| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 25, March 2024, pages 62-71
A novel combination against skin aging via promoting the synthesis of biological collagen
Bei-Bei Donga, b, Peng-Fei Zhangc, Wu-Yan Guod, *, Heng-Yu Zhenge, You-Nan Koua, b, Huan Zhangf, Ying-Chao Mag, Bo Zhangh, *
aCollege of Pharmacy, Nankai University, Tianjin, 300350, China
bTianjin Key Laboratory of Early Druggability Evaluation of Innovative Drugs, Tianjin International Joint Academy of Biomedicine, Tianjin, 300457, China
cTianjin Pharmaceutical and Cosmetic Evaluation and Inspection Center, Tianjin, 300191, China
dTianjin JiAnKang Bio&TCM-technology Development Co., Ltd. Tianjin, 300457, China
eBreast Disease Center, Peking University First Hospital, Beijing, 100032, China
fCollege of Bioengineering, Tianjin University of Science and Technology, Tianjin, 300457, China
gCollege of Marine and Environmental Science, Tianjin University of Science and Technology, Tianjin, 300457, China
hTsinghua University Institute of TCM-X, Beijing, 100084, China
*Corresponding author: Bo Zhang, Tsinghua University Institute of TCM-X, Beijing, 100084, China. E-mail: zhangbo.2007@tsinghua.org.cn; Wu-Yan Guo, Tianjin JiAnKang Bio&TCM-technology Development Co., Ltd. Tianjin, 300457, China. E-mail: guowuyan213@126.com
DOI: 10.31665/JFB.2024.18372
Received: January 2, 2024
Revised received & accepted: March 6, 2024
| Abstract | Top |
Supplementing collagen is considered to contribute to delaying skin aging. In this work, we developed a novel combination for improving skin aging by stimulating the biosynthesis of collagen. By screening a library of Chinese herbal medicines (CHM), we found that Angelicae Dahuricae Radix (Baizhi), Lilii bulbus (Baihe), Glycyrrhizae radix et rhizoma (Gancao), and Jujubae fructus (Dazao) substantially increased the mRNA expression levels of type I collagen, suggesting their potential anti-skin-aging activity. To keep the structural integrity of collagen, prolyl 4-hydroxylase (P4H), a key enzyme in collagen synthesis, was recombinantly expressed in Escherichia coli. In addition, the transfersome with added P-4H was prepared to improve the transdermal absorption of combination. Vitamin C (VC), the substrate required for the activity of P4H, was also incorporated into the combination. Eventually, an optimal combination, consisting of Baizhi, Baihe, Gancao, P4H transfersome and VC, was obtained by a series of combination experiments. Based on traditional CHM and modern biological agents, we developed a novel combination against skin aging by promoting the synthesis of collagen. Collectively, the combination show the high potential of application to delay skin aging.
Keywords: Chinese herbal medicines; Collagen; Hydroxyprolin; Skin aging; Transfersome
| 1. Introduction | Top |
The skin undergoes morphological and physiological changes with aging, such as discolorations and wrinkles. How to restore youthfulness of skin has attracted much attention. Skin aging is a spontaneous and complex process of skin degeneration. Both environmental and hormonal factors affect the skin aging (Schfer et al., 2020). Collagen, the most abundant extracellular matrix protein, accounts for 7080% of the dry weight of skin (Zhang et al., 2020). During the skin aging process, the collagen stability decreases greatly. Besides, the ability to replenish collagen naturally decreases by about 1% per year, also causing accelerated senescence in human skin (Edgar et al., 2018). Therefore, strategies of supplementing collagen hold great potential in delaying skin aging.
At present, topical treatments have become one of the most common methods in supplementing collagen (Kwatra, 2022). However, macromolecular collagen barely penetrates the skin due to the resistance of cuticle, so it merely plays a moisturizing role in the epidermis (Jepps et al., 2013). Besides, the collagen absorbed by skin is easily degraded by collagenases (Lagarto et al., 2020). Most topical supplements face the challenge of unsatisfactory therapeutic effects. It is urgent to develop an efficient topical collagen supplement.
We focused on supplementing skin collagen by endogenously promoting the biosynthesis of collagen and increasing collagenous stability. Among the various collagens, type I and III collagen are the most abundant (Chen et al., 2021). Different collagens have a common triple-helix conformation that consists of three almost identical polypeptide chains (Ricard-Blum, 2011). Proline (Pro) - hydroxyproline (Hyp) - glycine (Gly) is usually the most common triplet in collagens (Chattopadhyay and Raines, 2014). The interchain H-bondings between the hydrogen atom of Gly and the hydroxyl group of Hyp exert a critical action in stabilitizing collagen structure (Huang et al., 2009). It is noteworthy that the formation of Hyp is catalyzed by prolyl 4-hydroxylases (P4H) that act on Pro residues in peptides (Eriksson et al., 1999). So, P4H is the rate-limiting enzyme in the synthesis of collagen (Zhang et al., 2018). Vitamin C (VC), as a cofactor, participates in the synthesis of collagen (Stephens and Grande-Allen, 2007). In addition, some studies have confirmed Chinese herbal medicines (CHM) has a promoting role in collagen synthesis, such as Lycium chinense Mill. and Angelica dahurica (Lin and Chen, 2007). Transfersome can effectively protect the drug against undesired absorption into cutaneous blood vessels and are capable of retaining the drug in the skin (Elsayed et al., 2007).
