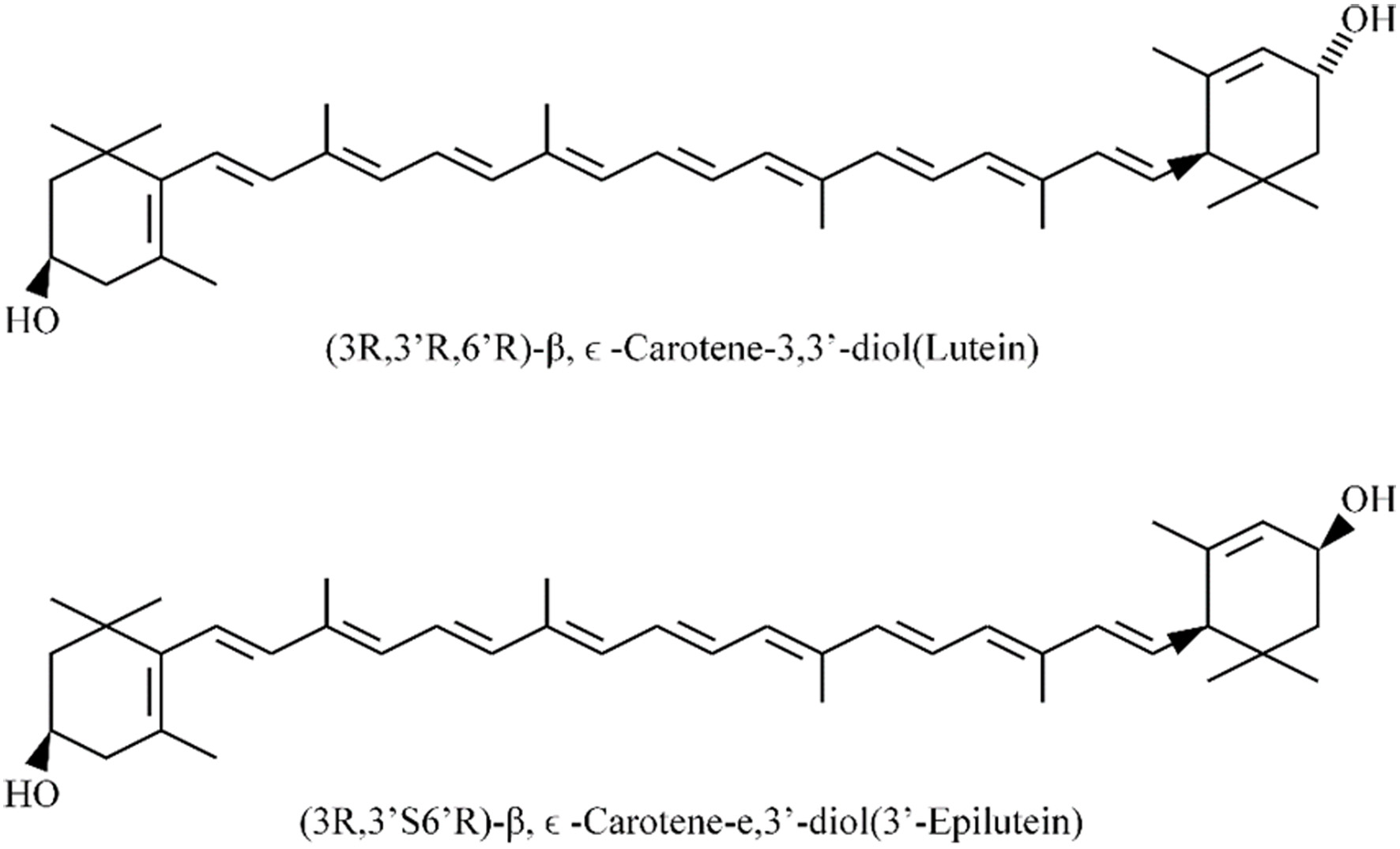
Figure 1. The molecular structures of lutein and zeaxanthin.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 26, June 2024, pages 7-23
The influence of food matrix and processing methods on the bioaccessibility of lutein: A review
Figures

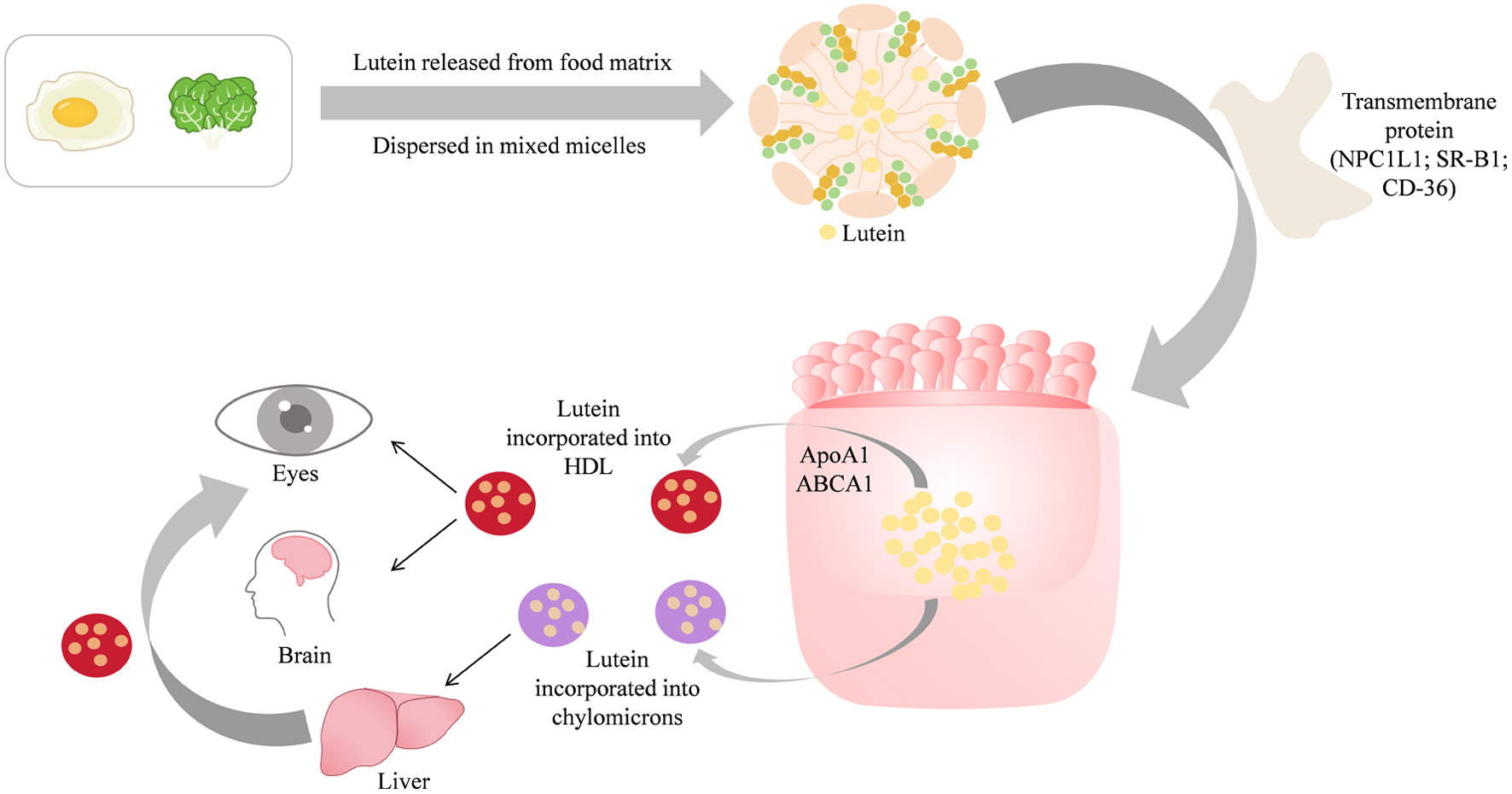
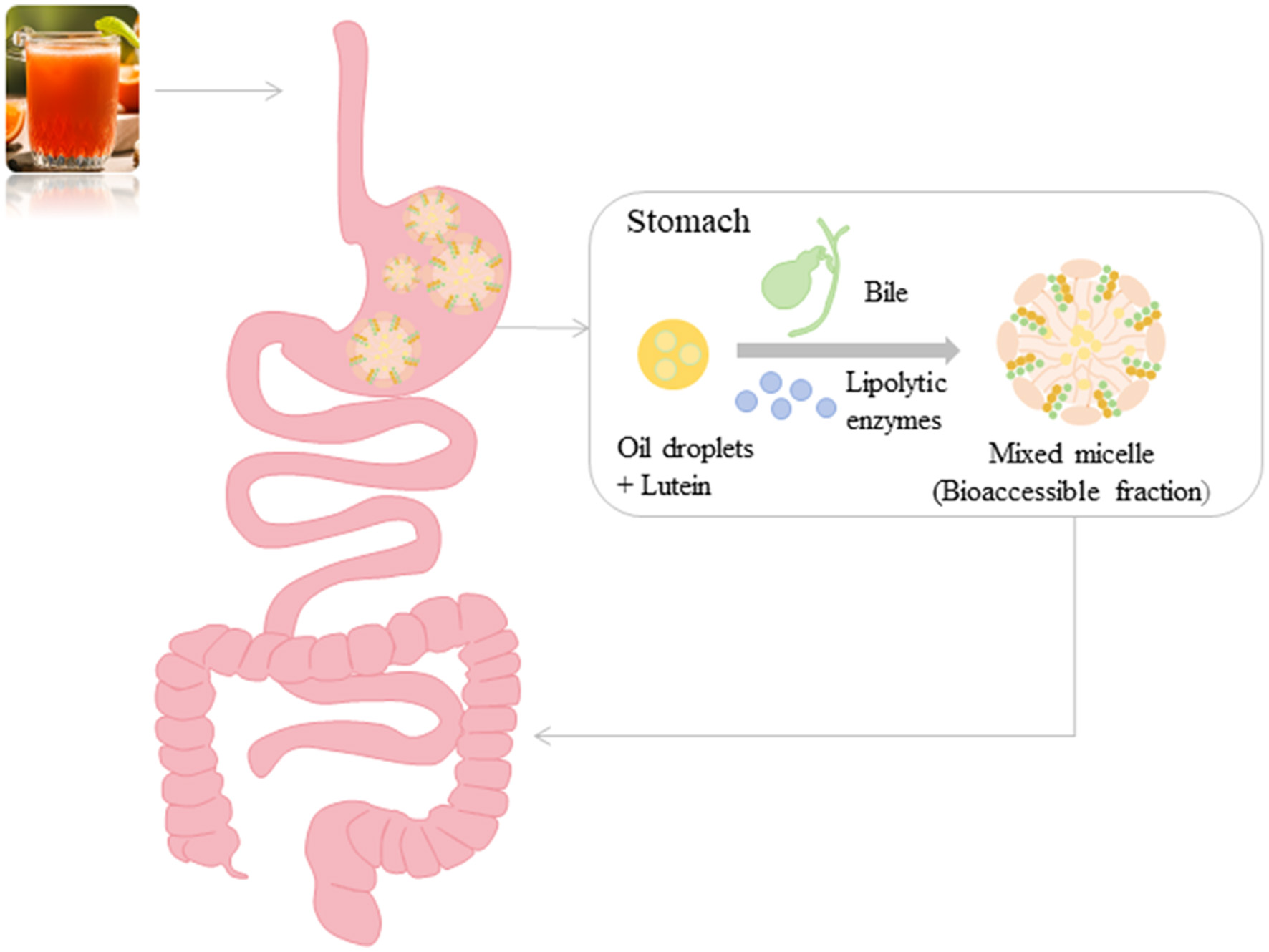
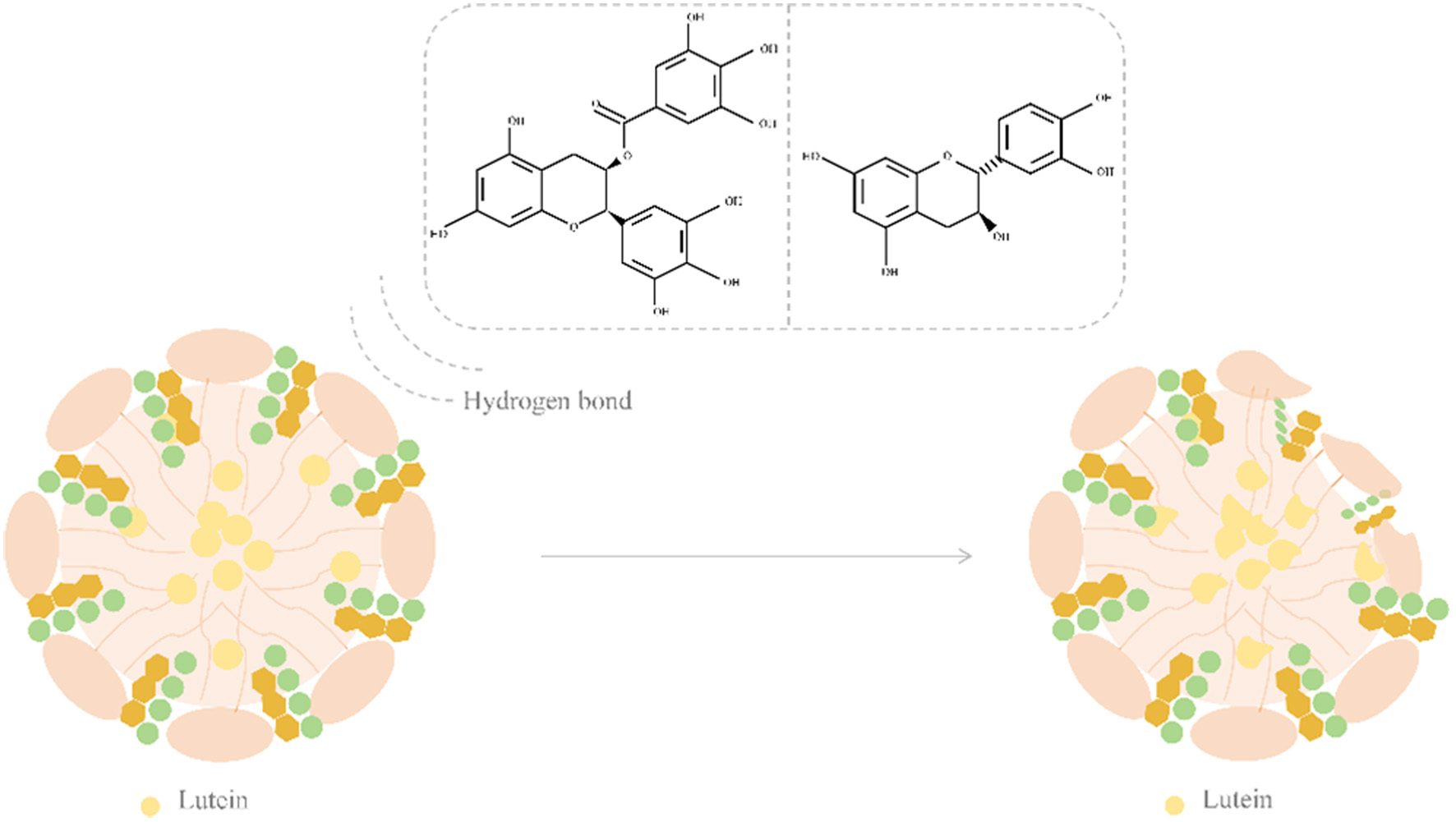
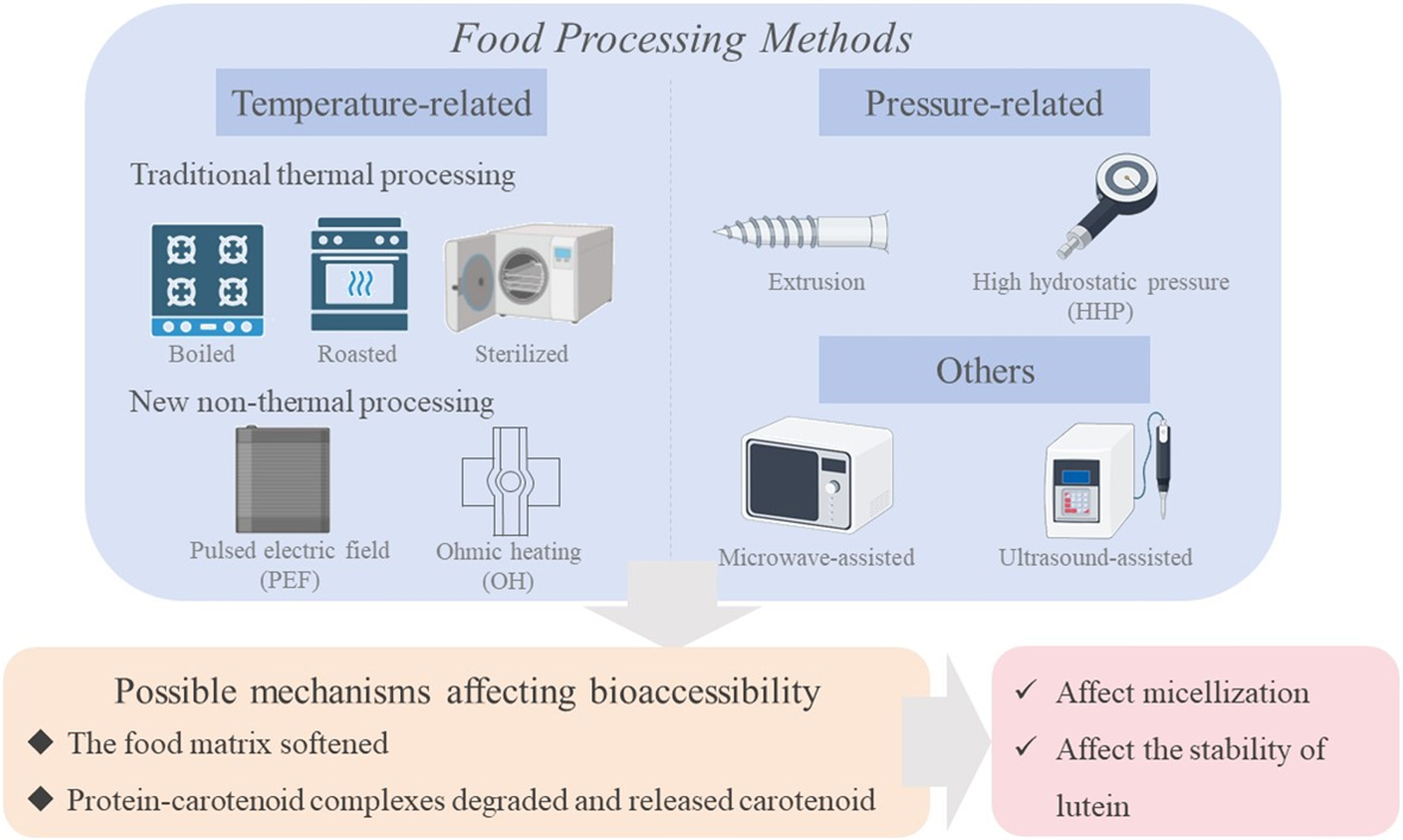
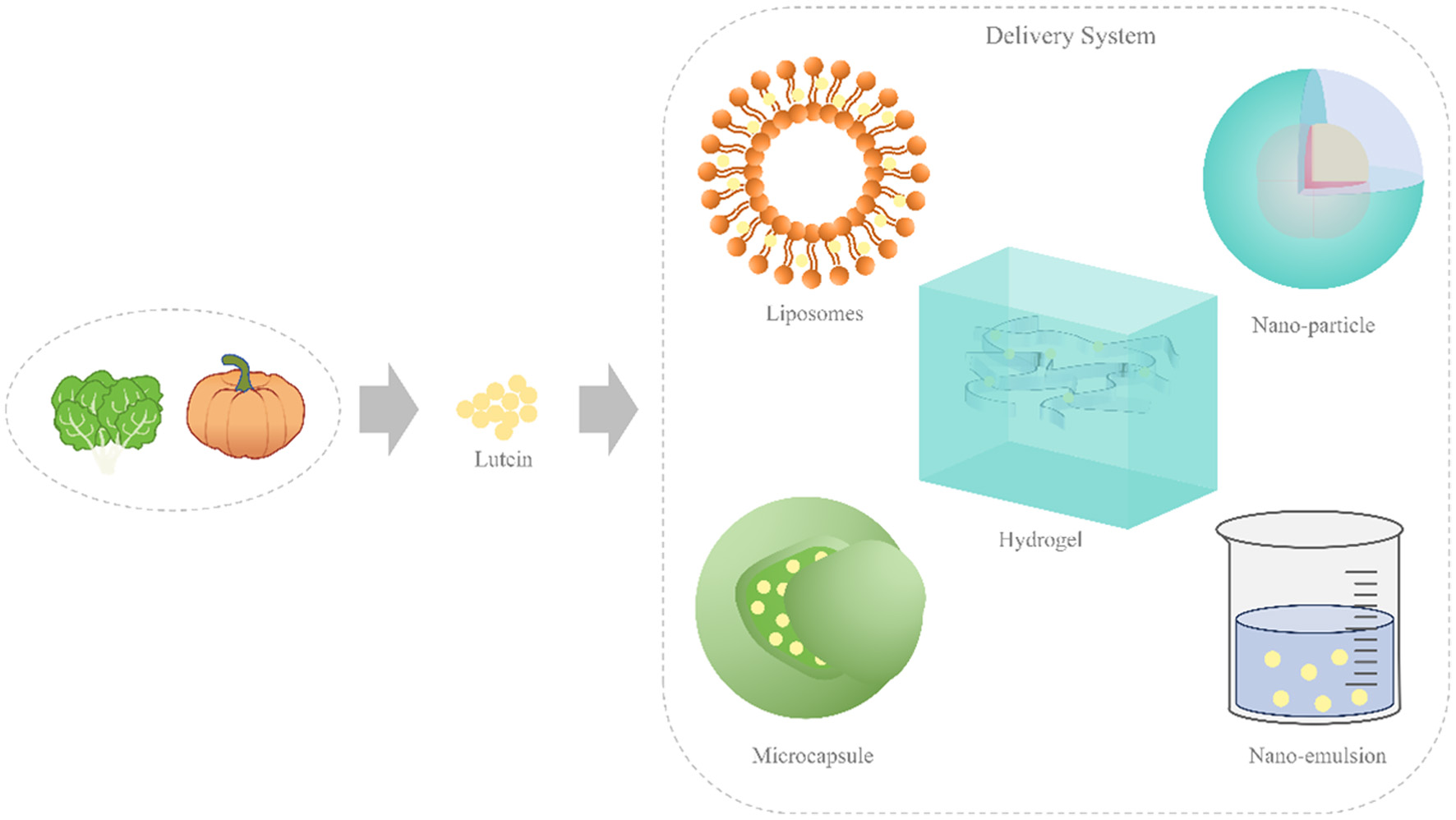
Tables
| Common lutein-riched foods | Content (μg/100 g) | Bioaccessibility (%) | Reference |
|---|---|---|---|
| Spinach | 7,142 | 19 | (Granado-Lorencio et al., 2007; O’Connell et al., 2007) |
| Kale | 2,860–3,500 | 8 | (de Sá and Rodriguez-Amaya, 2003; Schmidt et al., 2021) |
| Lettuce | 1,290–1,690 | 14 | (López et al., 2014; de Oliveira et al., 2020) |
| Red pepper | 1,411.5 | 54 | (O’Sullivan et al., 2010) |
| Pumpkin | 1,000 | 10 | (Bergantin et al., 2018) |
| Broccoli | 962 | 6 | (McInerney et al., 2007) |
| Green beans | 895 | 14 | (McInerney et al., 2007) |
| Maize | 587–593 | 51 | (Kuhnen et al., 2011; Zurak et al., 2021) |
| Carrot | 505 | 65 | (Molldrem et al., 2004) |
| Kiwi | 95 | 62 | (Granado-Lorencio et al., 2007) |
| Sweet Potato (Steamed) | 1,100 | 97 | (O’Connell et al., 2007; Donado-Pestana et al., 2012) |
| Egg yolk (cooked) | 1,051–1,622 | 80 | (Nimalaratne et al., 2012; Rodrigues et al., 2017) |
