| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 25, March 2024, pages 72-80
Exploring the anti-inflammatory effect of clove water extract in lipopolysaccharide-stimulated RAW264.7 cells and mouse peritoneal macrophages
Sellen Gurusmatikaa, Momoko Ishidab, c, Kosuke Nishia, b, c, Takuya Sugaharaa, b, c, *
aDepartment of Applied Bioresource Science, The United Graduate School of Agricultural Sciences, Ehime University, Matsuyama, Ehime 790-8566, Japan
bDepartment of Bioscience, Graduate School of Agriculture, Ehime University, Matsuyama, Ehime 790-8566, Japan
cFood and Health Function Research Center, Ehime University, Matsuyama, Ehime 790-8566, Japan
*Corresponding author: Takuya Sugahara, Department of Bioscience, Graduate School of Agriculture, Ehime University, Matsuyama, Ehime 790-8566, Japan. Tel: +81-89-946-9863; E-mail: sugahara.takuya.mz@ehime-u.ac.jp
DOI: 10.31665/JFB.2024.18373
Received: January 31, 2024
Revised received & accepted: March 6, 2024
| Abstract | Top |
Clove (Syzygium aromaticum L) is a precious spice that has been extensively used by many countries over the centuries to add flavor and for medicinal purposes. Because of its abundance of phytochemical compounds, clove has been shown to have positive benefits on human health. Hence, we investigated the anti-inflammatory activity of clove water extract (CWE) in LPS-stimulated RAW264.7 cells and mouse peritoneal macrophages. The results showed that CWE significantly inhibited the production of IL-6 and TNF- in a dose-dependent manner, as well as the production of nitric oxide (NO), without any cytotoxic effects at less than 20 mg/mL, through down-regulating IL-6, TNF-, and iNOS gene expression. Moreover, CWE impeded the MAPKs and inhibited the translocation of NF-B from the cytosol to the nucleus. These results suggest that CWE possesses anti-inflammatory properties by inhibiting the MAPKs and NF-B pathways.
Keywords: anti-inflammation; cytokines; clove; macrophages; MAPK; NF-B
| 1. Introduction | Top |
Inflammation plays a key role in the bodys immune defense mechanism (Venkatalakshmi et al., 2016) and thus has been of special concern to scientists around the world for a long time. It is an immune response that recognizes and neutralizes invasion by harmful microbes, prevents infection, and initiates the wound-healing process (Duque and Descoteaux, 2014; Oishi and Manabe, 2018). Inflammation can be either chronic or linked to disorders such as mental illness, cardiovascular diseases, cancer, autoimmune diseases, and diabetes (Netea et al., 2017; Hirano, 2021). Cell and tissue damage may arise from an unbalanced response between immune stimulation and resolution, which causes excessive inflammatory cytokine production (Leiherer et al., 2013; Duque and Descoteaux, 2014).
Macrophages, important components of the immune system, are capable of eliminating pathogens, destroying dead cells, and initiating inflammation through cytokine signaling regulation and growth factors (Sigola et al., 2016; Hamidzadeh et al., 2017). Suppressing production of inflammatory mediators is one effective therapeutic approach for treating inflammatory disorders and combating harmful chronic inflammation (Scull et al., 2010; Oishi and Manabe, 2018). Recently, healthy food choices and therapeutic compounds from herbal medicine have gained interest for treating and reducing the risk of inflammation diseases. Natural products also have several other characteristics such as being non-toxic, effective, and secure in applying pharmacologically due to their pleiotropic immunomodulatory properties (Ali et al., 2008; Jiang, 2019; Ugbogu et al., 2021).
Clove (Syzygium aromaticum L) is a dried flower bud from the clove tree in the family Myrtaceae, and is commercially cultivated in Indonesia, Madagascar, India, and China. Since ancient times, clove has been largely used as a spice in cooking to improve flavor, as a cosmetic, and for medicinal purposes (Corts-Rojas et al., 2014). This plant is abundant in phytochemical compounds such as eugenol, gallic acid, and eugenol acetate. The essential oil of clove has been reported to possess positive biological activities such as antioxidant, antimicrobial, antinociceptive, anti-depressant, and anticancer (El-Maati et al., 2016; Hu et al., 2018; Haro-Gonzlez et al., 2023). However, there are only a limited number of studies on the potential of water extracts of clove. Hence, this study aimed to investigate the anti-inflammatory effect of clove water extract (CWE) in lipopolysaccharide (LPS)-stimulated RAW264.7 cells and mouse peritoneal macrophages (P-mac), and its mechanism of action involved in the iNOS, mitogen-activated protein kinase (MAPK), and NF-B signaling pathways.
| 2. Materials and methods | Top |
2.1. Materials
Clove powder was purchased from S&B Foods Inc. (Tokyo, Japan). Roswell Park Memorial Institute 1640 (RPMI 1640) medium and WST-8 reagent were obtained from Nacalai Tesque (Kyoto, Japan). Fetal bovine serum (FBS), Dulbecco's modified Eagles medium (DMEM), and LPS from Escherichia coli 026/B6 were purchased from Sigma-Aldrich (St. Louis, MO, USA). The enzyme-linked immunosorbent assay (ELISA) kits for IL-6 and TNF- were purchased from BioLegend (San Diego, CA, USA) and Invitrogen (Carlsbad, CA, USA), respectively. Goat anti-rabbit IgG antibody and antibodies against ERK1/2, phosphorylated ERK1/2, JNK, phosphorylated JNK, p38, phosphorylated p38, histone H3, NF-B p65, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Danvers, MA, USA). The reagents and chemicals used were of analytical grade.
2.2. Sample preparation
Based on previous research (Pandey et al., 2024) which mentions several components contained in clove and following the method by Nishi et al. (2021), the water extraction of clove components was performed as followings. Clove powder was suspended in distilled water at 100 mg/mL and homogenized overnight at 12C. The mixture was subsequently centrifuged at 70,000 g for 1 h at 4C, filtrated using a 0.45 m membrane, and freeze-dried. The freeze-dried clove extract was weighed and then dissolved in distilled water at 40 mg/mL. The clove extract was filtered using a 0.22 m membrane for sterilization. This water extract was referred to as CWE and used for further experiments.