Taken together, wwe developed a novel combination consisting of Baizhi, Baihe, Gancao, P4H transfersome and VC for promoting collagen production and stabilizing the collagen structure in skin. We then evaluated whether the combination functions on D-galactose-induced aging mice to determine whether the combination could promote collagen synthesis and improve skin conditions.Taken together, we attempted to combine the advantages of P4H and CHM to design a novel anti-skin aging combination.
| 2. Materials and methods | Top |
2.1. Materials
NIH-3T3 fibroblasts were purchased from Beijing Beina Institute of Biotechnology. All Chinese medicinal materials were provided by Anguo Kanghua Traditional Chinese Medicine sales Co. Ltd. Dulbeccos modifed eagle medium (DMEM), fetal bovine serum (FBS), dNTPs, T4 DNA ligase, Pfu polymerase, and Gene ruler were obtained from Thermo Fisher Scientific. RNA extraction kits, RNA reverse transcription kits, plasmid DNA mini-extraction kits, and gel recovery kits were purchased from Tiangen Biochemical Technology Company. Isopropyl-bate-D-thiogalactopyranoside (IPTG), VC, and soy lecithin (purity > 95%) were obtained from Shanghai Sangon Biological Company. FastDigest NcoI, FastDigest XbaI, GeneRulerT 1 kb DNA ladder, protein marker, FastDigest BamHI, and FastDigest XhoI were purchased from Thermo Scientific. Commercially available kits bicinchoninic acid assay (BCA) and hydroxyproline were provided by Nanjing Jiancheng Bioengineering Institute.
2.2. Extraction of 82 Chinese herbal medicines
Firstly, 80 g of each herb was pulverized and passed through a 60-mesh sieve. The pretreated dried powder was reflux-extracted twice with purified water (1 : 8, w/v) at 100C for 2 h each time. After 50h lyophilization (lyophilizer VS-502FD,Wuxi Woxin Instrument Co., Ltd.,China) the extracts were concentrated under reduced pressure at 45C using rotary evaporator (Heidolph Hei VAP Rotary Evaporator, Germany) and then freeze-dried. Finally, the lyophilizates were stored at 20C for back-up purposes.
2.3. Cell culture
NIH-3T3 fibroblasts grown in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were incubated at 37C in an atmosphere containing 5% CO2/95%. The growth of the cells was observed using an inverted microscope (Chongqing Optec Instrument Co. Ltd., China).
2.4. Cell viability assay
In 96-well culture plates, 1 104 cells/well were seeded for 24 h and treated with CHM extracts (5 mg/mL). After 24 h of treatment, cell viability was determined using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Schoeman et al., 2020). MTT reagent (20 L) was added to each well and incubated for 4 h and then terminated by adding 100 L dimethylsulfoxide (DMSO). Next, the 96-well plate was incubated for 15 min at room temperature. Cell proliferation was assessed by measuring the absorbance at 570 nm.
2.5. Quantitative real-time polymerase chain reaction (RT-PCR)
NIH-3T3 cells (2 105 cells/mL) plated for 24 h were exposed with concentrations of CHM extracts. After 24 h, total RNA was extracted from the NIH-3T3 fibroblasts using the TRIzol reagent. Then total RNA was used to synthesize cDNA by reverse transcription. We performed PCR on the resultant cDNA from each sample using specific primers (Table 1). The amplification was carried out on a Thermal Cycler (Applied Biosystems, Thermo fisher scientific, Waltham, MA, USA). The 20 L reaction mixture contained 5 L of cDNA, 1 L of sense and antisense primers, 200 M of each deoxynucleotide (DTT), 2 L of GoTaq polymerase, 2 L of 10 Taq polymerase buffer. The reaction was cycled 40 times for 50 s at 95C, 60 s at 58C, and 90 s at 72C. Data was analyzed using the 2-CT values method and normalized to the expression levels of GAPDH.
 Click to view |
Table 1. Primer list used in this study |
2.6. The expression and purification of P4H
P4H gene of eukaryotic algal virus, Paramecium bursaria Chlorella virus-1 (PBCV-1) was selected for as the target gene. Because it is small in size and simple in structure, and can be recombinantly expressed in prokaryotic expression system Escherichia coli (E coli) (Eriksson et al., 1999). Firstly, we obtained the sequence of P4H gene from GeneBank database. Plasmid vector pET16b(+) was used for expression in E coli. The Primer 5.0 was used to design primers (Primer sequences are provided in Table 1). Then, PCR was used to amplify fragments of the target genes.
The PCR product was digested with BamHI and XhoI, and ligated with into the corresponding sites of the expression vector pET16b(+). Subsequently, the ligation product was transformed into DH5 competent cells by heat shock method (Froger and Hall, 2007). E. coli DH5 cells grown at 37C in luria broth (LB) containing ampicillin. The monoclonal colony was picked and inoculated in LB medium for 6 h. The plasmid extraction followed the manual provided with the kit. The recombinant plasmids were identified by PCR, double restriction enzyme digestion and sequencing analysis, respectively.
Thereafter, the recombinant plasmid was transferred into BL-21 competent cells, and added IPTG (final concentration: 1 mM) to induce the expression of target protein. Then the bacterial solution was shaken at 28C, 220 rpm for 4 h. After centrifuging the bacterial liquid culture, the supernatant was discarded and the sediment was resuspended. The suspension was centrifuged and the supernatant was removed. A Ni column was used for affinity purification of the His-tagged P4H. Briefly, the supernatant was collected and applied to a Ni-NTA affinity chromatography column. Then the Ni-NTA column was washed with the wash buffer (His-Trap buffer supplemented with 60mM imidazole) and the protein was eluted with elution buffer (His-Trap buffer supplemented with 300mM imidazole). Eluted protein was concentrated using a 10 KD centrifugal filter to 1mL. Finally, the protein were further purified by Superdex-200 gel-filtration size column. Single peak fractions were collected, snap frozen and stored at 80C in aliquots. The purity of protein was examined by 12% SDS-PAGE.
