| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 26, June 2024, pages 1-6
Beneficial effects of Docosahexaenoic acid consumption on brain health: A mini-review
Jadyellen Rondon Silvaa, Isabella Cristina Prescilioa, Klenicy Kazumy Lima Yamaguchib, Evandson José Anjos Silvac, Anderson Oliveira Souzaa, *
aFederal University of Mato Grosso, Department of Chemistry, Brazil
bFederal University of Amazonas, Institute of Health and Biotechnology, Brazil
cState University of Mato Grosso, Brazil
*Corresponding author: Anderson Oliveira Souza, Federal University of Mato Grosso, Department of Chemistry, Brazil., E-mail: anderson.souza@ufmt.br
DOI: 10.31665/JFB.2024.18375
Received: April 30, 2024
Revised received & accepted: June 21, 2024
| Abstract | ▴Top |
The aging of the world population has increased the number of neurodegenerative diseases, such as Alzheimer’s disease (AD), which affects millions of people. Despite studies aimed at understanding this complex and progressive disease, there is still no cure. However, many compounds with pharmacological properties are recommended for patients to experience a reduction in Alzheimer’s symptoms. This study focuses on highlighting the importance of docosahexaenoic acid (DHA) and its mechanism of action, aiming to contribute to the advancement of research in new therapies for neurodegenerative diseases such as Alzheimer’s and Parkinson’s. The role of nutrition in preventing dementia and AD raises increasing hope, with particular interest in dietary intake of omega-3 fatty acids, as brain tissue membranes are rich in omega-3 fatty acids, including DHA. We searched the PubMed database for original articles published in English from 2018 to July 2023 using appropriate search terms: dietary fatty acids, DHA, Alzheimer’s disease, and cognitive impairment. Furthermore, this mini-review provides a synopsis of recent literature involved in the processes of neurodevelopment and neuronal plasticity.
Keywords: Phospholipid DHA; Functional food; Neuroprotection; Dementia; Amyloid-plaques; Biological effects; Healthy diet
| 1. Introduction | ▴Top |
1.1. Neurodegenerative diseases
Several mechanisms or dysfunctions in nervous tissue have been associated with neurodegenerative processes, such as depletion or insufficient synthesis of neurotransmitters (mainly serotonin and dopamine), oxidative stress, impairment of intracellular calcium flow, and inadequate activation of ubiquitination, resulting in neuroinflammation and, in some cases, tissue atrophy. When neurons involved in the memory process are damaged, it impairs the synapse, leading to a decrease in learning ability and other cognitive functions (Chen et al., 2021).
Nowadays, it is estimated that 44 million people worldwide have some type of neurodegeneration and this number could double within 20 years (Montoliu-Gaya and Villegas, 2015). Data from the World Health Organization (March, 2023) reveals that 55 million people worldwide suffer from dementia, with an increase of nearly 10 million cases each year, of which 60 to 70% are attributed to Alzheimer’s disease (Ministry of Health, 2013).
1.2. Alzheimer
Alzheimer’s disease (AD) was first reported by the neuropathologist Alois Alzheimer in 1906 as a neurodegenerative disease with symptomatology associated with sleep disturbances, memory-related cognitive impairment, and sporadic manifestations of intense aggression. The brain alterations characteristic of AD are attributed to aging, oxidative stress, alteration in the transmission of neurotransmitters, and formation of amyloid plaques resulting from the cleavage of the amyloid precursor protein (APP) (Cipriani et al., 2011). People affected with AD show disorientation, cognitive impairment, memory loss, and behavioral changes leading to poor-quality life (Atri, 2019).
Currently, it is the number one chronic neurodegenerative disease affecting about 50 million people worldwide. Recent research has identified more than 20 pathological factors that are promoting disease progression. It was described that around 3% of people aged between 65 and 74 years, 17% of people between 75 and 84 years, and 32% of people aged 85 and over suffer from AD, which is why age is considered the biggest risk factor (Chen et al., 2021).
Different hypotheses have been proposed in the literature for a better understanding of AD among many the amyloid and non-amyloid pathways (Figure 1) are presently studied under such molecular mechanisms a disease-modifying treatment has been developed, which aims to improve cognitive functions and reduce or prevent disease progression (Breijyeh and Karaman, 2020; Rahman et al., 2020), preventing the accumulation of amyloid plaques.
