| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 25, March 2024, pages 2-12
Bioactive peptides as antioxidants and antimicrobials: fundamentals and applications
Sarika Kumari, Fereidoon Shahidi*
Department of Biochemistry, Memorial University of Newfoundland, St. Johns, NL A1C 5S7, Canada
*Corresponding author: Fereidoon Shahidi, Department of Biochemistry, Memorial University of Newfoundland, St. Johns, NL A1C 5S7, Canada. E-mail: fshahidi@mun.ca
DOI: 10.31665/JFB.2024.18367
Received: March 14, 2024
Revised received & accepted: March 19, 2024
| Abstract | Top |
Bioactive peptides are well-known for their remarkable tissue affinity, specificity, and effectiveness in promoting health. Extracted from food proteins, these bioactive peptides have displayed significant potential as functional foods and nutraceuticals. Throughout the years, numerous potential bioactive peptides derived from food sources have been documented. These bioactive peptides offer a wide range of crucial functions within the human body, including acting as antioxidants, antimicrobial agents, anti-inflammatory compounds, anti-hypertensive substances, and immunomodulators. More recently, extensive research has been conducted to investigate the origins, bioavailability, potential physiological effects and functionality, as well as the mechanisms of action of bioactive peptides in rendering health benefits. Researchers have also delved into various technological methods for preparing, purifying, and characterizing these peptides. This contribution primarily centers on exploring the antioxidant and antimicrobial aspects of bioactive peptides.
Keywords: Bioactive peptides; Antioxidant activity; Antimicrobial activity and mechanism
| 1. Introduction | Top |
Bioactive peptides (BP) are composed of specific amino acid sequences that positively impact bodily functions or conditions that may influence health (Akbarian et al., 2022). BP commonly are made up of 220 amino acids, whereas proteins are polypeptides with higher molecular weights (MW) (Zaky et al., 2022). The sequence and composition of BP define their bioactivity characteristics. These peptides can be released from their precursor proteins by digestive enzymes during gastrointestinal digestion or by in vitro proteolytic processes with exogenous proteases (Shahidi and Zhong, 2015; Udenigwe and Aluko, 2012).
Bioactive peptides are isolated and generally produced from animal and plant proteins or other sources (Daliri et al., 2017, Awuchi et al., 2022). Animal proteins have traditionally been investigated for their high protein content and balanced essential amino acids (Qin et al., 2022). Milk and dairy products have been identified as potential sources of bioactive peptides (Punia et al., 2020). In addition, they can be produced from eggs and meat (Madhu et al., 2022). Various marine species, such as tuna, sardine, herring, and salmon, are an important source of BP (Mirzapour-Kouhdasht and Garcia-Vaquero, 2022). BP, derived from crustaceans, regulate various physiological processes such as cardiac function, pigmentation shifts, exoskeleton and internal muscle movements, metabolic activities, growth, transformation stages, and reproductive functions, some of these are also observed for those generated from other sources (Daliri et al., 2017).
Since plant sources have greater sustainability, low cost, and serve as an essential component in diet, the search for acquiring peptides from plant sources has increased (Rizzello et al., 2017). For example, Val-Tyr (VY), a multifunctional dipeptide, may be released from a protein source such as brewed sake or milk (Jakubczyk et al., 2020). The vegetable protein sources have also yielded favorable results using rice, soy, peanuts, peas, corn, algae, pseudo cereals, garlic, turmeric, spinach, and cocoa (Chalamaiah et al., 2019; Esfandi et al., 2019; Fernndez-Tom and Hernndez-Ledesma, 2019; Montesano et al., 2020; Phongthai et al., 2018; Rizzello et al., 2017; Shi et al., 2016; Wang et al., 2019).