2.7. Activity assay of P4H
According to literature, the P4H activity was evaluated by monitoring the hydroxylation of (PAPK)3, a short proline-rich peptide substrate (Myllyharju, 2003; Eriksson et al., 1999). The P4H enzyme activity reaction system (1.0 mL) was established in vitro. The system comprised the following components: Fe(NH4)2(SO4)2, 50 M; -ketoglutaric acid, 300 mM; ascorbate, 4 mM; tris (hydroxymethyl) aminomethane (Tris)-HCl, 50 mM; substrate, 300 g; bovine serum albumin, 2 mg; catalase, 200 ug; and nicotinamide-adenine dinucleotide (NADH), 200 g. The reaction mixtures were incubated at 37C for 4 h and then quenched by methanol. Finally, the molecular weight difference of sample was analyzed using MALDI-TOF MS.
2.8. Stability studies of P4H
The effect of temperature on the stability of the P4H was studied. The protease solution was placed at 20, 4, 25, and 50C for 8 days, respectively, and samples were drawn at 1, 2, 3, 4, 5, 6, 7, and 8 days. Separately, the protease solution was also placed in a 50C constant temperature water bath for 1 h and samples were taken at 5, 10, 15, 20, 25, 30, 40, and 60 min. Variation in P4H stability was confirmed qualitatively by 12% SDS-PAGE.
2.9. Preparation of P4H transfersome
P4H transfersome was prepared by the thin-film hydration method followed by extrusion. First, 1 mg/mL P4H solution was prepared using PBS as solvent. The different proportions of soy lecithin and cholesterol were dissolved in 5 mL organic solvent (methanol:dichloromethane = 1:3), and the solvent was evaporated under reduced pressure to obtain a thin lipid film in bottle bottom. Next, 5 mL P4H solution was add it to the bottle. The lipid film was eluted to obtain emulsion, ultrasonicated for 30 s, and filtered through 0.22 m microporous membranes to collect the P4H transfersome solution. After centrifugation, the P4H transfersome was obtained. The softening material (cholesterol) was replaced by an equal amount of distilled water to prepare the normal liposome.
Then, the P4H transfersome solution was centrifuged at high speed. We measured the free P4H content in the supernatant by the BCA method (Schoel et al., 1995). The factors affecting the encapsulation rate are mainly the ratio of soy lecithin and cholesterol (A), drug concentration (B), and lipid concentration in the aqueous phase (C). In this study, orthogonal experiment on above three factors was preformed (Table 2), and the optimal preparation process parameters were determined. Encapsulation efficiency was calculated by the following formula:
 Click to view |
Table 2. Factors and Levels |
The skin of nude mice was harvested and refrigerated at 4C for later use. P4H transfersome solution, P4H liposome solution, and P4H solution each was smeared by 0.5 mL to the skin surfaces and recovered after 12 h. Then the free P4H content in each solution was measured, and the transdermal efficiency was compared. Additionally, the morphological characteristics of P4H transfersome were observed under the transmission electron microscope.
2.10. Determination of hydroxyproline content
According to the composition of the formula (Table 3), all the ingredients are weighed in equal proportion and mixed. We pretreated NIH-3T3 cells with different formulae. After a 24 h incubation, cells were collected to detect the content of hydroxyproline. Similarly, the mice skin were collected, rinsed with normal saline, and cut into pieces. Then skin pieces were grinded using a tissue grinde (Thermo, USA) and the homogenate was centrifuged at 4,000 rpm for 10 min to obtain the supernatant. Subsequently, the hydroxyproline content of supernatant was measured using the ELISA kit.
 Click to view |
Table 3. The components of different combinations |
2.11. Animals and treatments
Kunming mice (female, 8weeks old, 2530 g) were obtained from Xuhe Pharmaceutical Technology Co., Ltd. (Tianjin, China) and randomly divided into 6 groups (10 per group). Mice were housed in a ventilated animal room where the temperature and humidity were maintained (temperature 25 1C and humidity control 60 5%). All mice experiments were performed according to the animal experiment guidelines approved by the Institutional Animal Care Committee at Nankai University and Tianjin Institute of Pharmaceutical Research New Drug Evaluation Co. Ltd. (Tianjin, China; approval ID: IACUC 2017532268, validity period: 22 March 2017 to 22 March 2022).
Mice were provided with water and pelleted diet and libitum. Drug dosage was calculated according to the calculation formula: DM (dose per kg body weight) = DH R (WH/WM), as detailed in The Methodology of Pharmacological Experiment. DM and DH are doses for mice and humans, and WM and WH are body weights of mice and humans, respectively (Lin et al., 2007). According to the method of pharmacology, the dosage for mice is adjusted to 9 times of the human dosage. In the control group, 0.2 mL of 0.9% saline water was injected subcutaneously in the back neck every day. The remaining mice received subcutaneous injections of 10% D-galactose every day for 42 d to prepare the subacute mouse aging model. The mice of drug treatment group were subcutaneously injected with different concentrations of CHM-3-4, respectively. The specific grouping situation was as Table 4 follows:
 Click to view |
Table 4. The experimental animial grouping |
2.12. HE staining
HE staining was performed to observe the changes of collagen fibers using a HE staining kit. Following sacrifice, the dorsal skin was excised from mice. Then the skin specimens (approximately 1 cm2) were fixed in the buffered neutral formalin (4%, w/v) for 24 h. After deparaffinization and rehydration, tissue sections were stained with hematoxylin solution for 5minutes and soaked in 1% acid ethanol (1% HCl in 75% ethanol) for 30 s and then rinsed in distilled water. Finally the sections were cleared by xylene and mounted. Pictures were taken with an inverted microscope (Nikon, Tokyo, Japan).