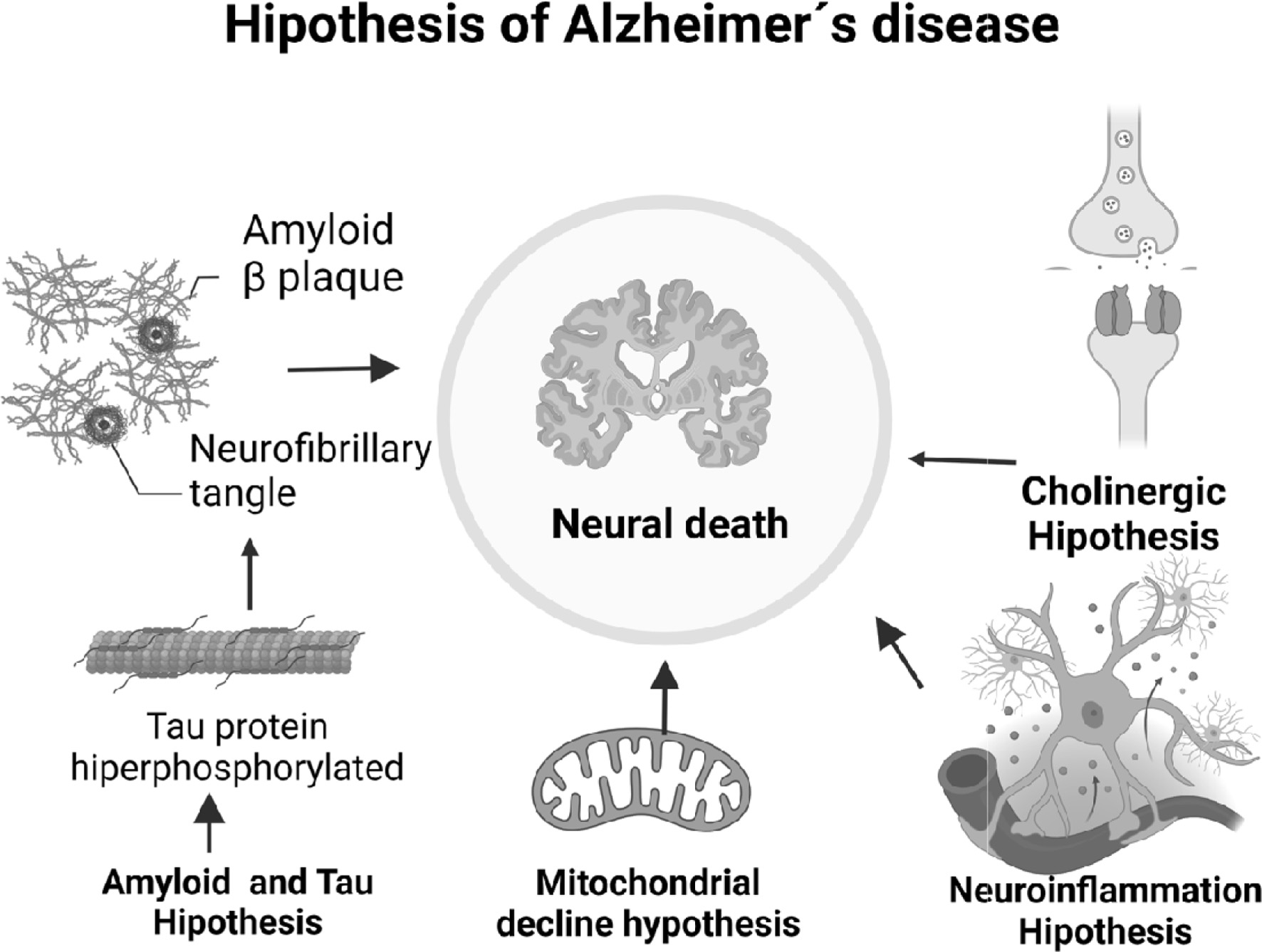 Click for large image | Figure 1. Neurodegenerative pathways in Alzheimer’s disease. |
1.2.1. Amyloid hypothesis
Amyloid precursor protein (APP) is a type I transmembrane glycoprotein, composed of a large ectodomain, an extracellular glycosylated N-terminus, and a shorter cytoplasmic C-terminus. This protein is expressed in several tissues; however, it is main expression is in the synapses of neurons, where it plays an important role in the pathogenesis of Alzheimer’s disease (Polanco et al., 2018; Chen et al., 2017).
