| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 25, March 2024, pages 42-51
Sesamolin has the ability to induce longevity effects on the Caenorhabditis elegans, and the mechanism depends on the SIR-2.1 and AAK-2 proteins
Chia-Yu China, Pei-Jing Leea, Nae-Cherng Yanga, b, *
aDepartment of Nutrition, Chung Shan Medical University, Taichung 402, Taiwan
bDepartment of Nutrition, Chung Shan Medical University Hospital, Taichung 402, Taiwan
*Corresponding author: Nae-Cherng Yang, Department of Nutrition, Chung Shan Medical University, Taichung 402, Taiwan. Tel: +886-4-3609-7673; Fax: +886-4-2324-8175; E-mail: naeman@csmu.edu.tw
DOI: 10.31665/JFB.2024.18370
Received: December 26, 2023
Revised received & accepted: February 4, 2024
| Abstract | Top |
Sesamolin, one of the prominent lignans in sesame seeds, offers diverse physiological benefits. However, the longevity effects and mechanisms of sesamolin remain unclear. We hypothesized that sesamolin can exert the longevity effects in the Caenorhabditis (C.) elegans when prepared with the -cyclodextrin to form an inclusion complex (named CD-SM). In this study, the CD-SM was prepared, and the lifespan assays, health indexes, and loss-of-function assays in the C. elegans or mutants were conducted. The results demonstrated that the CD-SM significantly extended the C. elegans lifespan and improved the health indexes, such as the pharyngeal pumping and body bends. The longevity effects of the CD-SM were found to depend on the signaling of the SIR-2.1 and AAK-2. In conclusion, the -CD inclusion is a crucial step for assessing the sesamolins longevity effects in the C. elegans. This study confirms that sesamolin exhibits the longevity effects, and its mechanism relies on the signaling of the SIR-2.1 and the AAK-2 proteins, suggesting its potential as a health-promoting ingredient.
Keywords: Sesamolin; Sesamin; Longevity effects; -cyclodextrin; Caenorhabditis elegans
| 1. Introduction | Top |
Sesame (Sesamum indicum L.) is one of the earliest oil crops cultivated and consumed by humans within the Pedaliaceae family (Wei et al., 2022). In addition to its rich nutritional content, sesame contains various lignans such as sesamin, sesamolin, and sesamol (Pathak et al., 2014; Wei et al., 2022). Sesamolin, therefore, is a lignan present in sesame second only to sesamin in abundance (Wei et al., 2022), and its structure is similar to that of sesamin (Michailidis et al., 2019). Researches have confirmed that sesamolin has several beneficial effects on human health, including neuroprotection, inhibition of lipid peroxidation in the liver and kidneys, suppression of proliferation, and the induction of apoptosis in colorectal cancer cells, regulation of lipid metabolism, inhibition of melanin production, and the enhancement of the NK cell lytic activity (Kushiro et al., 2002; Michailidis et al., 2019; Rosalina and Weerapreeyakul, 2021; Wu et al., 2019). Previous studies have demonstrated the ability of sesamin to extend the lifespan of the C. elegans (Nakatani et al., 2018; Yaguchi et al., 2014); however, it is currently unclear as to whether sesamolin possesses the similar longevity effects so as to extend the lifespan and to improve the health in the C. elegans.
Although there have been studies investigating the ability of sesamolin to extend the lifespan of the nematodes, they have reached negative conclusions (Kashima et al., 2012). Compounds suitable for testing in the nematode models typically require a water solubility, as the test components must dissolve in the nematode growth medium (NGM) or the Luria Bertani (LB) broth used to spray the testing agent on the top surface of the NGM plates. Previous research has indicated that the liposoluble components are not miscible with the water-soluble environment of the C. elegans model (Avery and Thomas, 1997). To address this issue, some studies have proposed using the -cyclodextrin (-CD) to resolve the insolubility of the liposoluble components in a water-soluble environment (Kashima et al., 2012). The -CD is a cyclic molecule composed of glucopyranose monomers, featuring a hydrophilic surface and a lipophilic central cavity (Saokham and Loftsson, 2017). It includes the liposoluble components within its central cavity, thereby enhancing the solubility in a water-soluble environment (Echezarreta-Lopez et al., 2002; Uekama et al., 1982; Vianna et al., 1998). Research results have demonstrated that the -CD is suitable when used in the model of the C. elegans (Kashima et al., 2012). In the two previously mentioned reports exploring the longevity effects and mechanisms of the sesamin, researchers used the -CD to include sesamin, which was then applied onto the NGM plates for the C. elegans (Nakatani et al., 2018; Yaguchi et al., 2014). Given that sesamolin also exhibits a high liposolubility, it theoretically cannot dissolve in a liquid medium or broth, and be applied in or onto the NGM plates. Therefore, we are interested in the possibility that the lack of the longevity effects observed in the previous studies on sesamolin (Keowkase et al., 2018) may be attributed to the absence usage of the -CD as a carrier.
Additionally, past research has proposed that, for the lifespan assay in the C. elegans, using dead bacteria as a food source is more suitable so as to avoid the potential effects of the live bacteria on the test substance (Collins et al., 2006; Garigan et al., 2002); for instance, live E. coli can be pathogenic to the older nematodes; hence, a substance that reduces the pathogenicity of E. coli might extend the lifespan of the C. elegans. Furthermore, metabolites produced by the live bacteria could be detrimental to the C. elegans (Garigan et al., 2002). Moreover, the test substance itself might undergo metabolic transformations by the live bacteria, influencing the lifespan of the C. elegans; this could directly or indirectly influence the nematodes, leading to the distorted research results (Collins et al., 2006; Garigan et al., 2002; Liao et al., 2011). Indeed, the paper reporting the inability of sesamolin to induce the nematode longevity used live bacteria (Keowkase et al., 2018). Therefore, we are also interested in investigating as to whether interference in the results occurred due to the usage of the live bacteria.
In the mechanistic aspect, previous research has revealed that the ability of sesamin to extend the lifespan is lost in the mutants such as the SIR-2.1, AAK-2, and DAF-15 (note: these are all the loss-of-function mutants). This suggests that sesamin can extend the lifespan of the nematodes through the signal pathways related to the calorie restriction, including the SIR-2.1, AAK-2, and DAF-15 (Nakatani et al., 2018). The SIR-2.1, AAK-2, and the DAF-15 correspondingly represent the homologous proteins of the human Sirtuin 1 (SIRT1), AMP-activated protein kinase (AMPK), and the regulatory unit of the mTOR (raptor) in the C. elegans. Additionally, sesamin also has been demonstrated to extend the lifespan of the C. elegans by modulating the IIS pathway (Yaguchi et al., 2014). However, it remains unclear whether sesamolin can also extend the lifespan of the C. elegans through the similar signaling mechanisms.