Bioactive peptides generated from food possessing provide excellent potential for creating functional foods and/or nutraceuticals to prevent or treat certain chronic diseases. The production and characterization of bioactive peptides with antimicrobial, anti-inflammatory, antihypertensive, anti-obesity, and antioxidant attributes have been extensively published. These are classified into two types of endogenous and exogenous peptides (Abril et al., 2022; Zambrowicz et al., 2013). Endogenous peptides are produced in different cells, such as neural cells (analgesic/opioid application), immune cells (role in inflammation and antimicrobial), or various glands, such as the pituitary and adrenal glands (Froehlich, 1997). Exogenous peptides enter the body from various sources, such as food, dietary supplements, and medications (Akbarian et al., 2022; Chakrabarti et al., 2018; Geissler et al., 2010; Satake et al., 2002). BP have received much attention due to their application for enhancing health and reducing the risk of diseases by producing healthy foods, drugs, and other products (Akbarian et al., 2022).
| 2. Action mechanism of bioactive peptides | Top |
2.1. Food processing
Bioactive peptides derived from food proteins can be obtained either by enzymatic hydrolysis or by fermentation using starter cultures. Few studies have shown a combination of these two methods to produce peptides with enhanced biological activities. Additionally, bioactive peptides can be chemically synthesized due to their low presence in nature and the growing commercial interest in creating synthetic bioactive peptides. Here, we are primarily focusing on enzymatic hydrolysis and microbial fermentation.
2.2. Enzymatic hydrolysis
Enzymatic hydrolysis is a common method for procuring protein and hydrolysates/peptides from various food sources, especially by using digestive enzymes (Luna-Vital et al., 2015; Cruz-Casas et al., 2021). Enzymatic hydrolysis can be carried out in three different ways: i) using immobilized enzymes, ii) under traditional batch conditions, and iii) using ultrafiltration membranes.
The immobilized enzymes in a two-phase system, where one phase exclusively contains the enzyme and the other solely the product, as described by Rizzello et al. (2016), offer substantial benefits. The immobilization of enzymes is more economically advantageous and simplifies the separation of enzymes and products, thereby minimizing the risk of product contamination and enabling enzyme reuse, thus lowering the production cost (Michalak et al., 2017). The method conducted under traditional batch conditions is the least favored. This is due to the high enzyme costs, low yields and productivity, as well as the production of unwanted secondary metabolites resulting from enzymatic autolysis that contribute to its limited use (Cruz-Casas et al., 2021).
Ultrafiltration membranes have become the most known method for obtaining bioactive peptides. Ultrafiltration membranes, which hold back the enzyme and protein substrate while facilitating the separation of peptides through the application of hydraulic pressure, play a crucial role (Boukil et al., 2018). Throughout hydrolysis, proteins are continuously transformed into bioactive peptides, which are then released and separated from the reactor based on the membranes molecular weight cut-off. By selecting a membrane with the appropriate pore size, typically recommended to be 36 times smaller than the molecular mass of the enzyme, the molecular weight of the final product can be precisely managed, ensuring the enzymes retention (Ewert et al., 2022; Mora and Toldr, 2023).
The final product of enzymatic hydrolysis depends on the type of enzyme used, the type of protein precursor, the degree of hydrolysis, and the separation method of the product. Even though crude and purified peptides are used for different applications to reduce the final production cost, the use of crude peptides is often preferred (Alavi and Ciftci, 2023).
2.3. Microbial fermentation
Microbial fermentation is a biotechnological method used to derive bioactive peptides. This technique employs microorganisms that produce proteolytic enzymes, aiming for these enzymes to break down proteins into smaller peptide fragments (Jia et al., 2021). The microorganisms typically utilized include bacteria, fungi, or yeasts, which might naturally occur in the substrate or be introduced as a starter culture (Najafian et al., 2021; Chakrabarti et al., 2018, Maghraby et al., 2023).
The microbial fermentation process encompasses various systems, with submerged fermentation and solid-state fermentation being the most prevalent. Submerged fermentation involves cultivating microorganisms in a liquid, nutrient-rich medium. This method is particularly effective for microorganisms that thrive in high-moisture environments, such as bacteria. It has the benefit of facilitating the purification of bioactive peptides produced during the process (Tolpeznikaite et al., 2023). On the other hand, solid-state fermentation consists of microbial growth on nutrient-rich solid substrates. It has the advantage of releasing nutrients and is suitable for fungi and microorganisms with minor moisture requirements (Subramaniyam and Vimala, 2012). During the microbial fermentation process, it is essential to handle the appropriate substrate, suitable microorganisms, and optimal environmental conditions, such as pH, temperature, and humidity, to generate peptides with better bioactivity (Melini et al., 2019). Table 1 shows the few industrial bioactive peptides and their antioxidant activity.