| Tomato paste (Sauce) | 83 | 92 | (Granado-Lorencio et al., 2007) |
| Nano-scale delivery system | Preparation conditions | Bioaccessibility | Reference |
|---|---|---|---|
| NE/NLC/SLN filled hydrogel | Rhamnolipid-stabilized nanoemulsion (NE)/nanostructured lipid carrier (NLC)/solid lipid nanoparticle (SLN) was incorporated into κ-carrageenan/konjac glucomannan (KC-KGM) composite hydrogel | The bioaccessibility of lutein was shown to be the highest in NE-FH (29.7%), followed by NLC-FH (28.2%) and SLN-FH (23.4%) | (Shu et al., 2024) |
| Lutein loaded composite nanoparticles | Zein/soluble soybean polysaccharide composite nanoparticles were fabricated using a facile antisolvent precipitation method to encapsulate lutein | The bioaccessibility of encapsulated lutein (32.1%) was greatly higher than that of non-encapsulated lutein (16.2%) | (Li et al., 2020) |
| lutein-loaded nanostructured lipid carriers (NLCs) | NLCs particles stabilized by ethyl lauroyl arginate, rhamnolipid, or tea saponin were fabricated by high-pressure microfluidization method. | During in vitro digestion, rhamnolipid-stabilized NLCs showed the slowest release of free fatty acids (50.9%) and provided an optimal sustained release for lutein with relatively high bioaccessibility (23.0%) | (Shu et al., 2023) |
| lutein-enriched nanoemulsions | Oleic-linoleic acid nanoemulsion | The composite nanoemulsion also showed exceptionally higher (87.4%) in vitro bioaccessibility than nonencapsulated or free lutein (15%). | (Toragall et al., 2021) |