APP in its structure presents a heparin-binding domain (HBD), copper ion binding domain (CuBD), region enriched in acidic amino acids (ACIDIC), Kunitz-type protease inhibition domain (KPI), central structural domain (CAPPD), and APP intracellular domain (AICD). Preliminary studies show that deletion of the ACIDIC domain reduces the aggregation of β-amyloid plaques (Liu et al., 2021).
According to the amyloid hypothesis, AD is characterized by the accumulation of senile plaques resulting from the increase in the β-amyloid peptide, which results in the hyperphosphorylation of the Tau protein, which contributes to the mechanisms of neuroinflammation (Onyango et al., 2021). The mechanism of accumulation of β-amyloid occurs through the amyloidogenic pathway, where the APP is cleaved by β-secretase, generating a short C-terminal fragment and soluble APPβ-secreted subsequently, the C-terminal fragment attached to the membrane is cleaved by γ-secretase, thus releasing the fragments that form senile plaques (Grimm et al., 2013; Grimm et al., 2017).
The APP protein has several isoforms of varying sizes from 305 to 770 amino acids. However, APP695 is the most abundant isoform in the brain and is mainly produced by neurons. APP is responsible for several functions, among them, the main ones are the regulation of synaptic formation and repair, neuronal transport, neurite outgrowth, neuronal cell differentiation, and cell adhesion (Rahman et al., 2020; Polanco et al., 2018; Chen et al., 2017; Liu et al., 2021). APP can be processed through two alternative pathways, the amyloid pathway, and the non-amyloid pathway (Figure 2).
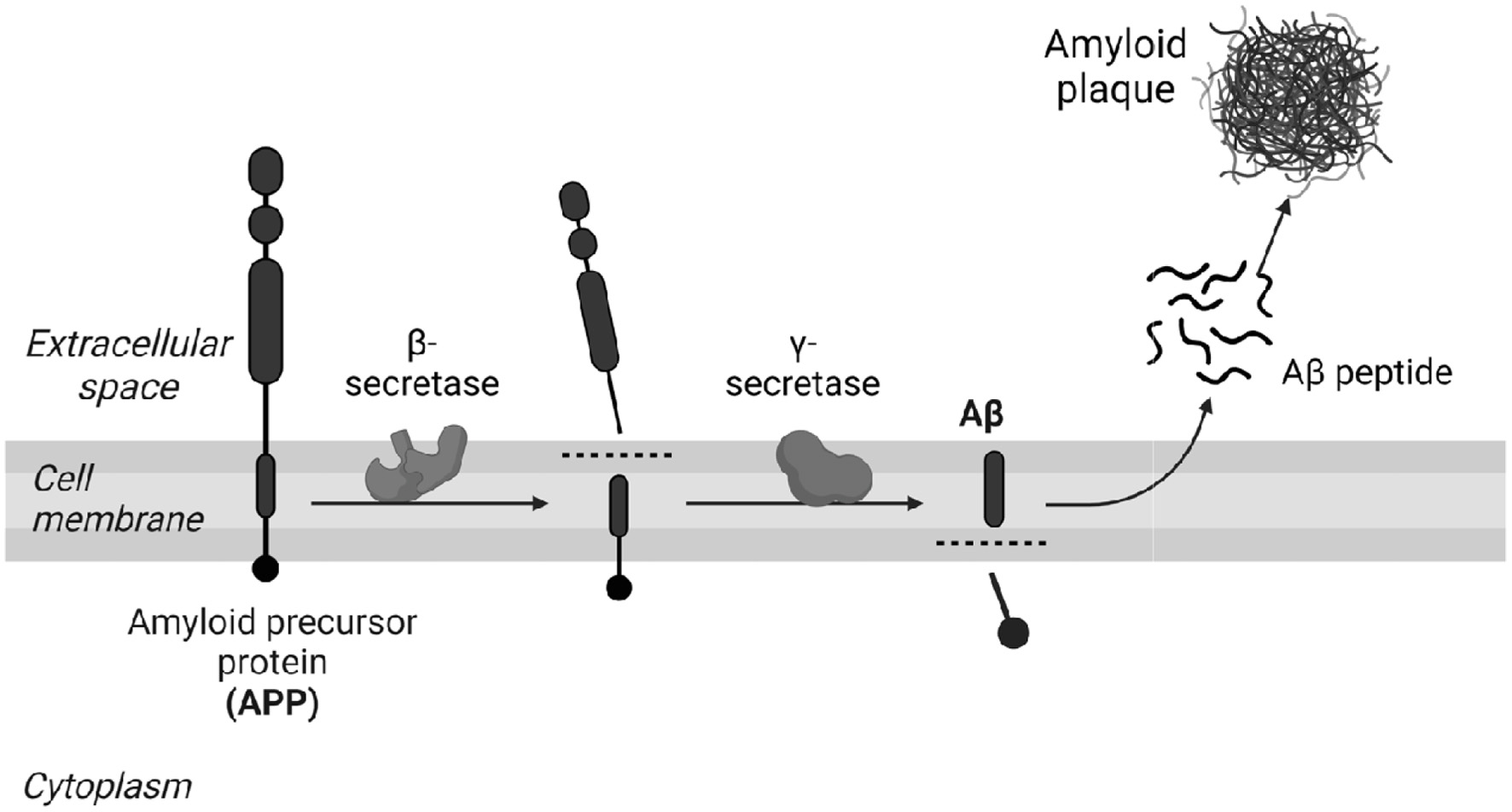 Click for large image | Figure 2. The non-amyloid and amyloid pathway. |
1.2.2. Non-amyloid pathway
