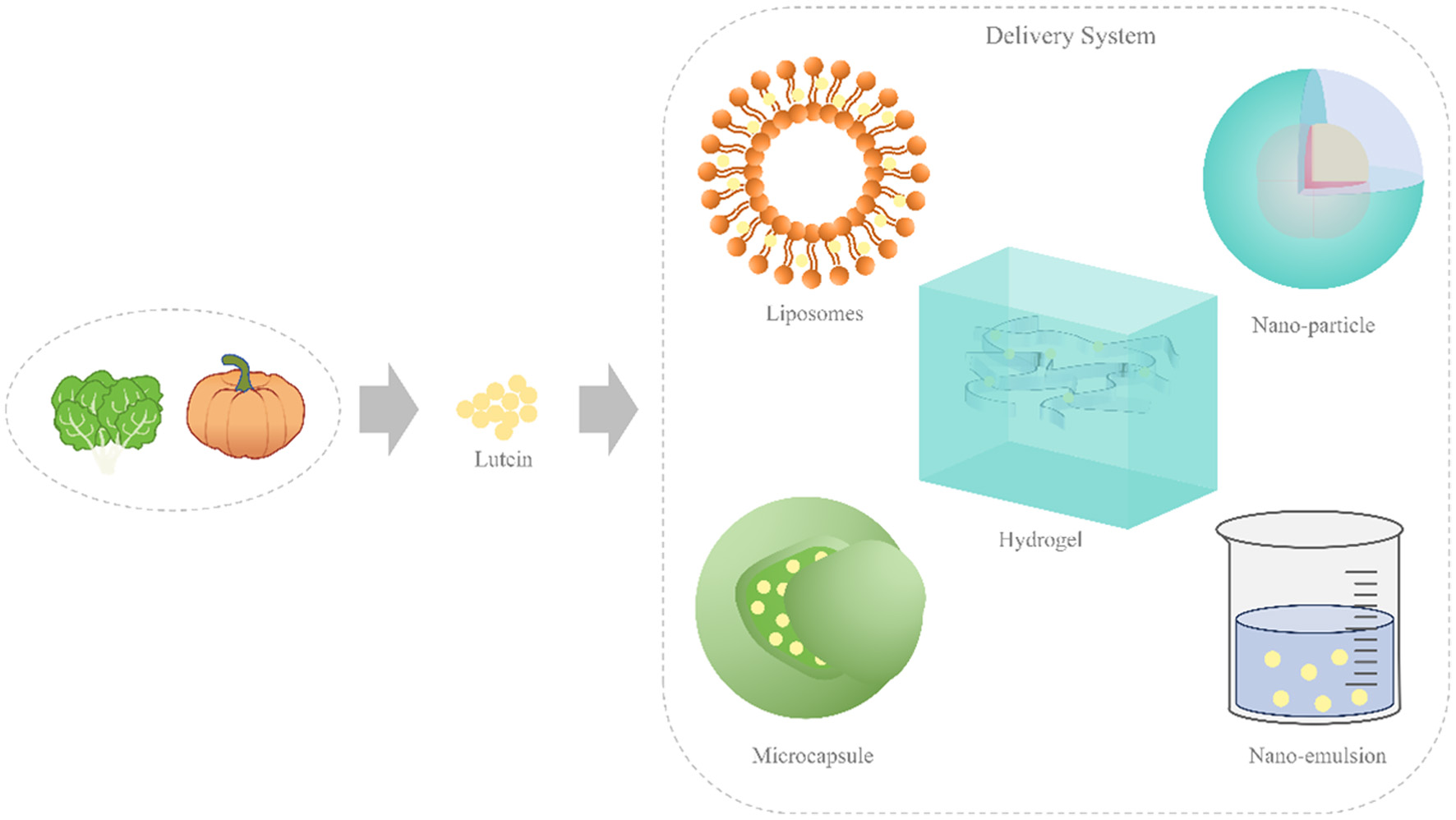 Click for large image | Figure 6. Methods for improving the bioaccessibility of lutein in food. |
| 7. Future perspective | ▴Top |
Food matrices may have a significant impact on the bioaccessibility of lutein. Some components in food matrices, such as matrix structure, proteins, lipids, dietary fiber, minerals, phenolic components, and other carotenoids, can undergo reversible or irreversible reactions with lutein. Although the exact mechanisms behind these observations are not fully understood, some studies have suggested that the reduced bioaccessibility may be due to the capture of lutein into the food matrix. The micellization and activation of transport proteins involved in lutein uptake may lead to an increase in the bioaccessibility of lutein. However, the investigation on this topic is limited, and some results are contradictory. Additionally, most studies only focus on a single food component, while the impact of multiple food matrices on lutein absorption needs further exploration. Elucidating how lutein interacts with food matrices is crucial for developing foods that offer greater health benefits to consumers.
Meanwhile, various food processing techniques can impact the chemical structure and bioaccessibility of lutein, as well as the composition of food matrices. Given these characteristics, many types of research have been conducted in the field of functional foods and excipient foods through food processing to improve the bioaccessibility of lutein. During food processing, lutein may be degraded, be released more fully or form new substances. Therefore, it is necessary to carefully consider the selection of appropriate food processing techniques to improve the bioaccessibility of lutein and reduce the lutein loss in chemical reactions. It should be noted that the bioaccessibility of lutein is a crucial factor in determining its bioavailability, i.e. the ability to pass through the intestinal epithelium and ultimately reach the target tissues to exert its biological activities. The nature of the processing operations employed also impacts the bioaccessibility of lutein in complex ways. It is worth highlighting that the level of bioavailability does not necessarily align with the level of bioaccessibility. Bioaccessibility primarily focuses on the release of lutein from food into an absorbable state, while bioavailability encompasses a series of metabolic, transport and other processes after absorption. Even if a processing method improves bioaccessibility, other factors (such as interactions with other substances, alterations in metabolic pathways, etc.) may affect the final bioavailability in subsequent in vivo processes. Thus, there is an urgent need for more comprehensive research through the development of new processing techniques to subsequently evaluate the mechanisms of gastrointestinal digestion, absorption, release, and overall bioavailability. Additionally, more in-depth in vivo studies are necessary.
Strategies to improve the bioaccessibility and bioavailability of lutein have been widely studied. These strategies include optimizing processing methods, such as using appropriate heat treatment, non-thermal and bioprocessing, or adding excipients; providing reasonable food formulation suggestions, such as combining with lipid-rich foods to promote lutein absorption; giving targeted nutritional advice, such as consuming lipid-containing lutein; developing formulations that can protect lutein or improve their absorption. The comprehensive application of these strategies may have positive or negative impacts on its bioaccessibility and bioavailability, depending on specific studies that emphasize the best ways to ensure that individuals consuming foods rich in lutein receive greater benefits.
Futhermore, although analytical methods for characterizing the biological fate of delivery systems have advanced significantly, the majority of research focuses on assessing the bioaccessibility of lutein through various in vitro static and dynamic models that simulate the gastrointestinal tract (GIT) conditions during digestion. However, the key stage of oral bioavailability is when lutein enters epithelial cells from the intestinal lumen and then enters the systemic circulation. Therefore, more methods such as cell culture models, animal studies, and clinical investigations are required to confirm the oral bioavailability of lutein encapsulated in various delivery systems.
The authors would like to extend their appreciation for the financial support provided by the project of Natural Science Foundation of Jiangsu Province (Project No. BK20201241) and Independent Innovation Fund Project of Agricultural Science and Technology in Jiangsu Province (Project No. CX (20)3047).
Conflict of interest
All the authors confirmed there is no conflicts of interest.
| References | ▴Top |