2.3. Cells and cell culture
The mouse macrophage-like cell line RAW264.7 cells were obtained from the European Collection of Authenticated Cell Cultures (ECACC, London, UK). The medium used to culture the cells was DMEM supplemented with 10% FBS and antibacterial compounds (100 U/mL of penicillin and 100 g/mL of streptomycin). The cells were incubated at 37C under humidified 5% CO2 in air. Meanwhile, to collect P-mac, 8-week-old female BALB/c mice (Japan SLC, Hamamatsu, Japan) were injected into the peritoneum with 3 mL of sterile 3.0% thioglycolate and left for 3 days to deteriorate the peritoneum. Three days after injection, peritoneal exudate cells were collected through lavaging the peritoneal cavity by injection of 3 mL of phosphate-buffered saline (PBS, pH 7.4). Harvested cells were centrifuged at 200 g for 5 min at 4C, and the cell pellet was washed with RPMI 1640 medium and centrifuged again. The cell pellet was then suspended in 10% FBS-RPMI 1640 medium and incubated for 2 h. After incubation for 2 h, adherent cells were used as P-mac. In the subsequent experiments, P-mac was detached by pipetting in cold PBS. All animal experiments described were conducted by protocols approved by the Ehime University Animal Care and Use Committee and were performed under applicable guidelines and regulations for the Care and Use of Laboratory Animals of Ehime University.
2.4. Cell viability
The cytotoxicity of CWE against RAW264.7 cells and P-mac was analyzed using WST-8 reagents according to a published method (Ishida et al., 2022). The RAW264.7 cells and P-mac were seeded in 96-well culture plates (Violamo, Osaka, Japan) at a concentration of 2.0 105 cells/well in 10% FBS-DMEM and 10% FBS-RPMI 1640 medium, respectively, and cultured for 18 h in a CO2 incubator. After removing the medium, 200 L of fresh 10% FBS-DMEM or 10% FBS-RPMI 1640 medium containing 1 g/mL of LPS and serial concentrations of CWE were added to each well and cultured for 6 h. Subsequently, after washing the cells with fresh medium, 200 L of fresh medium containing 5% WST-8 was added to each well, and the cells were incubated for 30 min at 37C in the dark. Cell viability was measured at 450 nm with reference at 655 nm using an iMark microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
2.5. Cytokine production
The amounts of IL-6 and TNF- produced by the LPS-stimulated RAW264.7 cells and P-mac were measured according to a previous study (Gurusmatika et al., 2023) by using the mouse IL-6 and mouse TNF- ELISA kits, respectively. The RAW264.7 cells and P-mac at a density of 2.0 105 cells/well were seeded in 96-well culture plate in 10% FBS-DMEM for the RAW264.7 cells or 10% FBS-RPMI 1640 medium for the P-mac in a CO2 incubator. After 18 h of incubation and the culture medium was discharged, the cells were treated with fresh 10% FBS-DMEM for the RAW264.7 cells or 10% FBS-RPMI 1640 medium for the P-mac. To stimulate the cells, 1 g/mL LPS was added at the indicated periods in the presence or absence of CWE, then incubated for 6 h in a CO2 incubator. After incubation, the culture media were collected, and the concentrations of IL-6 and TNF- were then measured using the respective ELISA kits. The assays were done in triplicate.
2.6. Nitric oxide (NO) production
RAW264.7 cells were seeded into a 96-well culture plate (2.0 105 cells/well) in 10% FBS-DMEM and cultured for 28 h in a CO2 incubator. After incubation, fresh medium containing 1 g/mL of LPS, 20 ng/mL of IFN-, and serial concentrations of CWE were administered to change the medium, followed by incubation for 24 h in a CO2 incubator. The NO concentration in the culture medium was analyzed by using a Griess Reagent System kit (Promega, Madison, WI, USA) according to the manufacturers instructions. The absorbance of the mixture solution at 540 nm was measured using the iMark microplate reader.
2.7. Real-time RT-PCR
RAW264.7 cells were cultured in a 24-well culture plate at 3.0 105 cells/well in 10% FBS-DMEM for 18 h in a CO2 incubator. After preculture, the cells were treated with 1 g/mL of LPS and serial concentrations of CWE or distilled water as control in 10% FBS-DMEM, then incubated for 6 h in a CO2 incubator. Total RNA was collected from the cells using Sepasol-RNA I super G (Nacalai Tesque) and used as a template for cDNA synthesis. As described by Ishida et al. (2019), real-time RT-PCR was performed with slight modifications. In brief, the reagents were prepared as follows: 10 L of Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan), 1 M forward primer, 1 M reverse primer, and 0.1 g of a cDNA sample. The PCR amplification conditions were 3 s at 95C and 30 s at 60C. The PCR was performed using a StepOnePlus System (Applied Biosystems, Foster City, CA, USA). Relative gene expression was normalized to the -actin gene expression level. Specific oligonucleotide sequences used for each gene are as follows: mouse -actin, 5-CATCCGTAAAGACCTCTATGCCAAC-3 (forward) and 5-ATGGAGCCACCGATCCACA-3 (reverse); mouse TNF-, 5-CTACTCCCAGGTTCTCTTCAA-3 (forward) and 5-GCAGAGAGGAGGTTGACTTTC-3 (reverse); mouse IL-6, 5-AAGCCAGATCCTTCAGAGAGAT-3 (forward) and 5-TTGGATGGTCTTGGTCCTTAGC-3 (reverse); mouse iNOS, 5-CCAAGCCCTCACCTACTTCC-3 (forward) and 5-CTCTGAGGGCTGACACAAGG-3 (reverse).
2.8. Western blotting
RAW264.7 cells were seeded in a 6-well culture plate at 5.0 105 cells/well in 10% FBS-DMEM and cultured in a CO2 incubator for 18 h. After the cells were twice washed with sterilized PBS, the medium was changed to 10% FBS-DMEM containing 1 g/mL LPS and serial concentrations of CWE or distilled water as control, then incubated for 15 min in a CO2 incubator. The cells were lysed using a homogenizer and centrifuged at 12,000 rpm, 4C for 1 min (cytosol) and 5 min (nucleus). The cytosolic and nuclear proteins were prepared using a CelLytic NuCLEAR Extraction kit (Sigma-Aldrich). After processing the protein separation using SDS-PAGE, the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Hybond-P; GE Healthcare, Buckinghamshire, UK) using a transblotting instrument. Immunoblotting with various antibodies was performed as previously described (Gurusmatika et al., 2017). Specific protein and serial concentrations of CWE or distilled water as control bands were visualized using a ChemiDoc XRS Plus apparatus (Bio-Rad Laboratories), and the chemiluminescent intensity was quantified using Quantity One software (Bio-Rad Laboratories).