In the non-amyloid pathway, APP cleavage occurs through the proteolytic enzyme α-secretase within the Aβ sequence, releasing the ectodomain soluble amyloid precursor protein α (sAPPα) into the extracellular medium and the fragment attached to the membrane by the carboxylic terminal 83 (CTF-83). Subsequently, the CTF-83 extracellular domain is cleaved by γ-secretase forming the P3 peptides and the intracellular domain (AICD). In this way, it impedes the formation of the β-amyloid peptide (Aβ) that is found in the brain of patients with Alzheimer’s (Nicolas and Hassan, 2014; Wilkins and Swedlow, 2017; Soldano and Hassan, 2014; Daekins and Small, 2014).
The non-amyloid pathway, in addition to preventing the generation and aggregation of β-amyloid plaques, the pathway leads to the formation of sAPPα, which has neuroprotective functions (Zhang et al., 2020). Furthermore, the pathway is linked to reduced toxic calcium signaling, potassium (K+) channel activation, decreased glutamate excitotoxicity, and decreased glucose deprivation (Wang et al., 2016).
APP cleavage occurs mainly by the activity of the α-secretase enzyme, a member of the disintegrin and metalloproteases (ADAM) families, with ADAM9, ADAM10, and ADAM17 being the main catalytic enzymes involved in the cleavage process (Zhang et al., 2020; Wang et al., 2016). The ADAM family are proteins that have characteristics of cell adhesion molecules and proteases. This family involves several processes, such as fertilization, angiogenesis, neurogenesis, and the proteolysis of some substrates, such as APP (Liu et al., 2021; Agostinho et al., 2015).
The three main proteins of the ADAM family act as secretases among them, ADAM10 is considered the main protease in neurons responsible for APP cleavage (Agostinho et al., 2015). The increase in ADAM10 activity protects the brain against the formation of β-amyloid plaques in Alzheimer’s disease (Dar and Glazner, 2020).