Therefore, this study aims to investigate as to whether sesamolin, included in the -CD, has the ability to extend the lifespan and improve the health in the C. elegans. Initially, we prepared pure samples of sesamolin and sesamin using a preparative HPLC. Subsequently, we prepared the -CD-sesamolin inclusion complex (CD-SM) and the -CD-sesamin inclusion complex (CD-SA), and the preparation efficiency of both the inclusion complexes that were then analyzed using the analytical HPLC. We first examined the effects of the sesamin dosage on the lifespan assay to reveal the required dosage and extrapolate the dosage range for the sesamolin experiments. We then explored the effects of different dosages of the sesamolin on the lifespan of the C. elegans, and investigated the influence of using live or dead bacteria on the longevity capabilities by the administration with the CD-SM and the CD-SA as a positive control. Next, we assessed the effects of sesamolin on the health indexes, including the pharyngeal pumping and body bends. We also investigated the signal pathways using the loss-of-function mutants, including the SIR-2.1, AAK-2, DAF-15, and AKT-1 mutants. The SIR-2.1, AAK-2, and DAF-15 are crucial proteins in the signaling mechanisms of the calorie restriction longevity effects, while the AKT-1 is an important signaling protein in the IIS pathway (Sun et al., 2017). These pathways have been reported to be involved in the mechanisms underlying the effects of the sesamin on extending the lifespan of the C. elegans.
| 2. Materials and methods | Top |
2.1. Materials
All chemicals used were of the analytical grade. The NaCl, KCl, NaOH, Na2HPO4, and HCl were purchased from Merck (Darmstadt, Germany). Ampicillin sodium salt, fluorodeoxyuridine (FUdR), cholesterol, sodium dodecyl sulfate, and sodium azide were obtained from Sigma (St. Louis, MO, USA). The KH2PO4, MgSO4, and CaCl2 were purchased from J.T. Baker (Phillipsburg, NJ, USA). The American bacteriological agar and yeast extract were obtained from Conda (Madrid, Spain). The vegetable peptone and tryptone were obtained from Fluka (Buchs, Switzerland). The -CD was purchased from Tokyo Chemical Industry (Tokyo, Japan). The wild-type C. elegans strain N2 was a gift from the C. elegans core facility (National Taiwan University, Taiwan). The AAK-2, AKT-1, DAF-15, SIR-2.1 mutants (note: all of these were loss-of-function mutants) obtained from the Caenorhabditis Genetics Center (University of Minnesota, USA). The sesame lignan samples were obtained from the Distinguished Professor Min-Hsiung Lee, Department of Agricultural Chemistry, National Taiwan University.
2.2. The preparation of sesamolin and sesamin samples
The obtained sesame lignan samples (prepared from waste sesame cakes by Professor Lee through processes such as grinding, extraction, and column chromatography) were subjected to purification through the preparative HPLC, as the system consisted of a WatersTM 600 controller and pump, a Reprosil C18 column (30 250 mm, 5 m), a Varian Prostar 704 fraction collector, and a WaterTM 486 tunable absorbance detector. The separation was achieved using a mobile phase of 40% methanol with isocratic elution for 150 minutes at a flow rate of 20 mL/min. UV signals were detected at a wavelength of 280 nm. The sample loop volume was 5 mL, and the column temperature was maintained at 25C. The quality of the prepared sesamolin and sesamin were evaluated by the obtained chromatograms by following the analytic HPLC method.
2.3. Preparation of the -CD and sesamolin or sesamin inclusion complexes
According to the previous studies (Nakatani et al., 2018; Yaguchi et al., 2014), a saturated solution of the -CD (230 mg/mL) was prepared and filtered under aseptic conditions so as to obtain a sterile saturated -CD solution. Moreover, a sterile solution of sesamolin or sesamin in ethanol (2.5 mg/mL) was prepared. Subsequently, the two solutions were mixed in a 10:1 ratio, stirred using a rotary mixer for 1224 hours, and then subjected to high-speed centrifugation (4C, 10,000 rpm, 20 min) to obtain the solid inclusion complex to be weighed. To evaluate the efficiency of the inclusion, 5 mL of pure water containing a 0.5-mL sterile solution of sesamolin or sesamin in ethanol (equivalent to 3.4 mol sesamolin or 3.5 mol sesamin in the solutions, respectively), or the supernatant after the centrifugation of the -CD and sesamolin or sesamin mixtures, was subjected to extraction using 5 mL of ethyl acetate (EA) three times. The collected extracts were combined, and 1 mL was evaporated using nitrogen gas. Subsequently, 100 L of EA was added for the reconstitution, and the concentration of sesamolin or sesamin was analyzed by the HPLC. Five concentrations of purified sesamolin or sesamin were used to establish the calibration curves for the quantifications. The efficiency was determined using the equation: [the total amount of sesamolin or sesamin in the 15-mL EA extracts for the pure water sample] (the amount of sesamolin or sesamin in the suspension)/[the total amount of sesamolin or sesamin in the 15-mL EA extracts for the pure water sample]*100%. It has been calculated that approximately 1 mg of the solid inclusion complex contains 9.0 g of sesamolin and 8.9 g of sesamin.
2.4. Analytical HPLC method
The sesamolin and sesamin were analyzed by using an analytical HPLC system that included a Shimadzu system controller (Osaka, Japan), Shimadzu LC-10AD pump, Athena C18 column (4.6 250 mm, 5 m), and Shimadzu SPD-10A UV-VIS detector. The analysis conditions involved a mobile phase of 60% methanol solution with isocratic elution for 40 minutes at a flow rate of 1 mL/min. UV signals were detected at a wavelength of 280 nm. The sample loop volume was 20 L, and the column temperature was maintained at 25C.
2.5. Assay of the lifespan of C. elegans
The nematodes lifespan was assessed by following the previous methods with slight modifications (Sutphin and Kaeberlein, 2009). Synchronized larvae at the L2-3 stages were gently transferred onto a 5-cm Amp/FUdR plate (30 worms/plate; three plates per treatment, n = 90) with dead bacteria with the CD-SM or CD-SA at varying doses of sesamolin or sesamin on the surface, and cultured at 20C. Plates were replaced every four days, and survival was assessed every two days. Nematodes were considered deceased if unresponsive to a platinum wire touch or lacking pharyngeal pumping. Survival data, along with an average, median, and maximum lifespan values, were analyzed using the SPSS, and the survival percentage was plotted using the Sigma Plot 8.0. As we described in the previous report (Yang et al., 2023), we used the proper concentrations of the CD-SM or CD-SA in the LB medium, and spread 200 L of the corresponding solutions onto the surface NGM. Because the LB medium would dry out after spreading onto the surface of the NGM, thus, the dosage unit of nmol/plate was used rather than the molar concentration of the tested samples. All of the other handling procedures for the C. elegans have been described in our previous paper including the NGM plates with the dead bacteria that was prepared by the exposed plates to the UV at two doses of 9,999 100 mJ/cm2 to eliminate bacteria (Yang et al., 2023).