2.9. Statistical analysis
All experiments were repeated in triplicate. Data were shown as mean standard deviation (SD). Differences among groups were tested using the Tukey multiple comparison test. *p < 0.05, **p < 0.01, and ***p < 0.001 against control were considered statistically significant.
| 3. Results | Top |
3.1. Effect of CWE on cell viability of LPS-stimulated RAW264.7 cells and P-mac
The cytotoxic effect of CWE was evaluated using a WST-8 assay to determine the optimal concentration that is acceptable for subsequent experiments. A cell viability test was performed using a WST-8 kit after 6 h incubation of the RAW264.7 cells and P-mac with the addition of 1 g/mL of LPS and serial concentrations of CWE. As shown in Figure 1a, the survival rate was from 83.5 to 113%, showing that there was no significant cytotoxicity of CWE to the LPS-stimulated RAW264.7 cells at the tested concentrations. Similarly, Figure 1b shows that CL also did not affect the cell viability of P-mac. The WST-8 assay results indicated that the viability of the RAW264.7 cells and P-mac is not affected by CWE at the various concentrations tested. Therefore, the subsequent experiments were performed at less than 40 mg/mL of CWE.
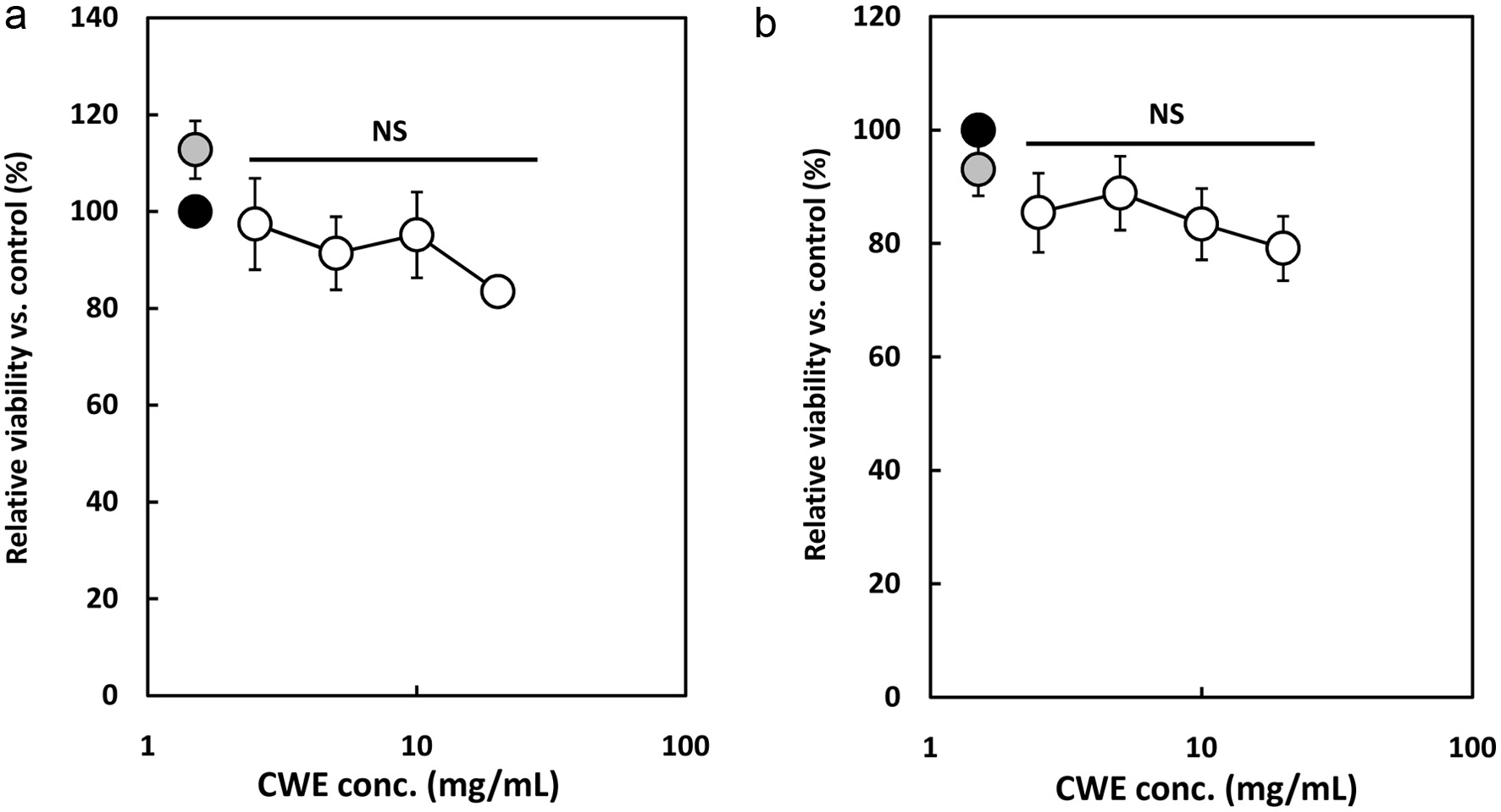 Click for large image |
Figure 1. Effect of clove water extract (CWE) on the cell viability of lipopolysaccharide (LPS)-stimulated RAW264.7 cells and mouse peritoneal macrophages (P-mac). The RAW264.7 cells and P-mac were treated with 1 g/mL of LPS and serial concentrations of CWE for 6 h. Relative viable cell number was then measured using a WST-8 reagent. Data are presented as the mean SD (n = 3). NS indicates no statistical significance against control (LPS) using the Tukey test. (a) RAW264.7 cells. (b) P-mac. Black circle: LPS without CWE (control); grey circle: distilled water without LPS (blank); open circles: LPS with CWE. |
3.2. Effect of CWE on cytokine production in LPS-stimulated RAW 264.7 cells and P-mac
Macrophages regulate inflammation and trigger the production of pro-inflammatory cytokines such as IL-6, IL-1, and TNF- through several inflammation signaling pathways when undergoing LPS stimulation (Scull et al., 2010; Duque and Descoteaux, 2014). Therefore, the effect of CWE on IL-6 and TNF- production in the LPS-stimulated macrophages was analyzed. As shown in Figure 2a, LPS treatment stimulated the production of IL-6 and TNF- by the RAW264.7 cells compared with control, as well as in the P-mac experiment (Figure 2b). CWE significantly suppressed the production of IL-6 and TNF- by the RAW264.7 cells in a dose-dependent manner (Figure 2a). Similar results were also observed in P-mac (Figure 2b). According to these results, CWE has anti-inflammatory properties by inhibiting IL-6 and TNF- production by not only the macrophage cell line but also the primary macrophages.