| High internal emulsion | Lutein-enriched high internal phase emulsions (LE-HIPE) stabilized by egg yolk-modified starch complex | In vitro digestion results showed that high level of hydroxypropyl distarch phosphate (HPDSP) in LE-HIPE was beneficial to the improvement of the bioaccessibility of lutein. The highest bioaccessibility of lutein in egg yolk-HPDSP 4 was due to their smallest droplet size | (Xu et al., 2021) |
| Lutein composite nanoparticles | The nanoparticles were prepared with Stauntonia brachyanthera seed albumin (SBSA) through a heat induced self-assembly method which were modified by gum Arabic (GA) and carboxymethylcellulose (CMC). | The bioaccessibility of lutein increased from 17.5% to 46.8% after encapsulation into nanoparticles. | (Yu et al., 2022) |
| Lutein ternary composite nanoparticles | The controlled pH response method was used to treat insoluble goat milk casein insoluble polypeptide aggregates to obtain soluble nano delivery carriers (GMCs). Self assembly method for designing ternary nanoparticles (NP) of zein-GMC-chitosan (Z-GMC-CS) | Compared with Lut-Z-CS NPs, Lut-Z-GMC-CS NPs significantly enhance the protective effect of lutein, without any significant aggregation or degradation, and maintain the original spherical structure during digestion | (Du et al., 2023) |