2.6. Assay of the pharyngeal pumping in C. elegans
Pharyngeal pumping was determined as previously described (Iwasa et al., 2010; Zhao et al., 2017). The 10 nematodes for each group were randomly chosen to record the pharyngeal pumping with a charge-coupled device (CCD) video camera under a microscope on the 6th, 8th, 10th, 12th and 14th day of life. The number of pharyngeal beats within 60 seconds was calculated based on the slow-motion playback.
2.7. Assay of the body bends in C. elegans
Body bend was determined by the swimming test as described previously (Iwasa et al., 2010). The 10 nematodes for each group were randomly picked up to determine the body bends on the 10th day of life. After crawling on an NGM plate without the OP50 for 30 seconds to remove the excess E. coli from the worms, each nematode was placed in a well of a 24-well plate containing 1 mL of M9 buffer. The motility of the nematodes was recorded with a CCD video camera under a microscope, and the number of body bends within 30 seconds was calculated based on the slow-motion playback. Body bends were defined as the number of repeated twists at the center point of the nematode.
2.8. The loss-of-function test
One of the powerful advantages of the C. elegans model is used for the loss-of-function mutants to reveal the signaling pathway of a certain tested molecule. If a certain function, such as the lifespan-extending effect disappears in a certain loss-of-function mutant, the result indicates that the signaling mechanism of the tested molecule is via the mutated protein. The SIR-2.1, AAK-2, DAF-15, AKT-1 mutants were used for the loss-of function test in this study. With the exception that the wild-type nematodes were replaced with the mutants, all the procedures performed were the same as the above assay of the lifespan, pharyngeal pumping, and the body bend of the C. elegans.
2.9. Statistical Analysis
Data were analyzed using the Students t-test or analysis of variance (ANOVA), followed by the Duncans test for group mean comparisons using the SPSS v.14.0 software (SPSS, Inc., Chicago, IL, USA). The differences in the lifespan of the nematodes between the groups were analyzed using the Cox regression. The pharyngeal pumping was analyzed using a simple regression analysis method. A difference with a p < 0.05 was considered statistically significant.
| Results | Top |
3.1. Preparation of sesamolin and sesamin samples
Initially, the sesame lignan samples obtained from Prof. Lee were subjected to the HPLC analysis, revealing a composition of approximately 60% sesamolin and 40% sesamin (Figure 1a). Subsequently, a preparative HPLC was employed for further separation and purification of the sesamolin and sesamin, followed by an analytical HPLC to assess the purification results. From the chromatograms (Figures 1b and c), we confirmed that, aside from the solvent signals, only the signals corresponding to the sesamolin and sesamin were present. This indicates that the purified sesamolin and sesamin samples should be of sufficient quality and purity for use in this study.
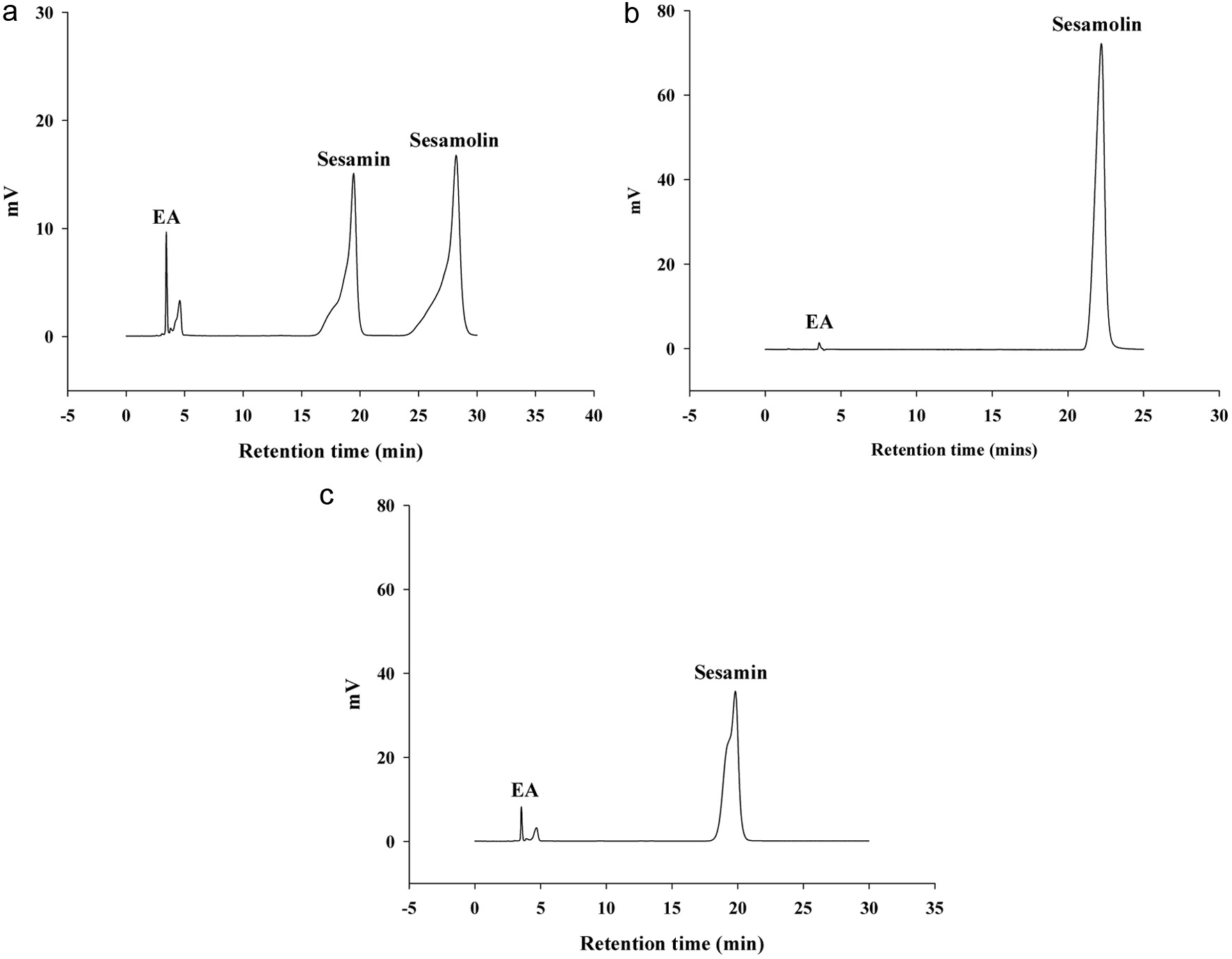 Click for large image |
Figure 1. Preparation of sesamolin and sesamin samples. (a) The HPLC chromatogram for the initial obtained sesame lignan samples, which was prepared in ethyl acetate (EA) with a concentration of 0.35 mg/mL in the sample. (b) The HPLC chromatogram of the purified sesamolin sample by using the preparative HPLC, which was prepared in EA with a concentration of 1 mM sesamolin. (c) The HPLC chromatogram of the purified sesamin sample by using the preparative HPLC, which was prepared in EA with a concentration of 1 mM sesamolin. |
3.2. Preparation efficiency of -CD and sesamin or sesamin inclusion complex
After confirming the quality of the samples, we utilized the -CD for the preparation of the sesamolin or sesamin inclusion complex. Using the analytical HPLC, we evaluated the efficiency of the -CD inclusion complexes by comparing the amounts of the sesamolin or sesamin in the EA extracts for the pure water sample or after the high-speed centrifugation. The more lignans included in the -CD, the lower the amount of the lignans in the supernatant after the centrifugation. The analysis results indicated that, for the -CD including sesamolin, the residual amount of not included lignans is only about 7.2% i.e., the concentration decreased by approximately 92.8% after the high-speed centrifugation (Figures 2a and b; Table 1). Similarly, for the -CD including the sesamin, the residual amount of not included lignans is only about 7.4% i.e., the analysis showed a concentration reduction of about 92.6% after the high-speed centrifugation (Figures 2c and d; Table 1). This suggests that, for both sesamolin and sesamin, after the inclusion and the high-speed centrifugation, the preparation efficiency of approximately 93% for both the sesamolin and sesamin in the solid inclusion complexes.