 Click to view |
Table 1. Bioactive peptides with their antioxidant properties |
Within the bacterial group, lactic acid bacteria (LAB) are notably prominent and acclaimed as some of the most effective microorganisms for producing bioactive peptides. Their significance stems from their remarkable ability to adapt to various environments and substrates, both animal and plant-based (Cruz-Casas et al., 2021; Melini et al., 2019). Furthermore, LABs are distinguished by their potent proteolytic system, which is a key feature contributing to their efficiency in peptide production (Kieliszek et al., 2021) and is used to produce BP. Their role in producing fermented products is not only due to their physiological effect but also their technological importance in developing texture and taste (Akbarian et al., 2022).
| 3. Antioxidant activity | Top |
Antioxidant peptides typically the size of these peptides plays a crucial role in determining their effectiveness and behavior in the body, affecting both how they are transported to their target sites and how the gastrointestinal system processes them (Tadesse and Emire, 2020). These factors can enhance their antioxidant capacity within the body. The antioxidant activity of the two aromatic amino acids tryptophan (Trp) and phenylalanine (Phe) has been related to their capacity to act as radical scavengers, and the antioxidant activity of tyrosine is attributed to the special capability of phenolic group that acts as a hydrogen donor. Moreover, the presence of histidine (His) in peptides is directly associated with their ability to donate hydrogen and trap lipid peroxyl radicals, further enhancing their antioxidant effectiveness (Ajibola et al., 2011). Bioactive peptides with antioxidant properties are found in plant-based proteins from industrial food processing and by-products. These sources include soybean, wheat germ, hemp seeds, rice bran, sesame bran, wheat bran, and rapeseed, highlighting the potential of plant-derived substances to contribute to health benefits through their antioxidant activities (Flynn et al., 2008, Toldr et al., 2018).
Many proteins, protein hydrolysates, and specific amino acids have demonstrated significant antioxidant activity. For example, it has been shown that a mixture of tryptophan and lysine inhibits the oxidation of butters fat (Amarowicz and Shahidi, 1997). Some non-polar amino acids, such as proline and methionine, also render antioxidant activity in sardine and vegetable oils (Harnedy and FitzGerald, 2012). Taurine, hypotaurine, carnosine and anserine have been demonstrated to exert an antioxidant effect in vitro (Surai et al., 2021). Proteins from animal, plant and microbial origin, such as gluten, egg albumin, casein and yeast protein, have also been shown to render antioxidant activity (Czelej et al., 2022). In almost all cases, low-molecular-weight peptides exhibit higher antioxidant activity than intact proteins.
Protein digests have different antioxidant activities depending on the size of the peptides and their amino acid sequences as well as, the protein source and the hydrolysis condition (Shahidi and Zhong, 2015). Some bioactive peptides and their antioxidant activity, such as soy peptides obtained from soy protein using different enzymes (papain, pepsin, chymotrypsin, Alcalase, and Flavourzyme), have resulted in different degrees of hydrolysis that dictate the outcome (Kneevi-Jugovi et al., 2022). Whey protein hydrolysates are known for their radical scavenging activity and lipid oxidation inhibition (Kleekayai et al., 2020). A study by Mann et al. (2015) assessed the antioxidant activity of flavoured milk enriched with whey protein hydrolysates (WPH) treated with Flavouzyme, Alcalase, and Corolase PP. The WPH evaluated for their degree of hydrolysis and antioxidant activity, using the ABTS method, showed higher antioxidant activity (Flavouzyme, 0.81; Alcalase, 1.16; and Corolase, 1.42) compared to whey protein concentrate (WPC, 0.19). Corolase-treated WPH exhibited the highest antioxidant activity. LC-MS/MS identified 15 -lactoglobulin, 1 -lactalbumin, and 6 -casein peptides in the WPH, contributing to the antioxidant effect. Meanwhile, strawberry and chocolate-flavoured milk with 2% WPH showed up to 42% increased antioxidant activity, suggesting WPH is an effective natural antioxidant for inclusion in food products (Mann et al., 2015).