2.13. Statistical analysis
All statistical analyses were performed by GraphPad Prism 9.0 software. Significant differences were evaluated via the one-way analysis of variance (one-way ANOVA) were performed with GraphPad Prism. The experimental results are presented as the means standard deviation (SD), and P value< 0.05 was considered significant.
| 3. Results | Top |
3.1. Glycyrrhizae radix et rhizoma (Gancao), Angelicae Dahuricae Radix (Baizhi), Lilii bulbus (Baihe), and Jujubae fructus (Dazao) increase the mRNA levels of type I collagen
In order to obtain the CHM that could promote collagen expression, we measured the mRNA levels of collagen type I and III after drugs action. The effects of 82 CHM extracts on NIH-3T3 cells viability were studied through the MTT assay to to preliminarily screen drugs (Table S1, Figure S1). The top ten CHM extracts with an obvious effect on promoting cell proliferation were determined, including Baihe, Pogostemincablin benth (Huoxiang), Baizhi, Dazao, Platycodonis radix (Jiegeng), Coicis semen (Yiyiren), Chaenomelis fructus (Mugua), Zaocys dhumnades (Wushaoshe), Gancao, and Lonicerae japonicae flos (Jinyinhua) (Figure 1a). And the above CHM extracts at 5 mg/mL increased cell viability by 246.0%, 210.1%, 209.3%, 154.9%, 152.8%, 130.0%, 119.6%, 109.8%, 90.0%, and 80.8%, respectively.
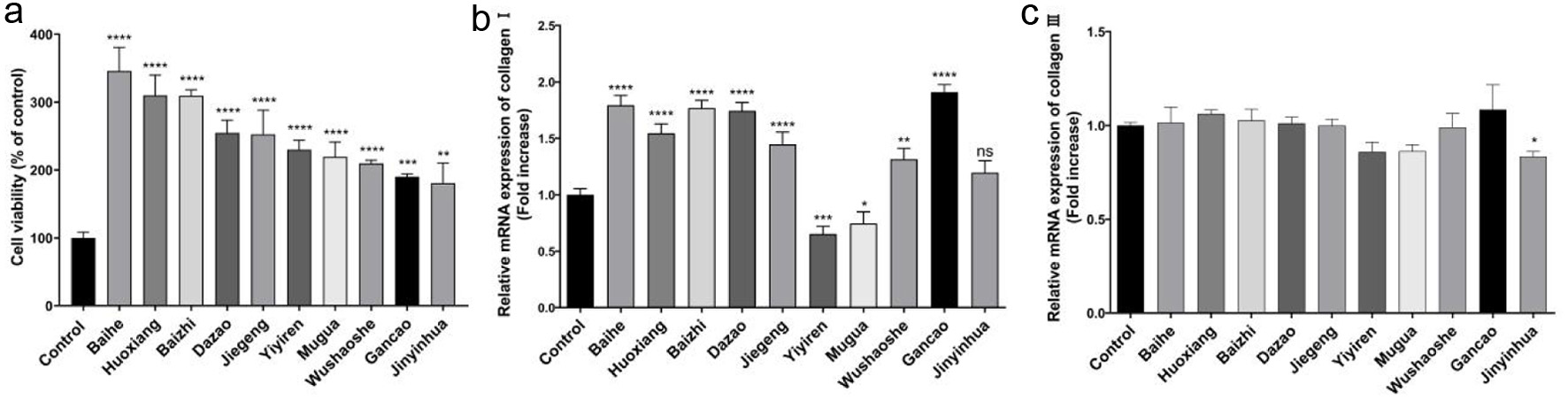 Click for large image |
Figure 1. The effect of Gancao, Baizhi, Baihe, and Dazao extracts on cell viability and the mRNA levels of collagen. (a) Determination of cell viability in NIH-3T3 cells pretreated with ten CHM extracts. The mRNA levels of collagen I (b) and collagen III (c) in NIH-3T3 cells. |
Collagen type I and III are the main constituent structure of the skin (Tiganescu et al., 2018). We further evaluated the mRNA levels of collagen type I and III under the ten above CHM extracts treatment. The qRT-PCR results showed that the extracts of Gancao, Baizhi, Baihe and Dazao significantly increased the mRNA levels of collagen I by 82%, 69%, 63%, and 60%, respectively (Figure 1b). Mugua and Yiyiren both inhibited the transcript levels of type I collagen, and the other CHM extracts had no significant effect (Figure 1c). In addition, the results showed that treatment with the ten CHM extracts did not appear to significantly affect the expression of type III collagen. In this preliminary screen, four kinds of CHM had significantly promoting effects on the mRNA of collagen type I.
3.2. Evaluation of the activity and stability of P4H
The initial screen unveiled four CHM extracts that promoted the expression of collagen. Next, we intended to improve the stability of collagen. The P4H was recombinantly expressed and its activity and stability was examined. Viral P4H has been cloned from PBCV-1 and it can hydroxylate several synthetic peptides that corresponding to proline-rich repeats coded by the PBCV-1 genome, such as (Pro-Ala-Pro-Lys)n and (Pro-Glu-Pro-Pro-Ala)5 (Myllyharju, 2003). Moreover, the proline in both positions in these repeats were hydroxylated by P4H (Eriksson et al., 1999). Mass spectrometry analysis indicated that the molecular weight of (PAPK)3 increased from 1,198.8 Da to 1,262.7 Da after incubation with P4H (Figure 2a). The result showed that the recombined P4H had the ability to selectively hydroxylate proline that located in the repeating position of (PAPK)3. Moreover, to study the stability of P4H, we measured the enzyme stability under different temperatures. We found the stability of P4H dropped as temperature increased. P4H is stable relatively when stored at 4 and 25C (Figure 2b). In conclusion, P4H is active in proline hydroxylation and stable at room temperature.