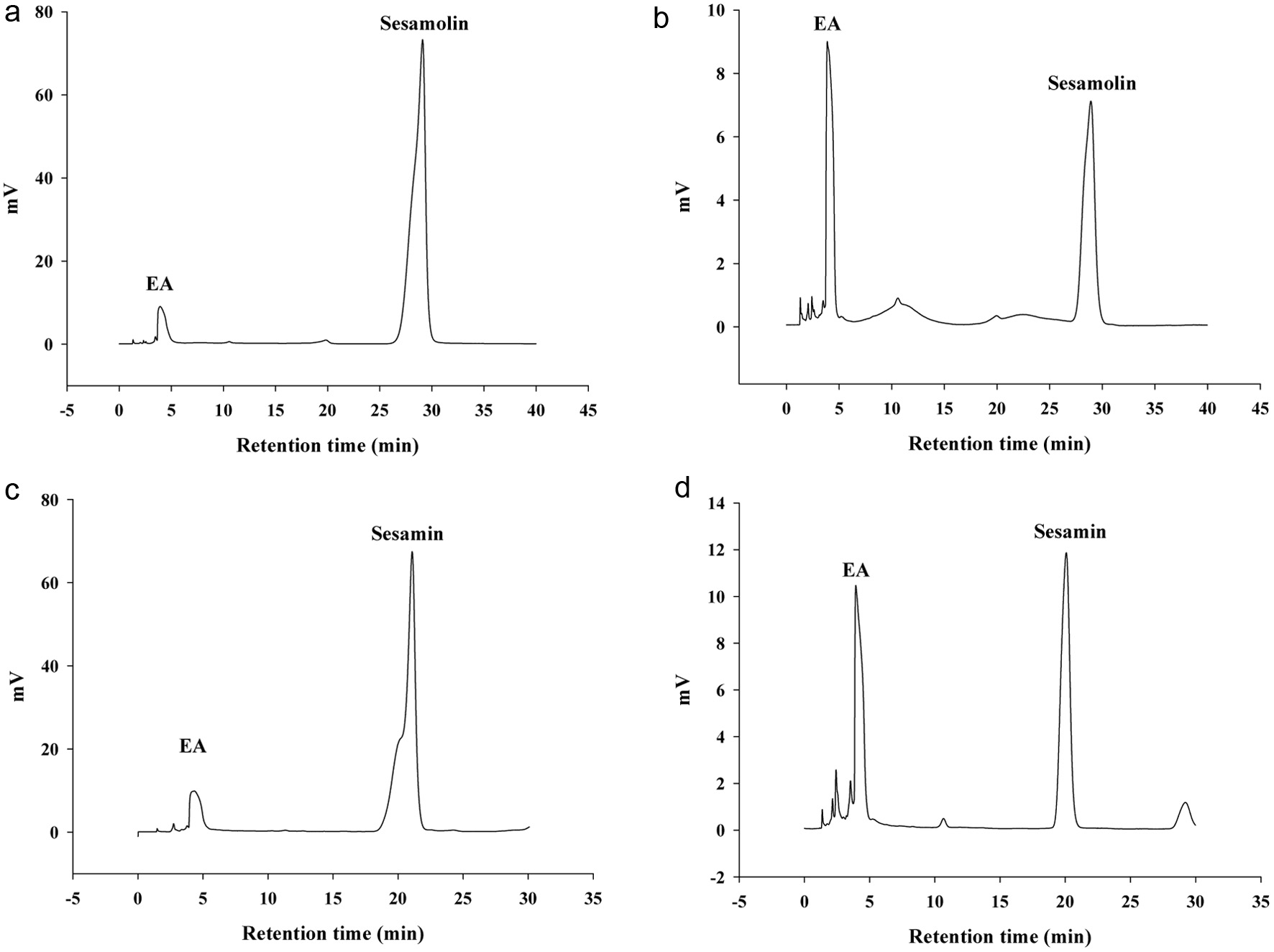 Click for large image |
Figure 2. Efficiency evaluation of the preparation of the -cyclodextrin (-CD) and sesamolin or sesamin inclusion complexes. (a) The HPLC chromatogram of sesamolin in a pure water sample containing the original concentration of sesamolin, which was then extracted by ethyl acetate (EA). The extract (1 mL) was evaporated, reconstituted, and analyzed by HPLC, as described in the Methods. (b) The HPLC chromatogram of sesamolin after mixing with the -CD for 12-24 hours, followed by centrifugation and the same procedures as described in the Methods. (c) The original HPLC chromatogram of sesamin, as described in the Methods. (d) The HPLC chromatogram of sesamin after mixing and centrifugation, following the same procedures as described in the Methods. |
 Click to view |
Table 1. Analysis of the preparation efficiency of the -CD and sesamolin or sesamin inclusion complexes |
3.3. Effects of sesamolin and sesamin on the lifespan in wild-type C. elegans
Subsequently, we investigated as to whether the sesamolin has the ability to extend the lifespan of the C. elegans by the administrated nematodes with the CD-SM that contained different doses of the sesamolin. The tested doses of the CD-SM included 4, 16, 64, and 256 nmol/plate sesamolin. The results showed a significant extension of the nematode lifespan in the groups treated with the CD-SM contained with 64 and 256 nmol/plate of sesamolin, with the mean lifespans extended by 15.7% and 18.7%, respectively (Figure 3b; Table 2). However, as the effects at 64 and 256 nmol/plate of sesamolin approached similarity, it suggests that the 256 nmol/plate of sesamolin is close to the dosage with the highest lifespan extension capacity. Additionally, we selected experimental doses for the CD-SA based on a previous study reporting the optimal dosage for extending the nematode lifespan with the CD-SA (Yaguchi et al., 2014). The report indicated that the optimal dosage for sesamin for extending the nematode lifespan was 6.3 g/plate, which, when converted to the units used in this study, equals 16 nmol/plate. Thus, we initially used the CD-SA contained 4, 16, and 64 nmol/plate of sesamin to perform the lifespan assay, and the results were similar to the previous report, showing that 16 nmol/plate of sesamin was the optimal dosage, extending the average lifespan by 11.5% (p < 0.05), while the 64 nmol/plate of sesamin only extended the mean lifespan by 2.6% (p > 0.05) (Figure 3a; Table 2). Therefore, for the subsequent study, we chose the CD-SA contained with 16 nmol/plate of sesamin as the dosage for the positive control, and the CD-SM were set at the dosages contained with 4, 16, 64, and 256 nmol/plate of sesamolin. When combining the results of these two lifespan assays, it demonstrates that sesamolin has a broader effective dosage range compared to sesamin, and the optimal dosage of sesamolin for extending the nematode lifespan is approximately 1.6 times higher than the optimal dosage for sesamin.