There is an increase in the production of sAPPα in response to electrical activity and the activation of muscarinic acetylcholine receptors, suggesting an increase in neuronal activity during the APP cleavage process by α-secretase (Chen et al., 2017). In addition, sAPPα promotes the proliferation of neuronal progenitors, neuroprotection, and synaptogenesis (Galvão et al., 2019). Also, sAPPα presents neuroprotective mechanisms against these damages, namely, through the regulation of intracellular Ca2+ homeostasis and an increase in the levels of cyclic nucleotides (cGMP) in neuronal cells, inhibiting the high levels of Ca2+, causing oxidative damage to mitochondria, neuronal dysfunction, and activation of proteins related to apoptosis through the inhibition of N-methyl-D-aspartate (NMDA) receptors (Liu et al., 2021; Habib et al., 2017). In addition, the neuroprotection mechanism evidenced by sAPPα is by limiting neuronal excitability through the activation of potassium (K+) channels and modulation of glutamate neurotoxicity by inhibiting NMDA currents (Habib et al., 2017).
The glutaminergic NMDA receptor is responsible for two actions in the brain: under physiological conditions, when activated it increases the secretion of sAPPα. However, when this activation reaches the state of excitotoxicity, the NMDA activity changes and becomes an inducer of the production of β-amyloid, with a lower activity of α-secretase (Galvão et al., 2019).
1.3. Therapeutic action in the amyloid pathway
Although more than a century has passed since the discovery of this pathology, until the year 2020 only two classes of drugs were reported for the treatment of the symptoms of the disease (Breijyeh and Karaman, 2020). However, no effective long-term treatments have been identified for this condition. Considering that the World Health Organization (WHO, 2023) reports that 55 billion people are affected by dementia, where 70% of cases are attributed to AD, and is expected to increase by 2050, it is necessary to advance research in the field.
Existing treatments for Alzheimer’s disease (AD) are based on symptomatic treatment, where the main pharmaceutical therapy available on the market is the use of cholinesterase inhibitors. Furthermore, they can cause adverse effects, as was the case with tacrine, donepezil, rivastigmine, and galantamine, none of these drugs can reverse, stop, or even slow down the damage and destruction of neurons that cause AD symptoms and make the disease fatal (Chen et al., 2021; Ferreira et al., 2022). Additionally, other therapies are described to reduce or prevent the progression of the pathology and it is established in treatments without drug intervention, among them physical activity and food supplementation (Breijyeh and Karaman, 2020).
1.4. Functional food
A diet of plants, animals, and microorganisms provides abundant bioactive compounds with complex structures and even pharmacological properties. Therefore, natural products and their isolated compounds have been studied for the treatment of neurodegenerative diseases, such as AD (Chen et al., 2021; Yamaguchi and Souza, 2020).
1.5. Omega-3
Lipids are the main constituents of the membrane, and they have a possible effect on the processing of APP, therefore, lipid alterations (impairment or reduction) imply cell malfunction, and in the case of neural cells, damage is neurodegenerative diseases (Grimm et al., 2017).
Among all tissues of the mammalian body, the nervous system has the largest lipid content and complexity, of which the last can be attributed to polyunsaturated fatty acids. Numerous aspects of brain function, structure, and metabolism rely on adequate eicosapentaenoic acid and docosahexaenoic acid levels (Custers and Kiliaan, 2022). Docosahexaenoic acid [22:6(n-3)] (DHA) is a polyunsaturated fatty acid accounting for ∼15% of the fatty acid composition in the adult human prefrontal cortex (McNamara, 2010).
DHA is the major constituent of the phospholipid portion of receptor cells, synthesized from alpha-linolenic acid (18:3 n-3), but with reduced production in mammals due to low expression or absence of the enzyme required for its biosynthesis. Therefore, it is commonly obtained through the diet, especially by consuming marine animals from cold waters. The chemical structure with strategic unsaturations in its molecule (Figure 3) gives DHA an important role in cell membranes and acts as a transporter in the blood-brain barrier, in low concentrations in the brain it has been associated with neurodegenerative diseases such as Parkinson’s and Alzheimer’s (Xiao et al., 2022; Sugasini et al., 2020). Despite being a constituent of the cell membrane, its endogenous production is limited, and it is necessary to obtain it from exogenous sources (Patrick, 2019).