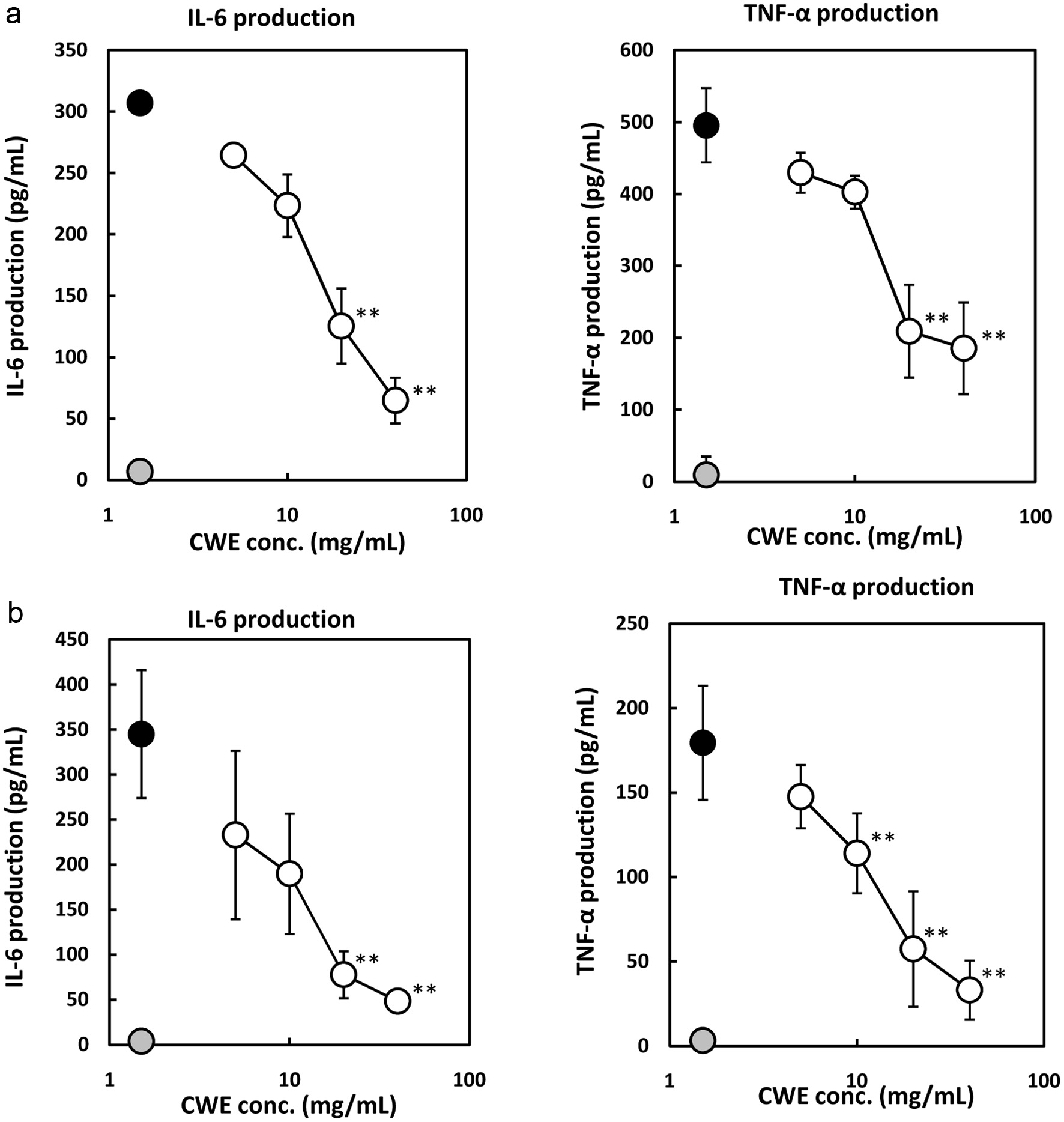 Click for large image |
Figure 2. Effect of CWE on IL-6 and TNF- production by LPS-stimulated RAW264.7 cells and mouse peritoneal macrophages (P-mac). The RAW264.7 cells and P-mac were treated with 1 g/mL of LPS and serial concentrations of CWE for 6 h. The culture medium was subsequently used for measurement with the ELISA kits. Data are presented as the mean SD (n = 3). **p < 0.01 against control (LPS) using the Tukey test. (a) RAW264.7 cells. (b) P-mac. Black circle: LPS without CWE (control); grey circle: distilled water without LPS (blank); open circle: LPS with CWE. |
3.3. Effect of CWE on mRNA expression of the LPS-stimulated RAW264.7 cells
The mRNA gene expressions of IL-6 and TNF- were analyzed using RT-PCR to determine the mechanism of the anti-inflammatory effect of CWE. Similarly to the previous result in the ELISA experiment, as shown in Figure 3, LPS treatment (control; black circle) markedly increased the gene expression levels of IL-6 and TNF- compared with blank (distilled water without LPS; gray circle), while CWE treatment significantly downregulated their gene expression levels at 10 mg/mL (p<0.01) in the LPS-stimulated RAW264.7 cells (open circles). These results suggested that the mode of action of the anti-inflammatory activity of CWE was related to the inhibition of inflammatory gene transcription.