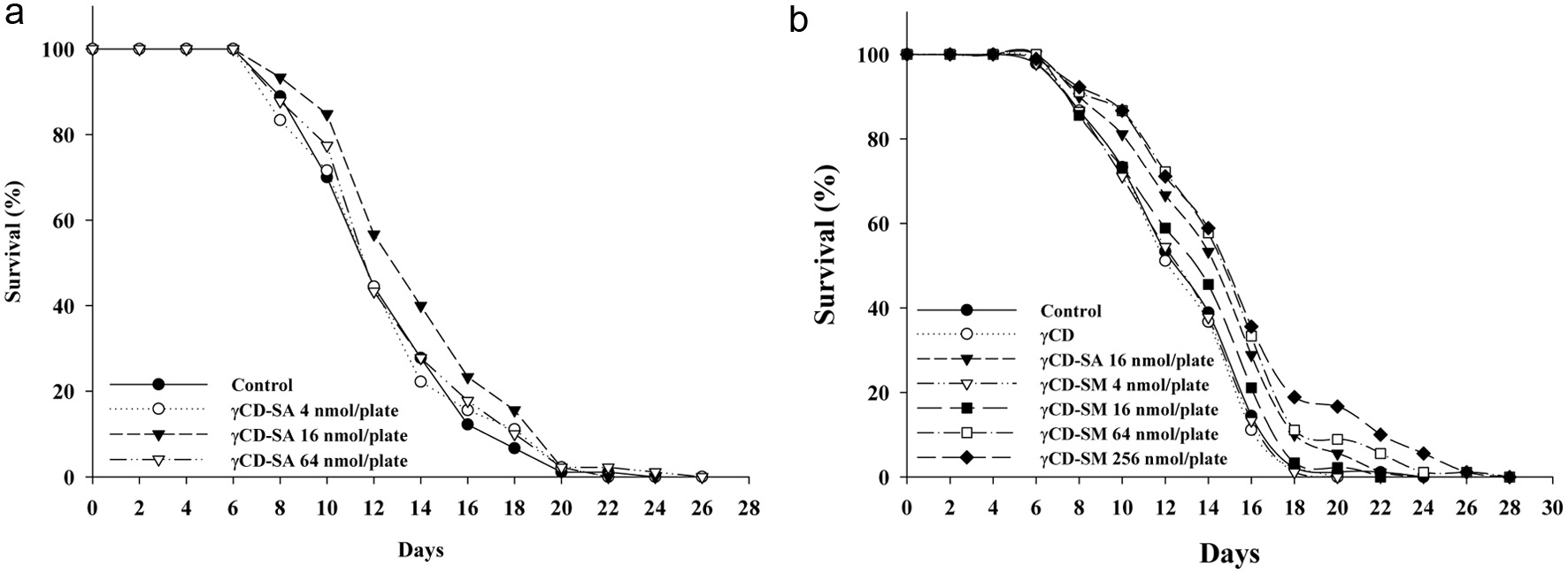 Click for large image |
Figure 3. Effects of sesamolin and sesamin on the lifespan of the wild-type C. elegans. The -CD and sesamolin or sesamin inclusion complexes (i.e., CD-SM and CD-SA) were prepared and the lifespan assay were conducted with (a) the CD-SM contained the doses of sesamolin from 0, 4, 16, 64 nmol/plate, or (b) the CD-SA contained the doses of sesamolin from 0, 4, 16, 64 to 256 nmol/plate. The method for the lifespan assay performed as described in Methods (n = 90). |
 Click to view |
Table 2. Effects of sesamin and sesamolin on the lifespan of wild-type C. elegans. |
3.4. Effect of sesamolin and sesamin on the lifespan of wild-type C. elegans under dead or live bacterial culture
In previous studies on the lifespan assay of sesamolin, the cultivation was carried out using live bacteria (Keowkase et al., 2018). Whether this could potentially be a reason for the previous inability of sesamolin to extend the lifespan of the C. elegans. Therefore, we further compared whether the CD-SM and CD-SA could also extend the lifespan of the C. elegans under live and dead bacterial culture conditions. Three independent lifespan assays were carried out for each of the bacterial culture conditions, and the resulting mean lifespans were subjected to a statistical analysis to assess the percentage increase in the mean lifespan. The results showed that, whether it was sesamolin or sesamin, there was no significant difference in the ability to extend the lifespan of the C. elegans under live or dead bacterial culture conditions (Figure 4; Figure S1).