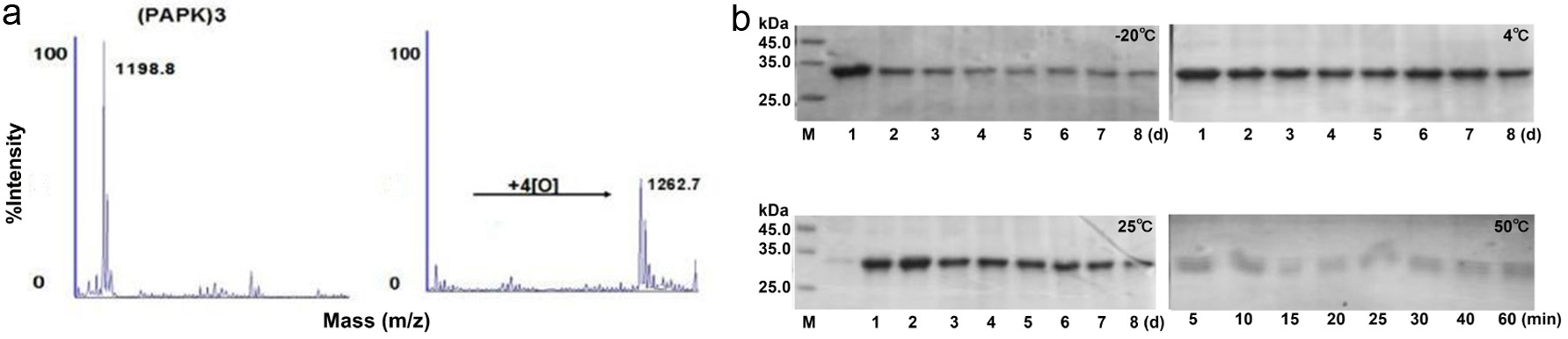 Click for large image |
Figure 2. The activity and stability of P4H. (a) MALDI-TOF-TOF mass spectra of (PAGP)3 before and after application of P4H. (b) Effect of different temperatures on P4H stability. |
3.3. Preparation and characterization of P4H transfersome
P4H has high molecular weight and is not easily absorbed by the skin. So, transfersome was prepared to make P4H achieve a higher transdermal permeability. Transfersome is highly flexible vesicle and is widely used for skin delivery systems (Azimi et al., 2019). The stability of transfersome is involved with the types and the ratio of constituents used in their preparation (Hua et al., 2017). An orthogonal test was designed to study the optimum conditions for the preparation of P4H transfersome (Table 5). According to the value of range R (Table 6), the order of importance that influenced encapsulation rate of P4H transfersome was found to be (B) total lipid concentration > (A) ratio of soy lecithin and cholesterol > (C) enzyme concentration. The optimum combination of factors was A1B1C2, namely, ratio of soy lecithin to cholesterol of 9 : 1, total lipid concentration of 6%, and enzyme concentration of 5 mg/mL, while the encapsulation rate of P4H transfersome was 36.9%. In addition, as shown in the electron micrograph (Figure 3a), the newly prepared P4H transfersome was uniform in size and shape.
 Click to view |
Table 5. Encapsulation efficiency of P4H at different factor levels of the ratio of soy lecithin and cholesterol (A), total lipid concentration (B), and drug concentration (C), respectively |
 Click to view |
Table 6. Intuitive analysis of the test results |
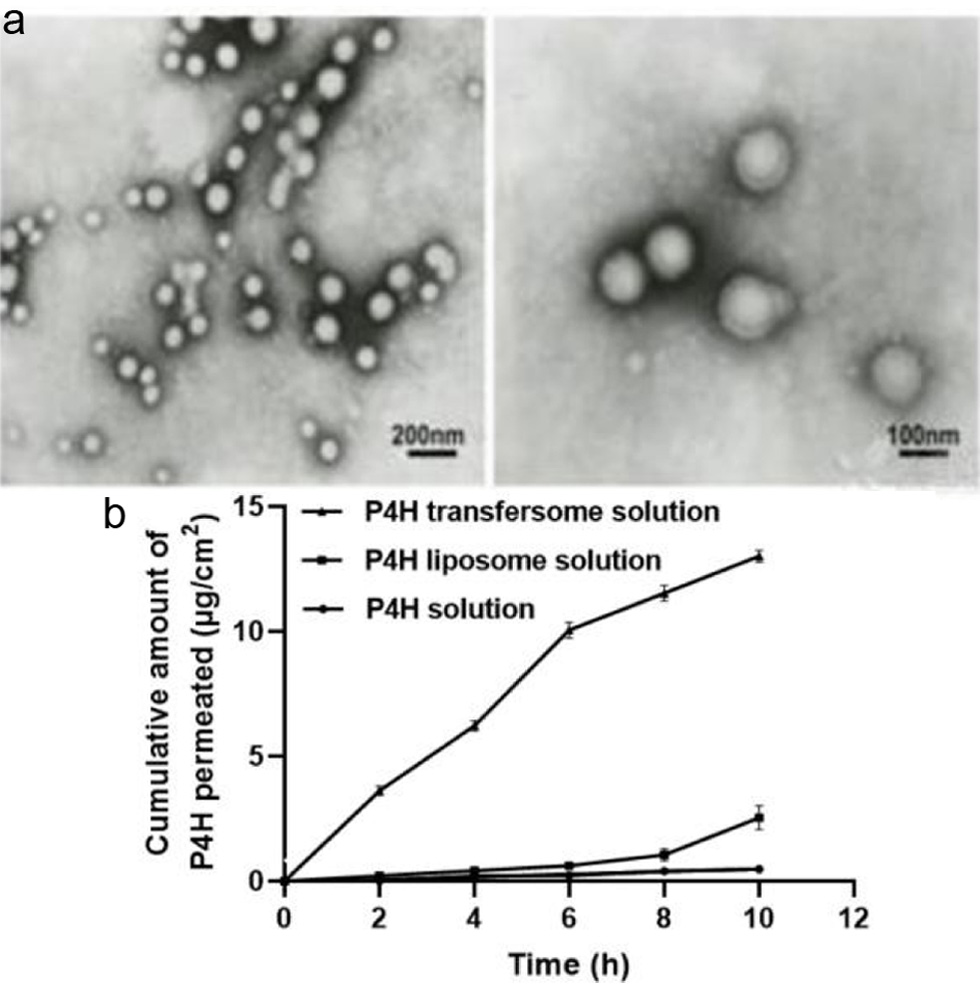 Click for large image |
Figure 3. The P4H with homogeneous particle size and good transdermal rate was successfully prepared. (a) Transmission electron microscopy image of the P4H transfersome. (b) Cumulative P4H permeation of P4H transfersome solution, P4H liposome solution, and P4H solution, respectively. |
3.4. Transdermal rate of P4H transfersome
Next, the transdermal permeation efficiency of different P4H solutions was compared. Result showed that P4H transfersome solution had a better transdermal rate in a time- and concentration-dependent manner, reaching 13.05% at 10 h (Figure 3b). In contrast, P4H liposome solution and P4H solution had no permeation of P4H basically. The transfersome greatly overcome the insufficient skin penetration of P4H.