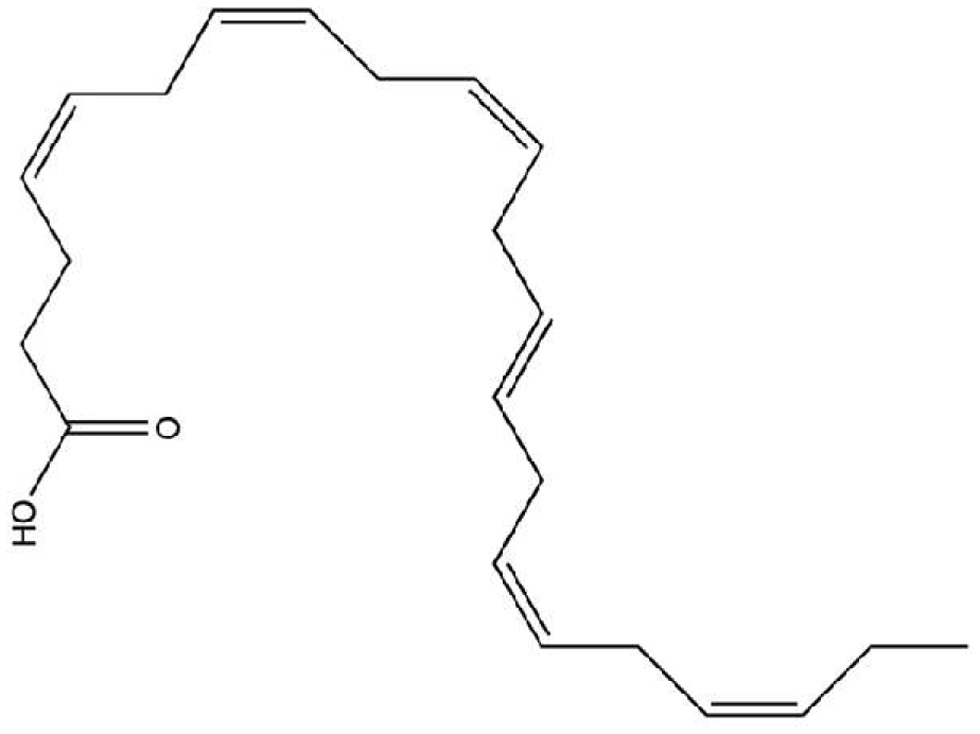 Click for large image | Figure 3. Docosahexaenoic acid [22:6(n-3)] (DHA) structure. |
Some mechanisms have been suggested for the action of DHA in AD, one of them being the repair of brain cells through neurotrophic (McNamara, 2010; Sun et al., 2018; Sona et al., 2018), anti-apoptotic (Xu et al., 2019; Díaz et al., 2021), anti-oxidative and anti-inflammatory signaling (Wiktorowska-Owczarek et al., 2015; Perís-Martínez et al., 2023), preventing the aggregation of Aβ and tau protein (Cardoso et al., 2016; Hashimoto et al., 2017) as well as through enhancing cholinergic signaling (Chen et al., 2021; Souza et al., 2017; Souza et al., 2019), however, this mechanism still has gaps. In addition to interacting with signaling agents, DHA is also related to lipid transporters, and membrane fluidity so its performance can be directly affected by its transport and delivery (Sun et al., 2018).
1.5.1. Neurotrophic activity
During perinatal development of the human brain, cortical concentrations of DHA increase sharply in association with active periods of neurogenesis, neuroblast migration, differentiation, and synaptogenesis (McNamara, 2010). In addition, DHA positively regulates cortical neurogenesis, neuroblast migration, neuronal differentiation/arborization, and brain-derived neurotrophic factor (BDNF). BDNF is involved in the regulation of energy homeostasis and is a useful biomarker for assessing neurologic disorders (Sugasini et al., 2020; Sun et al., 2018; Sona et al., 2018).
1.5.2. Anti-apoptotic signaling
DHA inhibits the expression of IL-1β, inhibits IL-1β-induced cell apoptosis, and has a certain inhibitory effect on the activation of the mitogen-activated protein kinase (MAPK) signaling pathway (Xu et al., 2019). In addition, pro-apoptotic proteins Bik and Bax are totally or partially counteracted by treatments with DHA. On the other hand, anti-apoptotic proteins Bcl-2, Bcl-xl, and Bfl-1 were enhanced by DHA (Díaz et al., 2021).