Plants are excellent sources of natural biopeptides, even though plant proteins are found in smaller quantities in crops eaten by plant-eating animals or in the leftover materials from agricultural and industrial processes (Csar et al., 2024). Nonetheless, due to their benefits, such as high production rates, cost-effectiveness, short harvesting times, lack of conflict with regional religious or cultural practices, and remarkable biological effectiveness, plant-based proteins are increasingly being explored as ideal starting materials for the production of bioactive peptides (Hossain et al., 2022; Senadheera et al., 2023; Yeo and Shahidi, 2020). Many plants and their waste products, including legumes, grains, vegetables, fruits, seeds, husks, and leaves, have served as sources for discovering antioxidant peptides. Thus, oilseeds such as rapeseed/ canola and soybean as well as cereals, and legumes stand out globally for their protein content, making them primary sources of phytochemical- containing proteins (Lizrraga-Velzquez et al., 2020). This situation presents more chances for access, cost-efficiency, and a variety of bioactive peptides, such as those with antioxidant properties, further evidenced by the extensive comparison between animal peptides and their protein sources. However, research has particularly highlighted that proteins from cereals and legumes are richest in peptide fragments known for their antioxidant capabilities (Zhu et al., 2022). Notably, antioxidant peptides have been found in major grains like oats (Du et al., 2016), wheat (Heo et al., 2022), rye (Leung et al., 2018), buckwheat (Luo et al., 2020), corn (Jin et al., 2016), and millet (Agrawal et al., 2016).
Antioxidative peptides from marine species and the by-products from the aquaculture industry have also been extensively studied (Awuchi et al., 2022; Jimeno et al., 2004; Noyer et al., 2011; Ngo et al., 2011). Furthermore, Amarowicz and Shahidi (1997) showed synergistic effects of capelin protein hydrolysates with synthetic antioxidants butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ) were observed (Amarowicz and Shahidi, 1997, 1999). Table 2 shows the food derived bioactive peptides with their antioxidant activity.
 Click to view |
Table 2. Bioactive peptides and their antioxidant activity |
Several in vivo studies have examined the biological effects of antioxidant peptides derived from various protein sources. A notable example is the research conducted by Chou et al. (2014), which investigated the antioxidant effects of peptides obtained from chicken liver through pepsin-assisted hydrolysis. This study focused on measuring the reduction of malondialdehyde (MDA) type compound accumulation, a known marker of secondary lipid oxidation, and the stimulation of antioxidant enzymes CAT, GPx, and SOD in mice subjected to chronic d-galactose intake, a condition known to increase the production of reactive species. The administration of chicken liver hydrolysate to mice at doses of 0.05 and 0.25 g/kg resulted in antioxidant status in their brain, heart, liver, and kidney of the mice that were equivalent to or better than those of the control or d-galactose-treated animals (Lorenzo et al., 2018).
| 4. Action mechanism of antioxidant peptides | Top |
Several studies have shown that the antioxidant activity of BP is due to their action as 1) radical scavenger inhibitors, 2) chelators of metal ions or 3) physical shielding (Rajapakse et al., 2005; Wu et al., 2003).
Antioxidant peptide molecules neutralize free radicals through two primary mechanisms: hydrogen atom transfer (HAT) and single electron transfer (SET). These processes enable the quenching of reactive oxygen and nitrogen species. Evaluation methods like the oxygen radical absorbance capacity (ORAC) and total radical-trapping antioxidant parameter (TRAP) assays utilize HAT principles to assess the capability of antioxidants to scavenge free radicals by donating a hydrogen atom (Zhu et al., 2022). The effectiveness of the HAT reaction depends on the bond dissociation energy and the ionization potential (IP) of the antioxidants hydrogen-donating group. On the other hand, SET-based assessments, such as the DPPH radical scavenging capacity, ferric reducing antioxidant power (FRAP), and ABTS, measure an antioxidants ability to reduce radicals, metals or carbonyls by electron donation (Prior et al., 2005; Lorenzo et al., 2018). This capacity is affected by the deprotonation of reactive groups and their IP. Nonetheless, it is recognized that both HAT and SET mechanisms often occur simultaneously in most instances, with the predominant mechanism influenced by the antioxidants structure, which affects its solubility and partition coefficient in the system (Akbarian et al., 2022; Awuchi et al., 2022; Sharma et al., 2011).