| Lutein entrapped by WPI nanoparticles within the inner water phase of a double emulsion (W-L/O/W) | The water/oil/water double emulsions were prepared by applying a two-step emulsification procedure. The inner aqueous phase of all double emulsions contained whey protein isolate (WPI) particles obtained by desolvation using ethanol, the oil phase was composed by sunflower oil added with polyglycerol polyricinoleate (PGPR), while the outer aqueous phase contained WPI and xanthan gum | The bioaccessibility of lutein incorporated into the single emulsion was considerably lower than those observed for the double emulsions | (Nunes et al., 2023) |
| Lutein hydrogel | Lutein hydrogels were cross-linked with varying amounts of Ca2+ | The formation of lutein mixed micelles was hindered by the hydrogel with a Ca2+ content more than 7.5 mM, and lutein bioaccessibility was reduced | (Luo et al., 2022) |
| Lutein-loaded nanoemulsions and excipient nanoemulsions mixed with lutein-based dietary supplements (capsules and soft gels) | - | The bioaccessibility of lutein from the capsules (1.5%) and soft gels (3.2%) was relatively low when they were administered alone. However, the co-administration of excipient nanoemulsions significantly increased the bioaccessibility of lutein from both the capsules (35.2%) and soft gels (28.7%) | (Dai et al., 2021) |