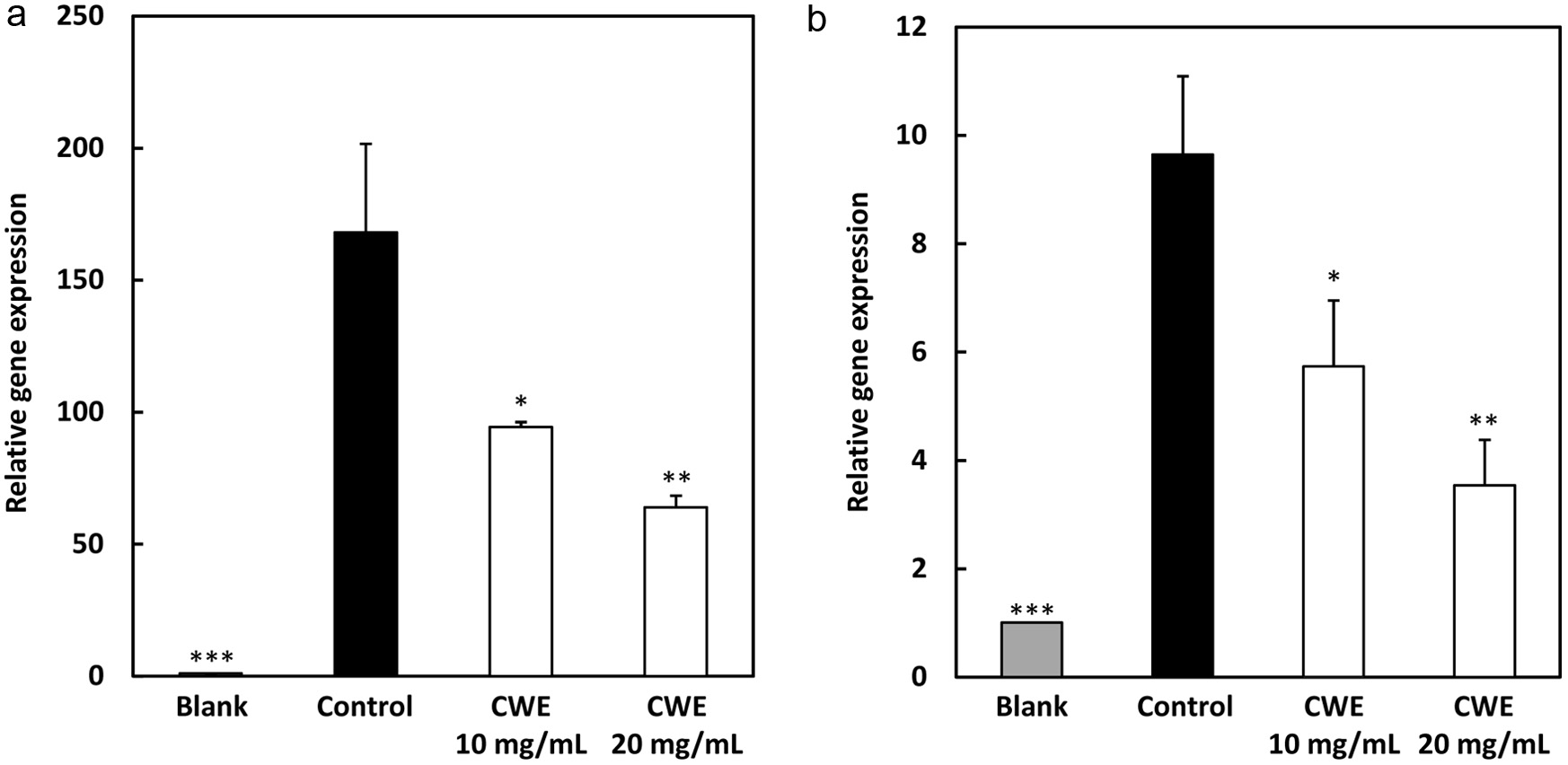 Click for large image |
Figure 3. Effect of CWE on mRNA expression levels of IL-6 and TNF- in LPS-stimulated RAW264.7 cells. The RAW264.7 cells were treated with 1 g/mL of LPS and serial concentrations of CWE and incubated for 6 h. After incubation, the mRNA expression levels of IL-6 and TNF- were evaluated using real-time RT-PCR. Data are presented as the mean SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 against control (LPS) using the Tukey test. Blank: distilled water without LPS; Control: distilled water with LPS. |
3.4. Effect of CWE on nitric oxide production and iNOS expression in LPS-stimulated RAW264.7 cells and P-mac
Nitric oxide (NO), an inflammatory mediator, can stimulate the production of pro-inflammatory cytokines, which can also stimulate the production of NO, creating a positive feedback loop that intensifies inflammation (Baek et al., 2020; Park et al., 2020). Therefore, inhibiting NO production reduces inflammation. In this study, NO production was evaluated using a Griess kit by measuring NO released in a culture medium. After treating the LPS-stimulated RAW264.7 cells with serial concentrations of CWE for 24 h, the NO concentration in the culture medium was subsequently measured. The result showed that NO production was suppressed by CWE in the LPS-stimulated RAW264.7 cells in a dose-dependent manner (Figure 4a).