3.5. The CHM-3-4 promotes collagen synthesis in NIH-3T3
The concept of the composition is to combine Chinese herbal medicines and P4H to explore a method of efficiently replenishing collagen in skin. To select the most efficient combination, a hydroxyproline assay was used to measure collagen content. The measurement of hydroxyproline levels can be used as an indicator of collagen content (Qiu et al., 2020). After 24 h of combination treatment, we assessed the levels of hydroxyproline in NIH-3T3 cells. Compared with the control group, the collagen content in combination treated groups was higher (P < 0.05), especially in CHM-3-4 treated group (Figure 4). Results suggested that CHM-3-4 treatment of NIH-3T3 fibroblasts could effectively promote collagen synthesis.
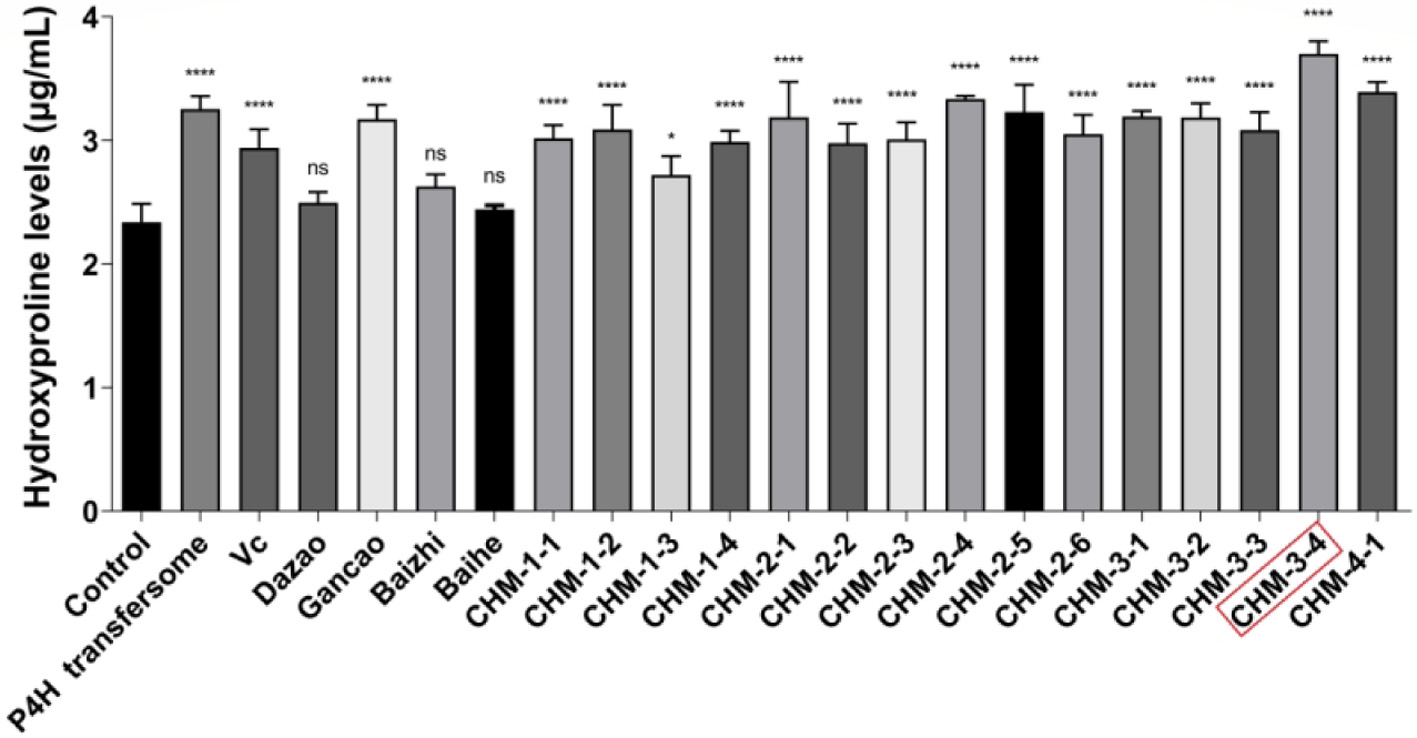 Click for large image |
Figure 4. The combinations improve the content of hydroxyproline in NIH-3T3 cells. |
3.6. The CHM-3-4 promotes collagen synthesis in the skin of D-galactose-induced aging mice
Mouse chronically injected with D-galactose has been widely used as an aging animal model for anti-aging pharmacology research. Excessive D-galactose accumulated in the body will be converted into galactitol which can not be metabolized normally. The galactitol will further affect the osmotic pressure of cells, resulting in increased levels of reactive oxygen species, and damage to cells, and finally leading to aging (Palacios-Pedrero et al., 2021). HE staining illustrated the increased epidermal thickness of mice skin caused by D-galactose (Figure 5). In the control group, the epidermal thickness of mice was uniform and the collagen fibers in the dermis were wavy and arranged in order. Compared with the control group, D-galactose altered the epidermal and dermal morphology, which was characterized by epidermal thickening and a fracture of collagen fibers. Compared with the model group, the mice skin treated with CHM-3-4 had thinner epidermal thickness and more normal collagen fibers. The result confirmed that CHM-3-4 provided effective protection against D-galactose-induced damages to skin. It indicated that CHM-3-4 could alleviate collagen degradation in skin aging caused by D-galactose to some extent.