As chelators of metal ions, BP inhibit the production of free radicals by removing metallic prooxidants metal ions (Wang et al., 2014) The peptides carboxyl and amino groups participate in the chelating function (Lpez-garca et al., 2022). Studies have shown that chelating peptides are rich in histidine (Holeek, 2020). The copper-chelating peptides are abundant in histidine and inhibit coppers oxidative activity. The imidazole ring of the histidine primarily facilitates the binding of copper. Copper chelating peptides can prevent copper-assisted oxidation activity that can damage the luminal of stomach cells and oxidation of low-density lipoprotein (LDL) if it reaches the bloodstream (Timoshnikov et al., 2022).
In the physical shielding mechanism, peptides inhibit lipid peroxidation by acting as a barrier or membrane. They can work as a surfactant component and split at the oil-water interface, forming a thick membrane coating to avoid the direct interaction of lipids with radicals and other oxidizing agents (Nikoo and Benjakul, 2015).
| 5. Antimicrobial activity | Top |
Antimicrobial peptides (AMP) are a diverse group of molecules with thousands of AMP sequences identified so far (Huan et al., 2020). These are mostly short-chain peptides with 1055 amino acids, with overall positive charge and structures containing both hydrophobic and hydrophilic regions (Li et al., 2021). AMP are categorized differently by structure, amino acid sequence, or biological function (Mishra et al., 2018).
The first AMP structures that were identified had an -helical configuration, and these have been extensively studied. An example of such an -helical AMP is magainins, derived from the African clawed frog Xenopus laevis, which is effective against many pathogens, including bacteria, fungi, yeast, and viruses (Brogden, 2005). However, magainin failed in clinical trials due to being less effective than the standard antimicrobial treatment (Dijksteel et al., 2021). In current clinical trials as a topical antimicrobial treatment for foot ulcers in diabetic patients, an analog of magainin 2 with an increased content of positively charged amino acids, which is the C-terminally modified MSI-78, referred to as a pexiganan peptide, is being used (Ting et al., 2020).
The second category of antimicrobial peptides have a secondary structure characterized by strands. Upon interacting with a lipid membrane, these AMPs adopt a sheet structure. However, the sheet peptides flexibility is limited by disulfide bonds between the -strands, imposing structural constraints (Pirtskhalava et al., 2021; Zasloff, 2002).
The third class of antimicrobial peptides do not adopt either -helix or -sheet secondary structures. One example is the cathelicidin family, which contains a significant amount of proline, an amino acid known for disrupting both -helical and -sheet structures (Xhindoli et al., 2016).
| 6. Action mechanism of antimicrobial peptides | Top |
The primary target of antimicrobial peptides is membrane permeabilization, and there are various models (see Figure 1) that describe how AMP primarily disrupt the cytoplasmic membrane. The action mechanisms of AMPs can be categorized into groups based on whether they target the cell membrane or non-membrane cellular components (Wimley, 2010; Espeche et al., 2024). In the case of AMP that specifically act on membranes, it is established that electrostatic interactions play a significant role, primarily facilitating the attraction between cationic AMP and negatively charged bacterial membrane components like lipopolysaccharide, peptidoglycan, and teichoic acid (Espeche et al., 2024). AMP that do not target the membrane act on intracellular targets (Matsuzaki, 2019).