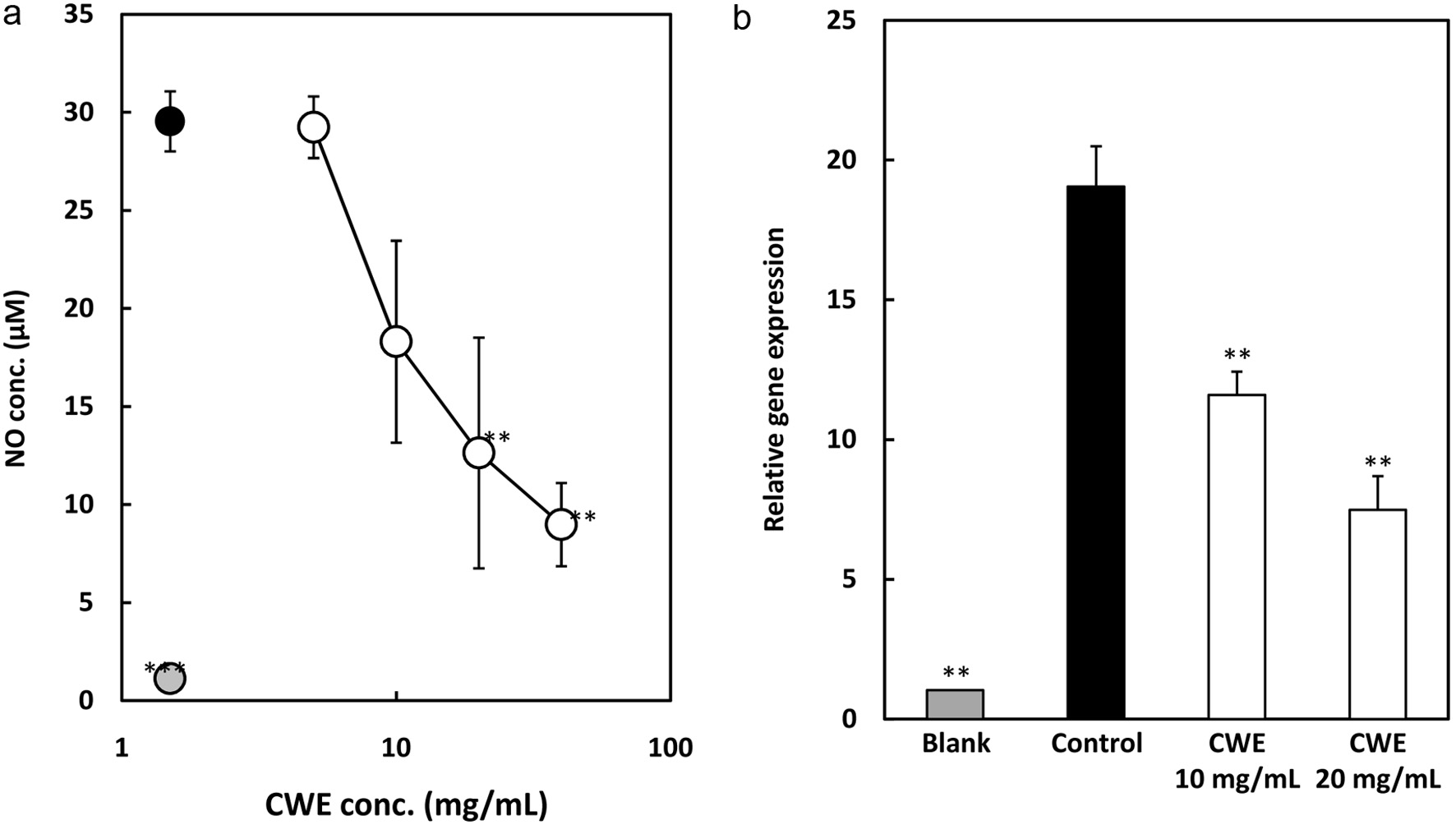 Click for large image |
Figure 4. Effect of CWE on NO production and mRNA expression of iNOS in LPS-stimulated RAW264.7 cells. The RAW264.7 cells were treated with 1 g/ml LPS and serial concentrations of CWE for 6 h. The culture medium was subsequently used to measure NO concentration. Data are presented as the mean SD (n = 3). **p < 0.01, ***p < 0.001 against control (LPS) using the Tukey test. (a) NO production. Black circle: LPS without CWE (control); grey circle: distilled water without LPS (blank); open circle: LPS with CWE. (b) iNOS gene expression level. |
In addition, the mRNA of iNOS was examined to explore the possibility that CWE suppresses the synthesis of NO by inhibiting the gene expression of the corresponding synthase, iNOS. As shown in Figure 4b, CWE inhibited iNOS mRNA expression at 10 mg/mL in the LPS-stimulated RAW264.7 cells, which is similar to the suppression of NO production. In brief, these data revealed that CWE suppresses NO production by downregulating iNOS gene expression in the LPS-stimulated RAW264.7 cells.
3.5. Effect of CWE on iNOS expression and MAPK and NF-B signaling pathways
Inflammatory signaling pathways such as MAPK and NF-B are important transcription factors in the inflammation-regulating system (Nishi et al., 2021). Therefore, to investigate the potential mechanism of the anti-inflammatory effect of CWE, the effect of CWE on the essential intracellular signaling pathways such as MAPK and NF-B, and the protein expression level of iNOS, were investigated using Western blot analysis. As shown in Figure 5a, the MAPK family such as JNK, p38, and ERK in the RAW264.7 cells induced by LPS were highly phosphorylated, while JNK and p38 phosphorylation was significantly inactivated by CWE at 10 mg/mL. In contrast, CWE treatment notably increased ERK phosphorylation at 10 mg/mL. Moreover, as depicted in Figure 4b, CWE treatment significantly suppressed the translocation of NF-B from the cytosol to the nucleus in the LPS-treated RAW264.7 cells. Next, we further analyzed the inflammation-related iNOS expression. iNOS is another key signaling pathway that regulates the release and gene expression of pro-inflammatory mediators and the inflammatory cytokine response. As shown in Figure 4c, the iNOS protein expression level was attenuated by CWE treatment compared with control. The results suggested that CWE inhibits the production of inflammatory cytokines and the inflammatory mediator NO by LPS-induced inflammation in the RAW264.7 cells via suppressing phosphorylation of JNK and p38, NF-B translocation, and iNOS expression.