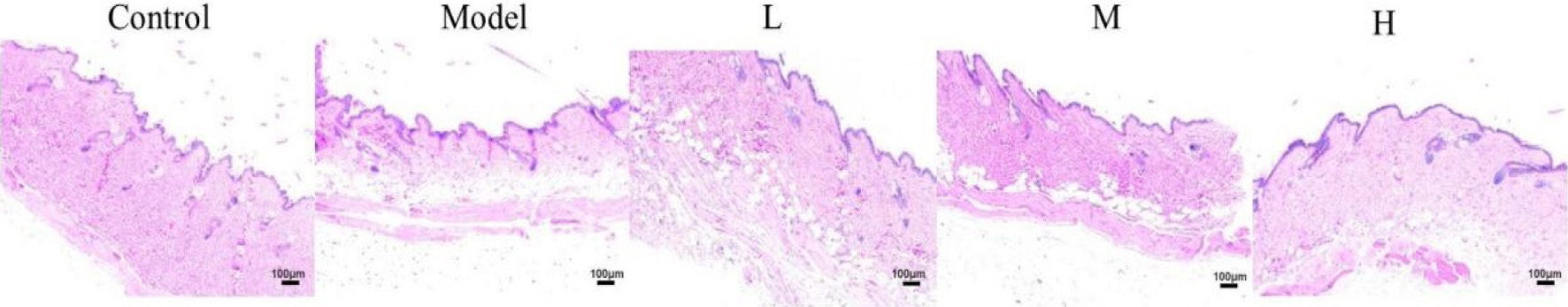 Click for large image |
Figure 5. HE staining of cross-sectional slices from the dorsal skin of mice, magnification, 100. |
In addition, we examined whether the collagen content was increased in mice skin after combination treatment. Compared with the control group, the collagen content of mice skin in the model group was decreased by 25.4% (P < 0.01) (Figure 6). Compared with the model group, skin treated with high-dose combination had high collagen content. We have also seen an increase of collagen content in mice skin treated with the low-dose and middle-dose combination. It can be concluded that the CHM-3-4 could promote collagen synthesis in mice skin.
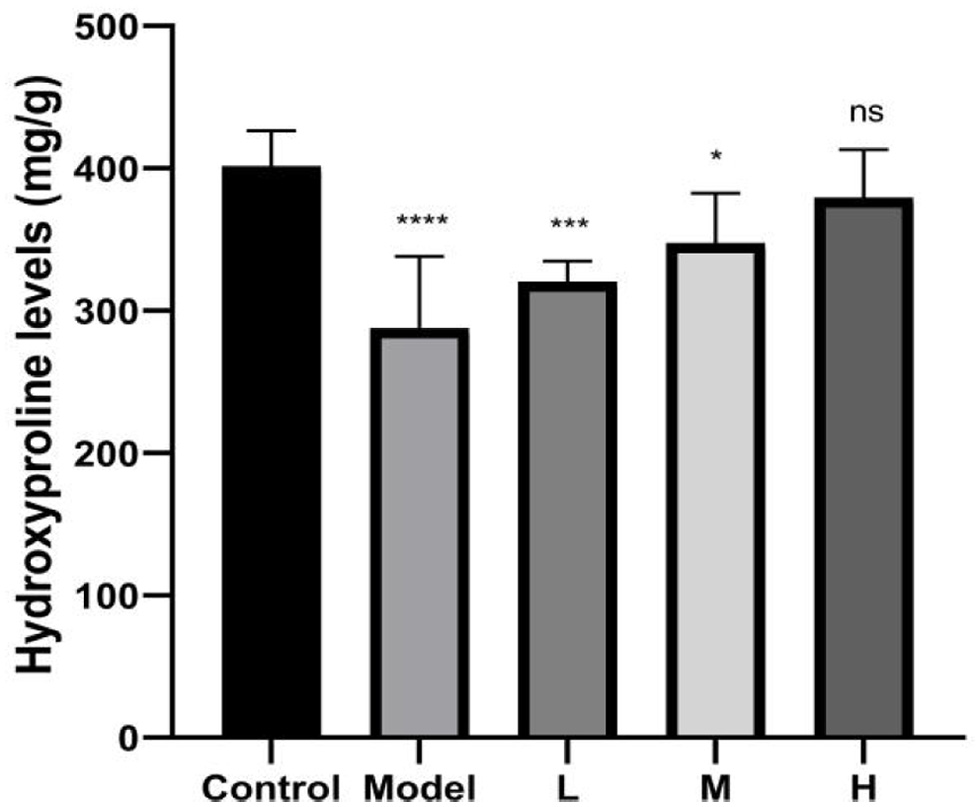 Click for large image |
Figure 6. The hydroxyproline content in skin tissue of mice. |
| 4. Discussion | Top |
In this study, we developed a novel combination consisting of Baizhi, Baihe, Gancao, P4H transfersome and VC for promoting collagen production and stabilizing the collagen structure in skin. We found that the combination showed an enhanced effect on the mRNA levels of type I collagen. Moreover, this combination also delayed the D-galactose-induced skin aging in mice. Thus, we suggested that this combination could be a potential candidate to be used as an anti-skin-aging product.