| Lutein microparticles | Biopolymer microparticles were fabricated for the encapsulation and delivery of lutein by electrostatic complexation of sodium caseinate (NaCas) and sodium alginate (ALG) | Gastrointestinal tract (GIT) studies showed that the microparticles presented higher free fatty acids (FFAs) release and lutein bioaccessibility (51.3%) than emulsions (12.8%). | (Hao et al., 2022) |
| Lutein O/W emulsion system | Using oil-in-water emulsions stabilized with biopolymers. Commercially available octenylsuccinylated (OS) starches, including capsule TA® (CTA), HI-CAP®100 (HC), and Purity Gum® 2000 (PG), along with gum Arabic (GA) variants Ticaloid acacia Max® (TAM), TICAmulsion® 3020 (TM), and pre-hydrate gum Arabic (PHGA), were chosen as emulsifiers | The lutein emulsions stabilized by octenylsuccinylated starches, and gum arabic displayed 1.3∼2.3-fold, and 2.3-fold higher lutein bioaccessibility, respectively, compared to free lutein | (Zhang et al., 2024) |
| Nanostructured lipid carriers with different interface structures | This study compounded natural macromolecules whey protein isolate with small molecule surfactant Tween 80 to construct lutein nanostructured lipid carriers (Lutein-NLCs) with three different interface structures, including single-interface (S-Lutein-NLCs), composite-interface (C-Lutein-NLCs) and double-layer interface (D-Lutein-NLCs) Lutein-NLCs | The bioaccessibility of lutein in monolayer interface nanoliposomes reached 63.1%, significantly higher than that of bilayer and composite interface structured nanoliposomes (39.6% and 30.1%, respectively). Perhaps