1.5.3. Anti-inflammatory signaling
The involvement of inflammatory mediators interleukins (IL), tumor necrosis factor-alpha (TNF-α), matrix metalloproteinases (MMP-9), oxidative stress-related products, and nutritional and/or metabolic imbalance, affects a variety of metabolites. DHA reduced values of inflammatory markers, including interleukin (IL)-4, IL-6, and vascular endothelial growth factor (VEGF-A) (Perís-Martínez et al., 2023; Yum et al., 2016; Lv and Xu, 2022). The metabolism of docosahexaenoic acid (DHA) gives prostanoids series 3 (PGE3, PGI3, TXA3) that show anti-inflammatory properties (Wiktorowska-Owczarek et al., 2015).
1.5.4. Antioxidant effect
During mitochondrial metabolism, oxygen receives four electrons resulting in water; however, 10% of the oxygen is univalently reduced, retaining unpaired electrons. This formed anion becomes known as a reactive species, interacting with other molecules and generating new reactive species. Therefore, these species are products of mitochondrial metabolism and perform various functions. However, the excessive generation of such species can cause tissue damage, as they interact with proteins and lipids (Halliwell, 1992).
Organisms have developed antioxidant defense mechanisms to control, neutralize, or inhibit the excess of reactive oxygen species (ROS) by storing and transporting iron and copper ions in forms that will not catalyze the formation of reactive radicals, enzymatic antioxidant system (Halliwell, 1992), transcriptional regulation (Tonelli et al., 2018). Another existing mechanism of antioxidant defense is exogenous, compounds derived from food with chelating, neutralizing, and conversion power into molecules with lower reactivity (Pisoschi and Pop, 2015), and the nuclear factor erythroid 2-related factor 2 (Nrf2) is the key transcription factor identified for transmitting the inducer signal to the antioxidant response element (Borgonovi et al., 2023).
The antioxidant acts as scavengers or neutralizers of ROS or reactive nitrogen species (RNS), making them potential agents to prevent oxidative stress, thereby playing a therapeutic role in various diseases including cancer, diabetes, and neurodegenerative diseases as well as aging (Apak et al., 2016). DHA activates peroxisome proliferator-activated receptor-γ and induces catalase, which inhibits oxidative stress-mediated inflammatory signaling (Jeong and Kim, 2017). Also, transcriptional regulation of brain antioxidant defense by DHA affects other members of the glutathione/glutaredoxin system as well as the thioredoxin/peroxiredoxin system (Díaz et al., 2021).
| 2. Materials and methods | ▴Top |
2.1. Search strategy
We conducted a systematic bibliographic search for studies that examined the role of omega-3 PUFAs in neurodegenerative processes using “docosahexaenoic acid”, “DHA”, “fatty acid, omega-3”, and “Alzheimer” as keywords. In addition, only reviews (described from July 2013 to 2023) were identified by searching electronic databases such as PubMed, and Scielo, including publications in English, Spanish, and Portuguese. The studies eligible for this review included trials carried out on animal models, or cell culture exposed to molecules involved in the etiology of neurodegenerative diseases.
| 3. Conclusion | ▴Top |
This review provides an overview of the neuroprotective effects of docosahexaenoic acids and marketed as a functional food. Recent studies with the DHA described here have shown significant antioxidant, neurotrophic, and genetic regulators in the brain. Continuing research is encouraged to understand the mechanism associated with experimental assays and subsequent clinical analyses to comprehend the role of this biomolecule in neurodegenerative diseases. Therefore, new research should be conducted considering the enormous potential in the mechanisms to protect against oxidative stress or neuronal degeneration or even potentially delay and prevent associated pathologies through the diet of polyunsaturated acids.
Acknowledgments
The authors are grateful to the Federal University of Mato Grosso, the Federal University of Amazonas and the State University of Mato Grosso for the financial support. Also, thank you to the two anonymous reviewers for their constructive criticism and corrections on this manuscript.
Conflict of interest
The authors declare no conflicts of interest.
| References | ▴Top |