It has been reported that the therapeutic effect of reasonable combinations may be superior to single component (Ruan et al., 2006). Multicomponent interventions offer bright prospects for the control of complex diseases in a synergistic manner (Li et al., 2011). The concept of our study is to combine CHM with modern biotechnology to develop an anti-skin-aging agent. CHM, a kind of natural resources with abundant sources, has the characteristics of small side effects, simple and easy to use, and has been widely applied to skin maintenance (Li et al., 2022). Alternatively, mostly of topical collagen-containing products only focus on promoting collagen production and ignore to stabilize the structure of collagen. To compensate this defect, the recombinant P4H was successfully expressed in E. coli. At the same time, we added cofactors in the combination with P4H. In this study, the novel combination we prepared could play to the advantages of each component. It not only promoted collagen synthesis but also stabilized the structure of collagen. Besides, we prepared transfersome which greatly improved the transdermal absorption of combination. In a word, this combination holds great promise for the resistance of skin aging due to its excellent ability in collagen biosynthesis and structural stability.
The choice of CHM to promoting collagen production for this study is because some traditional Chinese medicines could effectively delay skin aging and were expected to be developed as anti-skin-aging drugs in the future. And we selected 82 CHM extracts to determine whether could be used for skin benefit (Wang et al., 2021). Immediately afterward, we utilized the cell viability experiments to screen ten CHM extracts. Then in the in vitro studies, we found that four kinds of CHM (Baizhi, Baihe, Gancao, and Dazao) among ten above could promote the expression of type I collagen in NIH-3T3 cells. This indicated that these CHM extracts have beneficial action for the alleviation of skin aging. It has been reported that glycyrrhizin, a natural extract from the licorice roots, is generally considered to offer protection against cellular senescence (Zhang et al., 2022). Matrix metalloproteinases (MMPs) are known to contribute to the degradation of collagen during ultraviolet irradiation (Yamada et al., 2021). The flavonoid aicalein from Baihe protects cells from UVB irradiationinduced MMP-1 expression. Ursonic acid from Dazao downregulates the transcriptional expression of gelatinases (MMP-2 and MMP-9) by inhibiting of ERK and CREB signaling pathways in NSCLC cells (Maione-Silva et al., 2019). Moreover, ursonic acid suppresses the transcriptional levels of MMP-1 through reduced activation of ERK and c-Fos signaling pathways in HaCaT keratinocytes (Son and Lee, 2020). From the available literature, we speculated that the combination may play an important role in collagen synthesis by downregulating MAPK signaling. Future studies will further explore how the combination promotes the expression of collagen and the underlying mechanisms involved.
In this study, P4H was recombinantly expressed in E. coli to maintain the collagen structure. Prolyl hydroxylation is a post-translational modification that affects the structure, stability and function of collagen. P4H can catalyse hydroxylation of proline to hydroxyproline and plays a central role in the formation and stabilization of the triple helical domain of collagens (Zou et al., 2017). VC is a cofactor of P4H that modifies newly synthesized collagen on the route for secretion (Zhao et al., 2019). Stability tests at different temperatures showed that P4H was highly stable at room temperature. Additionally, the skin permeation of P4H is poor, due to its high molecular weight and hydrophilicity. Therefore, we prepared the P4H transfersome for the purposes of increasing permeability. Transfersome, also known as flexible liposome, has good biocompatibility and biodegradability (Gai et al., 2020). In addition, the transfersome is highly hydrophilic, which helps the drug to permeate through the hydrophilic layer of the skin, i.e., epidermis (Krenczkowska et al., 2020). Here, we prepared the P4H transfersome by thin film hydration method. On the basis of the orthogonal experiments, the optimal conditions were determined as following: ratio of soy lecithin to cholesterol of 9 : 1, total lipid concentration of 6%, and enzyme concentration of 5 mg/mL. The encapsulation rate of P4H transfersome was 36.9% under these conditions. Soy lecithin and cholesterol are used as liposome wall materials and are able to enhance the bioactivity by improving drug solubility and bioavailability. This might be explained by the fact that if the ratio was small, cholesterol content was relatively high, and the formed film is relatively flexible (Park et al., 2019). After screening various combinations of solvents, soy lecithin: cholesterol in ratio 9 : 1 was selected to prepare the P4H transfersome.
The current study established an in vitro cell model using NIH-3T3 to investigate the functions of CHM extracts in regulating collagen synthesis. The murine cell line NIH-3T3 has been used as a model system in a multitude of different studies since its first description in 1963 (Salih et al., 2017). In practice, NIH-3T3 fibroblasts are often used as adesirable cell models for evaluating collagen expression. During the biological progression of skin aging, the aberrant production of collagen plays a crucial role. Thus we assayed cell proliferation and collagen production of CHM extracts in NIH-3T3. The effect of combination on the skin of aging mice was assessed. We are aware that there is still room for improvement in our study. Our current study showed the beneficial effects of CHM with the use of simple NIH-3T3 cells and animal models. And in the future, more diverse cell lines and more advanced experimental models such as human 3D skin equivalents will be used to optimize our combination. Moreover, novel technologies such as network pharmacology will be used to explore the mechanism of action of the CHM combination in the present work (Wang et al., 2022; Zuo et al., 2018).
| 5. Conclusion | Top |
In this study, we designed a novel combination (Baizhi+Baihe+Gancao+P4H transfersome+VC), which effectively promoted the mRNA levels of collagen in NIH-3T3 cells. Moreover, this conclusion also verified in animal models. Taken together, we obtained a combination that could delay skin aging by promoting the generation of collagen.These findings suggest the combination to be a promising component for use in cosmetics or supplements that is being developed for anti-aging applications.
| Supplementary material | Top |
Table S1. Chinese herbal medicines datebase.
Figure S1. Determination of cell viability in NIH-3T3 cells pretreated with CHM extracts.
Acknowledgments
We would like to thanks to Jishou University for the fund support.
This research was funded by China Postdoctoral Science Foundation (2020T130093ZX) and Open Project of Hunan Provincial Engineering Laboratory for Conservation and Comprehensive Utilization of Giant Salamander Resources (DNOC2101).
Conflict of interest
All authors reveal no conflict of interest in this study.
| References | Top |