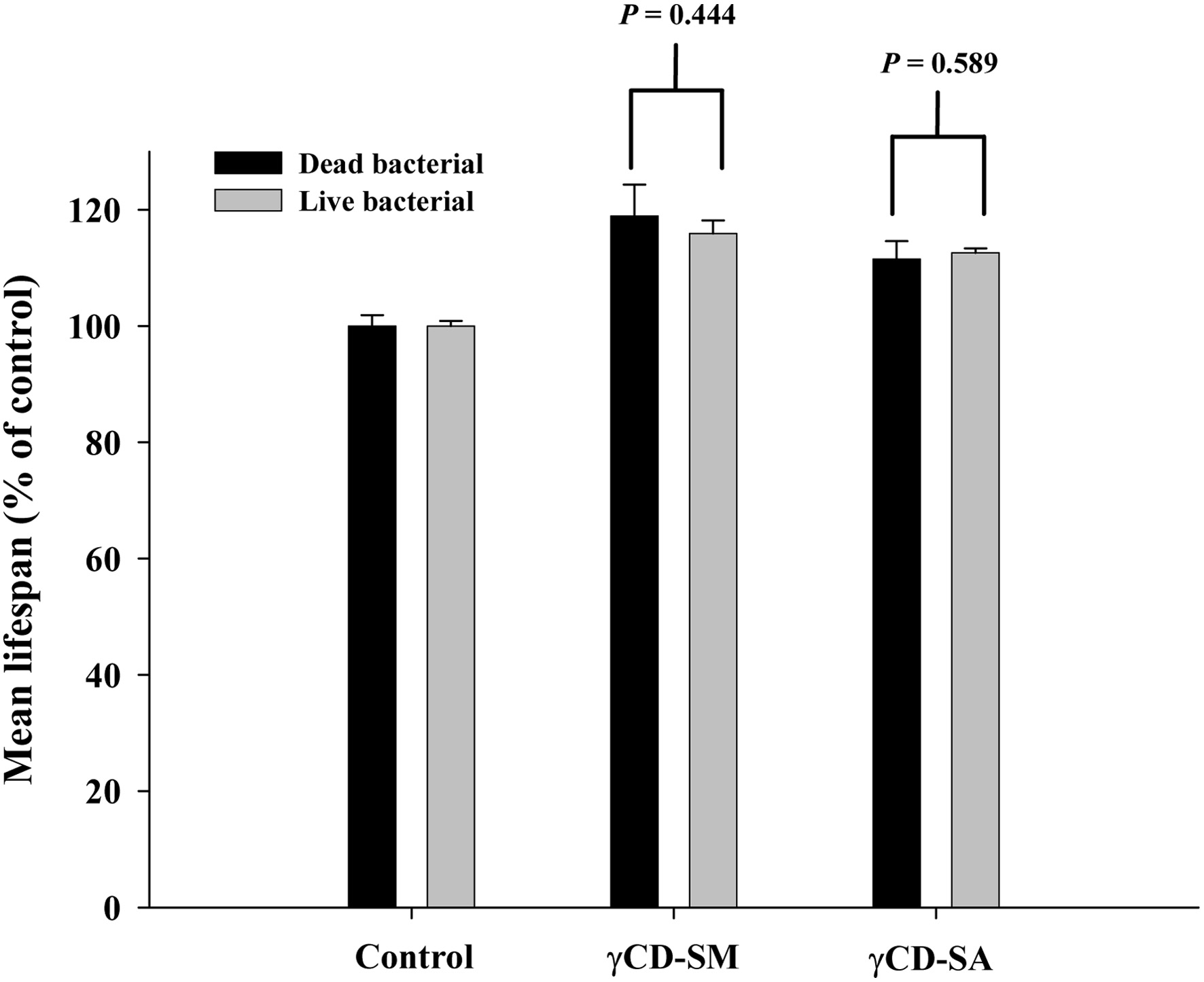 Click for large image |
Figure 4. Effects of sesamolin and sesamin on the lifespan of the wild-type C. elegans under the dead bacterial or live bacterial culture. The -CD and sesamolin or sesamin inclusion complexes (i.e., CD-SM and CD-SA) were prepared. The obtained mean lifespan presented as a percentage increase in the mean lifespan as compared to the control from the lifespan assay under the dead bacterial and live bacterial culture. The data are from three independent lifespan assays, with n = 90 in each group. P values are indicated in the figures. |
3.5. Effect of sesamolin on the health indexes of wild-type C. elegans
Furthermore, we investigated whether the sesamolin has the ability to improve the health indexes in the C. elegans, including the pharyngeal pumping and body bends. Experiments were conducted using the most efficacious dosage of the CD-SM, which contained a 256 nmol/plate of sesamolin. The results indicated that, compared to the control group, the CD-SM significantly increased the frequency of the pharyngeal pumping and body bends in the C. elegans (Figure 5a, b). Taken together, these results demonstrated the ability of sesamolin to improve the health of the C. elegans.
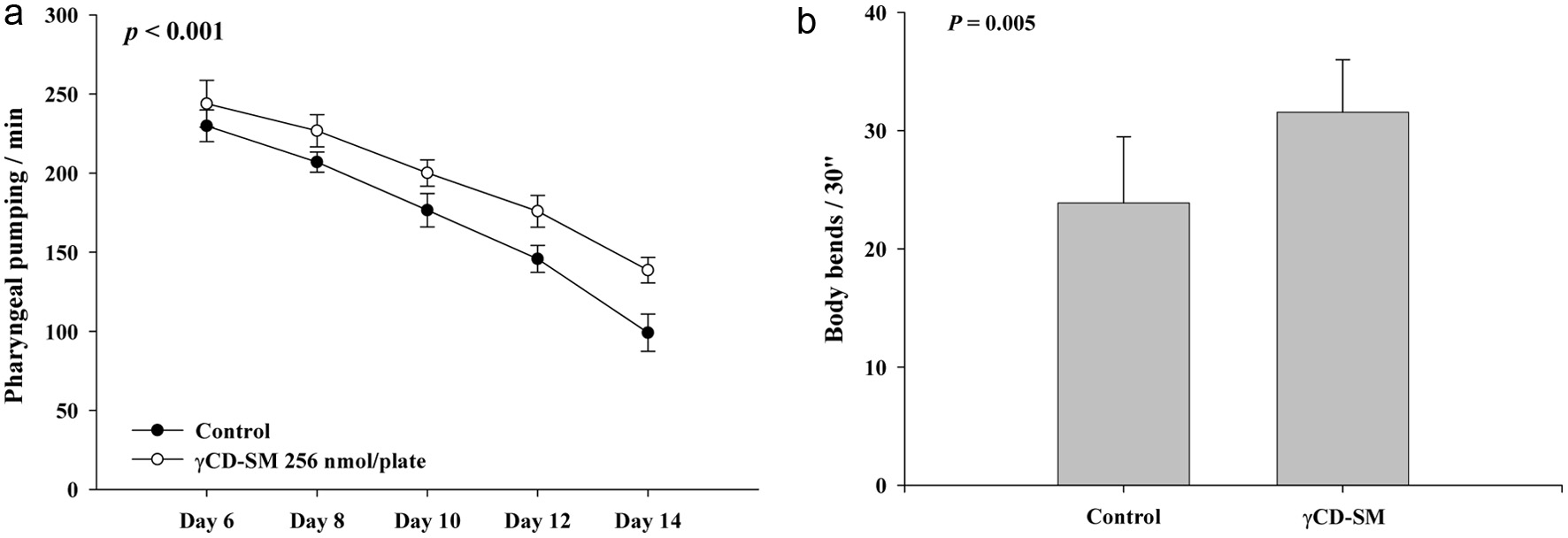 Click for large image |
Figure 5. Effects of sesamolin on the health indexes of the wild-type C. elegans. For health indexes analysis, the nematodes were grown on plates containing with or without 256 nmol/plate of sesamolin administrated by the prepared CD-SM. (a) The pharyngeal pumping and (b) the body bends were determined (n = 10) as described in Methods. P values are as shown in the figures. |
3.6. Evaluation whether the longevity function of sesamolin disappears in the SIR-2.1, AAK-2, DAF-15, and AKT-1 mutants
We aimed to reveal the signaling pathways for sesamolin to extend the lifespan of the C. elegans. The mutants including the SIR-2.1, AAK-2, DAF-15 and AKT-1 were used for the loss-of function test. The results revealed that the longevity-extending and health-improving capabilities of the CD-SM disappeared in the SIR-2.1 and the AAK-2 mutants (Figures 6a, b; Table S1). However, the longevity-extending and health-improving capabilities of the CD-SM were still observed in the DAF-15 mutant (Figure 6c; Table S1) and in the AKT-1 mutant (Figure 6d; Table S1). Since the longevity effects of sesamolin do not depend on the AKT-1 in the IIS pathway and the downstream protein DAF-15, mutually confirming that the IIS pathway was not involved in the mechanism of the sesamolins longevity effects. In summary, the longevity effects of sesamolin required the signaling mechanisms of the SIR-2.1 and AAK-2.