due to the dense interface structure formed by Tween 80 and whey protein isolate, the released lutein was restricted from being transferred from the digestive solution to micelles, thereby reducing its bioaccessibility | (Xu et al., 2023) |
| Lutein loaded in emulsions stabilized by egg yolk granules (EYG)- soy lecithin (SL) complex | Preparation of oil in water emulsion with EYG-SL dispersion, lutein and sunflower seed oil | The bioaccessibility of lutein in emulsion stabilized by EYG-SL complex was higher than that in emulsion stabilized only by egg yolk. Perhaps due to the increase in the proportion of lecithin, the surface tension of the digestive material decreased, which facilitated the micellization of lutein and improved its bioaccessibility | (Li et al., 2022) |
| Lutein-loaded emulsions stabilized by egg white protein-dextran-catechin conjugates (EWP-Dex-EC) | The conjugates were developed using a two-step method involving free radical grafting combined with the Maillard reaction. | The highest bioaccessibility of lutein was observed in the EWP-Dex-EC conjugate-stabilized emulsions, which was mainly attributed to the relatively smaller droplet size and stronger antioxidant activity. | (Gu et al., 2023) |
| Lutein-loaded nanoparticles based on zein and sophorolipid | Lutein was encapsulated in zein nanoparticles coated with sophorolipid (ZSLNPs). | In vitro studies showed that the ZSLNPs had great biocompatibility and bioaccessibility of lutein. ZSLNPs increased the bioaccessibility of lutein from 16.6% to 59.7%, possibly due to the formation of micelles in sophorolipids. | (Yuan et al., 2019) |