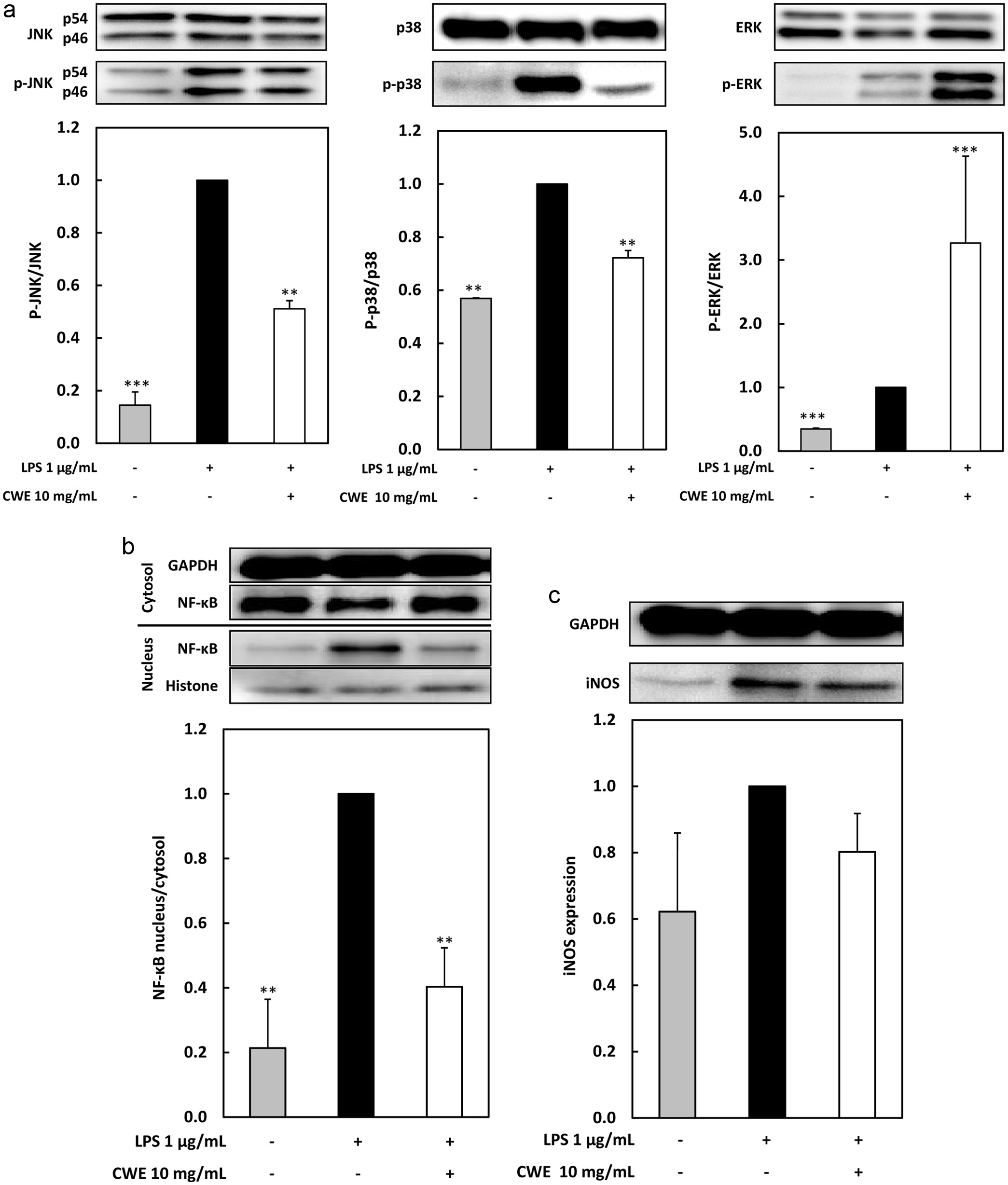 Click for large image |
Figure 5. Effect of CWE on MAPK and NF-B signaling pathways, and iNOS protein expression, in LPS-stimulated RAW264.7 cells. The RAW264.7 cells were treated with 1 g/mL of LPS and serial concentrations of CWE and incubated for 15 min. (a) The phosphorylated protein levels of ERK, JNK, and p38 were evaluated using immunoblot analysis. p-ERK, p-JNK, and p-p38 represent phosphorylated ERK, JNK, and p38, respectively. (b) The protein amounts of cytosolic and nuclear NF-B were evaluated using immunoblot analysis. (c) The protein amount of iNOS was evaluated using immunoblot analysis. Data are presented as the mean SD (n = 3). **p < 0.01, ***p < 0.001 against control (LPS) using the Tukey test. |
| 4. Discussion | Top |
Clove is a high-priced spice due to its large range of applications in food and beverages, use in fragrances, and for pharmacological purposes. Several studies have revealed the biological activities of compounds contained in clove. Haro-Gonzlez et al. (2021) reported that the main constituents of clove are eugenol, eugenol acetate, and caryophyllene. In addition, clove is one of the most abundant plant sources of phenolic compounds such as eugenol, eugenol acetate, and -caryophyllene (Uddin et al., 2017; Gaspar et al., 2018). The essential oil of clove has been reported to possess many positive benefits through its antioxidant, antimicrobial, anticancer, and wound-healing activities (Bachiega et al., 2012; Percival et al., 2012; Corts-Rojas et al., 2014; Zari et al., 2021). In this study, we investigated the anti-inflammatory activity and underlying mechanisms of CWE against LPS-stimulated RAW264.7 cells and P-mac.
Macrophages are key immune cells that play an important role in the inflammation system. Various cells can change their primary transcriptional program to combat harmful molecules, such as LPS (Sigola et al., 2016). Macrophages are pivotal in the inflammatory response through their ability to release inflammatory cytokines (IL-6, TNF-, IL-1) and mediators (NO) in response to LPS exposure (Guha and Mackman, 2001; Duque and Descoteaux, 2014). Therefore, we used mouse macrophage cell line RAW264.7 cells and P-mac as a model for this study. The RAW264.7 cells and P-mac treated with LPS were cultured in 10% FBS-DMEM and 10% FBS-RPMI 1640 medium, respectively, with the addition of CWE. The concentration of inflammatory substances IL-6, TNF-, and NO in the culture medium was measured. The synthesis of IL-6 and TNF- in macrophages generally increases after LPS exposure (Figure 2a, b). Our result showed that CWE significantly suppressed the production of IL-6 and TNF- in a dose-dependent manner without cytotoxicity (Figure 1a, b) in the LPS-stimulated RAW264.7 cells as well as in P-mac (Figure 2a, b). Pro-inflammatory cytokines such as IL-6 and TNF- play an important role in the inflammation system. The synthesis of TNF- in macrophages predominates and subsequently promotes the production of several other inflammatory cytokines, such as IL-6, IL-1, and IFNs (Baek et al., 2020). Many human disorders, including cancer, severe depression, Alzheimers disease, and inflammatory bowel disease, have been linked to the deregulation of TNF- release (Susanto et al., 2018; Heloneida et al., 2019). Consequently, measurement of TNF- attenuate activity is crucial for investigating potential anti-inflammatory agents as long as this cytokine contributes to the inflammation mediation system. Furthermore, to confirm the relationship between the inhibition of the mRNA level and the anti-inflammation effect of CWE, real-time RT-PCR analysis was conducted. CWE at 20 mg/mL and 10 mg/mL significantly inhibited the mRNA expression levels of IL-6 and TNF- compared with control treated with LPS only (Figure 3). These findings indicated that the anti-inflammatory activity of CWE is mediated through transcriptional regulation of inflammatory cytokines.