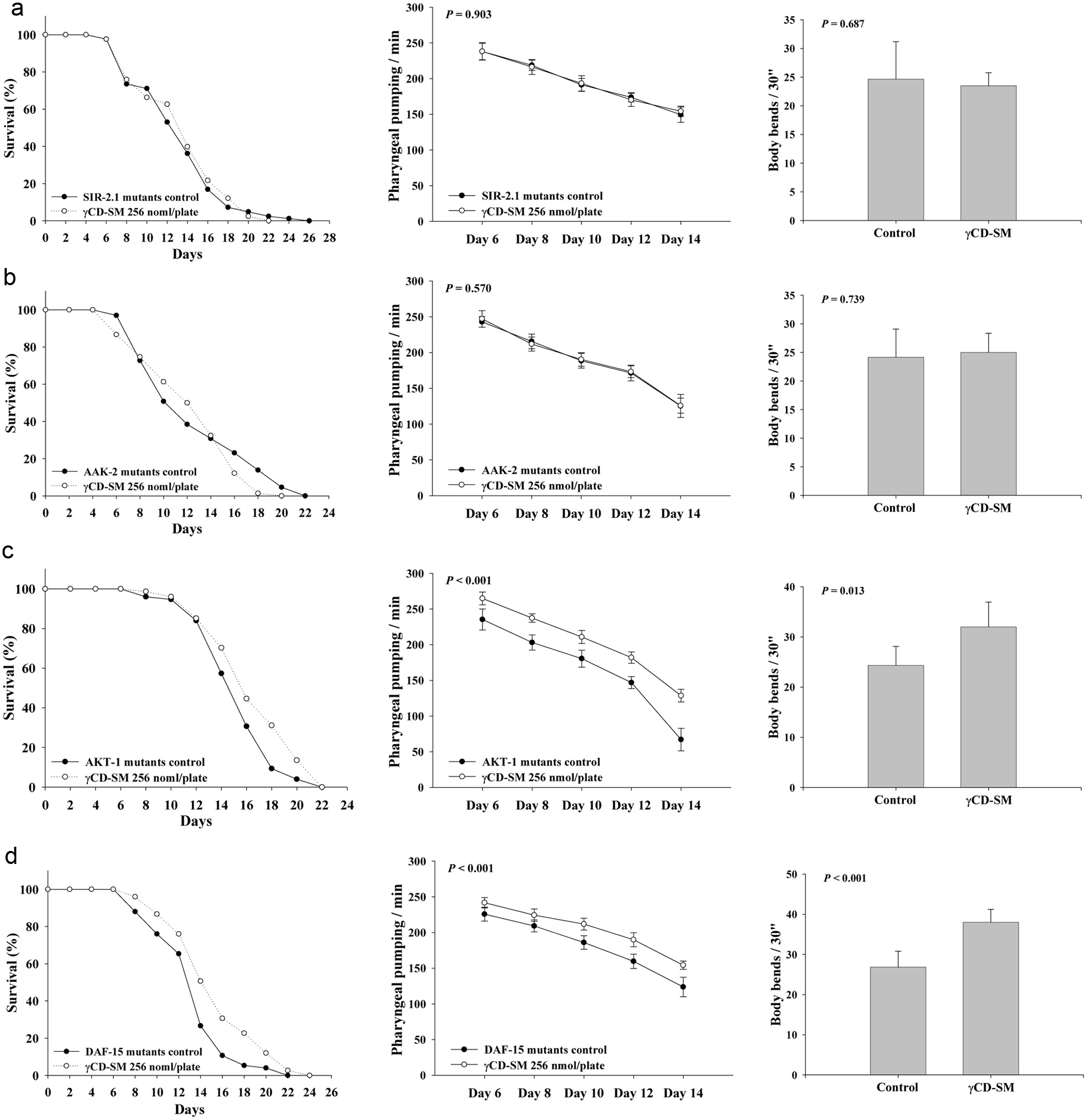 Click for large image |
Figure 6. Effects of the sesamolin on the lifespan and health indexes of the SIR-2.1, AAK-2, AKT-1 and DAF-15 mutants. For the loss-of-function tests in the lifespan assay, the (a) SIR-2.1, (b) AAK-2, (c)AKT-1, and (d) DAF-15 mutants were grown on plates with or without 256 nmol/plate of sesamolin administrated by the prepared CD-SM (n = 90) as described in Methods. For the loss-of-function tests in the health indexes analysis, the nematodes were grown on plates containing with or without 256 nmol/plate of sesamolin administrated by the prepared CD-SM. The pharyngeal pumping and the body bends were determined (n = 10) as described in Methods. P values are as shown in the figures. |
| 4. Discussion | Top |
The amount of sesamin and sesamolin in the sesame seeds is 200500 mg/100g and 200300 mg/100g, respectively (Hadipour et al., 2023). Previous studies have confirmed the longevity-enhancing ability of the sesamin. However, the sesamolin, structurally similar to the sesamin, does not exhibit a lifespan-extending effect in the C. elegans. Therefore, this study aims to explore as to whether the previous experiments did not use the -CD to include the sesamolin so as to increase the solubility in the medium or broth. The results indicate that the CD-SM has a longevity effect in the C. elegans, significantly extending the lifespan of the C. elegans, with 256 nmol/plate of sesamolin showing the highest efficacy. The CD-SM also significantly improves the health indexes, including the pharyngeal pumping and body bends. Furthermore, we found that whether using live or dead bacteria, there is no significant difference in the lifespan-extending effects of the CD-SM or CD-SA, indicating that the bacterial does not significantly interfere with the results. Additionally, through the loss-of-function tests with the mutants, we further explored the possible pathways for the longevity effect of the sesamolin. The results showed that under supplementation with the CD-M, the ability of the sesamolin to extend the lifespan and improve the health indicators disappears in the SIR-2.1 and AAK-2 mutants. This suggests that the longevity effect of the sesamolin in the C. elegans relies on the signaling pathways of the SIR-2.1 and AAK-2. To the best of our knowledge, this study is the first to demonstrate that sesamolin, a lignan in sesame, has the potential to extend the lifespan and improve the health indexes, suggesting its potential as a health-promoting substance for slowing the aging process.