| Lutein-oleic acid -ovalbumin ternary complex | Construction of oleic acid-ovalbumin complex using ultrasound coupled pH driven method and encapsulation of lutein through non covalent interactions | The encapsulation efficiency of lutein micelles by oleic acid-ovalbumin complex increased by 56.9%, and its bioaccessibility reached 35.9–42.5% | (Liu et al., 2024) |
| Lutein loaded emulsions stabilized by corn fiber gums | A coarse oil-in-water emulsion was prepared by mixing each aqueous phase and each organic phase using a high-speed shear homogenizer for 2 min at 13,000 rpm. The resulting coarse emulsions were homogenized immediately using a single-pass laboratory-scale jet homogenizer three times at 50 MPa to obtain fine emulsion samples | Compared with corn oil, the emulsion prepared from corn fiber gum increased the bioaccessibility of lutein from 13.8% to 24.9–32.4%, and the emulsification significantly improved the bioaccessibility of lutein in emulsion | (Feng et al., 2017) |
| Chitosan-EGCG covalently modified lutein nanoliposomes (C-CS-EGCG-LNLs) | Preparation of covalent complex modified lutein nanoliposomes by free radical grafting method | The bioaccessibility of lutein modified with covalent complexes was higher, possibly due to chitosan inhibiting lipid release, and covalent complexes improving the interface layer of lutein nanoliposomes, preventing enzyme diffusion and decomposition, enhancing their ability to enter nanoliposomes and lutein micellization | (Yan et al., 2023) |
| Pickering emulsion | Hydrothermally prepared tea seed cake protein nanoparticles (TSCPN) were used to fabricate Pickering emulsion | Pickering emulsion stabilized by TSCPN significantly improved the bioaccessibility of lutein, up to 56.0%. This was attributed to the high hydrolysis of tea seed cake protein nanoparticles | (Liang et al., 2022) |
| Lutein-albumin nanoparticles | Chitosan-modified albumin nanoparticles containing lutein ware synthesized by a self-assembly technique | The bioaccessibility of lutein encapsulated by modified albumin nanoparticles increased from 19.3% to 57.3%, attributed to the deprotonation of chitosan amine groups in an alkaline environment, weakening the interaction between chitosan and albumin, resulting in the expansion of nanoparticles and the release of lutein | (Yu et al., 2022) |
| Soy protein isolate (SPI)-lutein coassembled nanoparticles | The samples underwent ultrasonication and then were disassembled into subunits before being reassembled by 40% (v/v) ethanol | The presence of SPI enhanced the bioaccessibility of lutein, primarily beacuse the protein encapsulation increased the solubility of lutein | (Cheng et al., 2024) |