Next, we also explored the potential role of CWE in the modulation of another inflammatory mediator, NO, and its gene expression of the corresponding synthase, iNOS. The result showed that CWE inhibited iNOS gene expression (Figure 4b) and iNOS production (Figure 5c). In the human immune system, NO formation is biosynthesized by iNOS in macrophages. In addition, LPS-exposed macrophages induce inflammatory mediators, such as COX-2, an essential enzyme in the production of PGE2, and inflammatory cytokines such as IL-6 and TNF- (Baek et al., 2020; Kang et al., 2022). Pathogenesis is the process in which an infection leads to disease from prolonged inflammatory responses modulated by inflammatory cytokines including IL-6 and TNF-, and inflammatory mediators like NO and PGE2. During the inflammatory process, iNOS and COX-2 gene expression is markedly up-regulated in the accelerated synthesis of NO and PGE2, respectively. Furthermore, IL-6 is suspected to be involved in LPS-stimulated intracellular action, while TNF- triggers a variety of inflammatory consequences, such as sepsis and cytotoxicity. These inflammatory cytokines promote overexpression of NO and iNOS, and excessive inflammatory mediators are linked to tissue damage and multiple organ failure. Hence, the efficacy of anti-inflammatory medication may be assessed by blocking the synthesis of pro-inflammatory mediators (Jeong et al., 2019; Lee et al., 2021).
Selective intracellular systems, such as the signaling cascade that initiates the activation of MAPKs and the nuclear factor NF-B, are triggered by macrophages to recognize and react to extracellular stimuli. The family of serine-threonine kinases known as MAPKs has a vital role in transducing signals to control immune responses and inflammatory factors in response to an extensive range of extracellular stimuli, involving cytotoxic agents and oxidative stress (Zhang and Liu, 2002; Jeong et al., 2019). NF-B signaling is a transcription factor that, when activated, can regulate pro-inflammatory cytokines and mediators in LPS-stimulated macrophages (Harbeoui et al., 2019; Gurusmatika et al., 2023). Hence, in this current research, we analyzed proteins related to MAPKs and NF-B to elucidate the anti-inflammatory mechanism. Our finding showed that the phosphorylation level of JNK and p38 MAPK was attenuated by CWE, whereas the phosphorylation level of ERK was elevated (Figure 5a). In addition, as shown in Figure 5b, CWE inhibited the translocation level of NF-B from the cytosol to the nucleus in the LPS-treated RAW264.7 cells. These results suggested that JNK and p38 MAPK signaling cascade, are involved in the anti-inflammatory effect of CWE, as well as NF-B translocation. Several studies have revealed that MAPK regulates the secretion of various inflammatory mediators including NO and cytokines. As a result, p38 and JNK are molecules that have an anti-inflammatory effect (Ishida et al., 2013; Ishida et al., 2022; Gurusmatika et al., 2017).
GTP-binding proteins initiate the JNK cascade, similar to the ERK pathway. While Ras generates ERK, small GTPases that resemble Ras, such as Rac and Cdc42, generate signals that trigger JNK. On the other hand, the kinase p38 is described as a MAPK that is involved in regulating inflammation responses. In the cytoplasm, the binding process of NF-B to its inhibitory protein IB is inactivated under normal physiological circumstances, while the IKK complex is activated by pro-inflammatory cytokines, causing the phosphorylation of the IB protein to auto-ubiquitination and proteasome destruction. Released NF-B is subsequently translocated from the cytosol into the nucleus, where it binds to nucleotropic DNA to initiate expression factors to produce inflammation mediators and cytokines (Xu et al., 2021; Liu et al., 2022).
NO, resulting from iNOS protein expression, is a pro-inflammatory mediator that is released by macrophages. It initiates the inflammatory response, increases vascular permeability, and promotes the production of pro-inflammatory mediators and cytokines. Our findings indicated that CWE imparts anti-inflammatory activity by suppressing IL-6 and TNF- production by in the LPS-stimulated RAW264.7 cells and P-mac. In addition, inhibition of iNOS gene and protein expression undoubtedly affects NO synthesis, thereby reducing NO production through down-regulation of iNOS gene expression by CWE treatment in the LPS-stimulated RAW264.7 cells. Furthermore, suppression is regulated by the inhibition of transcription factor NF-B and iNOS in parallel with the attenuation of the p38 and JNK pathways. Clove consists of many natural compounds, such as phenolics, proteins, vitamins, and minerals. Although many studies have shown the anti-inflammatory activity of eugenol, the major component in clove, in the present study we conducted a sample extraction with water. Eugenol is optimally extracted in ethanol solvent but low water solubility (Pandey et al., 2024). In addition to some flavonoids like kaempferol and its derivatives, clove also contains the phenolic acids caffeic, elagic, and ferulic. Therefore, phenolic substances such as gallic acid, flavonolglucosides, phenolic volatile oils (eugenol, acetyl eugenol), and tannins are responsible for the health benefits of cloves (El-Maati et al., 2016; Chniguir et al., 2019; Zari et al., 2021). Active compounds in CWE have not yet been studied, and will be explored in future studies.
| 5. Conclusion | Top |
The current study provides evidence that clove water extract exerts anti-inflammatory activity in LPS-stimulated RAW264.7 cells and peritoneal macrophages. The extract significantly attenuated the production of IL-6, TNF-, and NO by LPS-stimulated macrophages. The anti-inflammatory activity of the extract was attributed to inhibiting the gene expression of IL-6, TNF-, and iNOS, which are interrupted through JNK and p38 MAPKs, the NF-B signaling cascade, and iNOS protein. The results of this study show that clove water extract is a safe and better natural alternative candidate as a therapeutic agent for chronic inflammation and oxidative stress-related diseases.
Acknowledgments
The animal experiment was conducted at the Division of Genetic Research Support of the Advanced Research Support Center (ADRES), Ehime University.
Conflict of interest
The authors declare no conflict of interest.
Conceptualization, T.S.; Methodology, S.G.; Software, S.G.; Validation, S.G.; Formal Analysis, S.G., M.I., K.N. and T.S.; Investigation, S.G.; Data Curation, S.G.; Writing Original Draft Preparation, S.G.; Writing Review & Editing, M.I., K.N. and T.S.; Visualization, S.G.; Supervision, K.N. and T.S.
| References | Top |