Previous studies have shown that under the conditions of the inclusion of sesamin with the -CD and culturing nematodes with live E. coli OP50, there is a significant extension of the lifespan of the C. elegans (Yaguchi et al., 2014). However, past research has indicated that in the lifespan experiments, using dead bacteria as a food source is more appropriate so as to avoid interference with the test substance, being mainly due to the effects of the live bacteria, either directly or indirectly affecting the nematodes (Collins et al., 2006; Garigan et al., 2002; Liao et al., 2011). Nevertheless, the results of this study demonstrate that using live or dead bacteria does not affect the ability of sesamin to extend the lifespan of the C. elegans. Additionally, previous research showed that sesamolin, when cultured with nematodes without the -CD inclusion and live E. coli OP50, did not significantly extend the lifespan of the C. elegans (Keowkase et al., 2018). This study also confirms that using live or dead bacteria as a food source has little impact on the ability of sesamolin to extend the lifespan of the C. elegans. However, the results indicated that the primary alteration in this study, using the -CD to include sesamolin, is sufficient to demonstrate the longevity effect of the sesamolin. This highlights the critical step of ensuring the solubility of the lipophilic components, such as sesamolin, in aqueous solutions. Although the effect of applying live or dead bacteria on the lifespan extension of sesamin and sesamolin is not significant, it is still recommended to use dead bacteria when evaluating other nutrients using the model of the C. elegans.
However, previous studies have demonstrated that the mechanism underlying the lifespan extension by sesamin relies on the IIS signaling and proteins such as the SIR-2.1, AAK-2, and DAF-15. It has also been shown that sesamins ability to extend the lifespan of the C. elegans disappears under the conditions of caloric restriction (Vianna et al.), suggesting sesamins potential as a CR mimetic. The CR involves reducing the calorie intake by approximately 2530% without depriving the essential nutrients (Pignatti et al., 2020) and has been demonstrated to extend the healthspan and the lifespan in rodent and primate models (Acosta-Rodriguez et al., 2022; Mattison et al., 2017). The CR generally achieves its effects by (1) downregulating the IIS pathway, (2) reducing the mTOR signaling, (3) activating the sirtuin 1 pathway, and (4) modulating the AMPK pathway (Green et al., 2022). Increasing evidence indicates that the mTOR is a downstream molecule in the IIS pathway (Testa et al., 2014). Thus, the downregulation of the IIS pathway and the reduction of mTOR signaling under the CR likely represents the coordinated effects within the same signaling pathway. In this study, we found that the sesamolins longevity effect on the nematodes relies on the assistance of the SIR-2.1 and AAK-2, indicating that the sesamolin may achieve its longevity effect through the SIR-2.1 and AAK-2 pathways. However, unlike sesamin, sesamolins mechanism does not depend on the IIS pathway or its downstream TOR protein, suggesting that sesamolin may not completely act in the same manner as sesamin. Interestingly, the results of this study indicate that sesamolins longevity effect seems to be superior to sesamin. For instance, the optimal dosage of sesamolin can extend the nematode lifespan by approximately 18.7% under conditions of dead bacteria cultivation, while the optimal dosage of sesamin only extends the nematode lifespan by 11.5%. Furthermore, this study also demonstrates that sesamolin has a broader effective dosage range when compared to sesamin. Therefore, the health-promoting longevity effect of sesamolin appears to be better than that of sesamin. This suggests that the longevity effect of the sesame extract may at least be the sum of the effects of various lignans, including sesamin and sesamolin. Future research will further evaluate the longevity effects of the sesame extract, including assessing the effects of other sesame lignans and the potential synergistic effects of the different lignans.
| 5. Conclusion | Top |
In conclusion, this study confirms that sesamolin, when included in the -cyclodextrin, possesses the ability to extend the lifespan and improve the health in the C. elegans, demonstrating the longevity effect of sesamolin. Moreover, sesamolins capacity to extend the lifespan of the C. elegans appears to be more significant than that of the sesamin, and it exhibits a broader effective dosage range as compared to sesamin. Mechanistically, sesamolins longevity effect on the C. elegans relies on the signaling mechanisms of the SIR-2.1 and AAK-2. However, the usage of live or dead bacteria as a food source does not significantly affect the ability of the sesamolin and sesamin to extend the lifespan of the C. elegans. In summary, sesamolin shows potential for development as a functional ingredient for the longevity.
| Supplementary material | Top |
Figure S1. Effects of sesamolin and sesamin on the lifespan of wild-type C. elegans under dead bacterial or live bacterial culture.
Table S1. Effects of sesamolin on the lifespan of mutants.
Acknowledgments
This work was supported by grants from the National Science Foundation (MOST 109-2813-C-040-022-B), Taiwan. We thank distinguished professor Min-Hsiung Lee from the Department of Agricultural Chemistry, National Taiwan University for the gift of sesame lignan samples. The AAK-2, SIR-2.1, DAF-15 and AKT-1 mutants were provided by the Caenorhabditis Genetics Center (CGC), which is funded by the National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40OD010440). The C. elegans strain N2 was a gift from the C. elegans core facility of Taiwan (National Tsing Hua University, Taiwan).
Conflict of interest
The authors declare no conflict of interest.
Conceptualization, N.C.Y.; methodology, N.C.Y. and C.Y.C.; formal analysis, N.C.Y., C.Y.C. and P.J.L.; investigation, N.C.Y., C.Y.C. and P.J.L.; resources, N.C.Y.; writingoriginal draft preparation, N.C.Y., C.Y.C. and P.J.L.; writingreview and editing, N.C.Y.; supervision, N.C.Y.; funding acquisition, N.C.Y. All authors have read and agreed to the published version of the manuscript.
| References | Top |