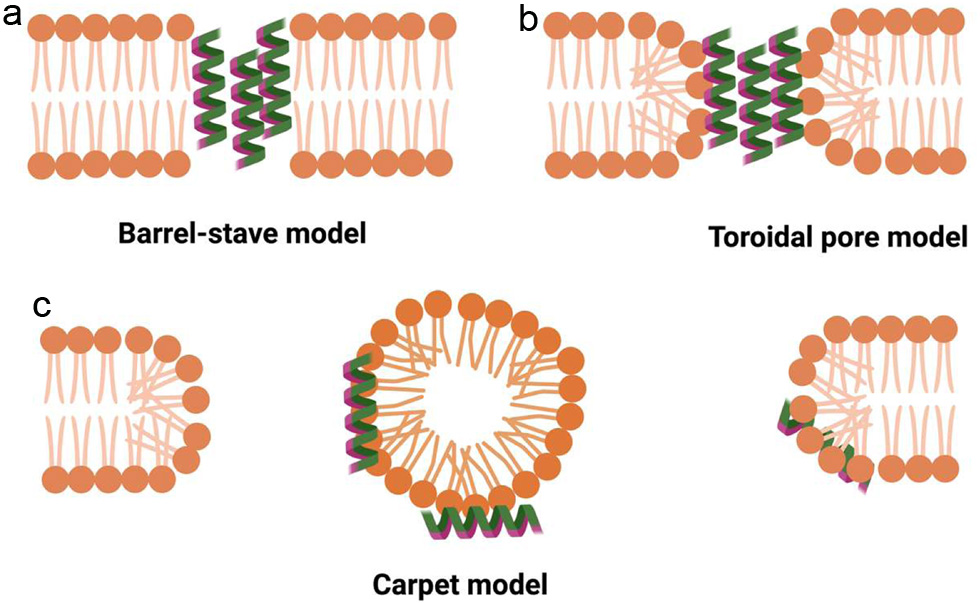 Click for large image |
Figure 1. Models representing different mechanisms of action of antimicrobial peptides (AMP) in Barrel-stave model (a); Toroidal pore model (b); and Carpet model (c). |
The cellular membranes of both Gram-positive and Gram-negative bacteria contain high levels of phospholipids, phosphatidylglycerol, and cardiolipin. These components possess negatively charged principal groups that effectively draw in positively charged antimicrobial peptides. Among AMP, the cationic amphipathic -helix represents one of the most prevalent forms. There are multiple theories detailing the mechanisms by which amphipathic -helix peptides function. In the barrel-stave model (Figure 1a), AMP organizes into a barrel-like ring around an aqueous pore (Chen et al., 2019; Epand et al., 2016). Initially, the AMP are oriented parallel to the membrane; however, they ultimately become inserted perpendicularly into the lipid bilayer, as observed in studies by Epand et al. (2016). Only a few numbers of AMP have been demonstrated to adopt barrel-stave models, with alamethicin being one example (Laver, 1994), pardaxin (Rapaport and Shai, 1991), and protegrin (Brogden, 2005) as examples.
Another model, known as the toroidal pore model (Figure 1b), provides valuable insights into peptide-membrane interactions. In this model, AMP bind to the polar head groups of lipids, causing a separation of the headgroups and introducing a positive curvature strain (Kabelka and Vcha, 2021; Matsuzaki, 2019). AMP like Aurein 2.2 (Cheng et al., 2009), melittin (Raghuraman and Chattopadhyay, 2007), and several other AMP have been shown to form toroidal pores.
The third model, referred to as the carpet model (Figure 1c), is based on the concept that AMP can exert their action without forming specific pores in the membrane (Steiner et al., 1981; Shai, 2004). In this model, AMP accumulate parallel to the lipid bilayer, reaching a maximum concentration where they envelop the membrane surface, essentially creating a carpet. This transformation results in unfavourable interactions on the membranes surface, causing alterations in phospholipid interactions that increase membrane fluidity and ultimately lead to membrane disruption and loss of membrane integrity (Nakajima, 2003).
Several AMP have been shown to interact with bacteria without disrupting the membrane to cause substantial permeabilization. Such peptides usually cross the membrane and reach one or more intracellular targets (Kumari and Booth, 2022; Huang, 2000; Matsuzaki, 1998; Shai, 2004). These AMP first interact with the cytoplasmic membrane and then accumulate intracellularly to block cellular processes such as DNA and RNA replication and protein synthesis; this disruption could effectively kill bacteria (Singh et al., 2022).
Lactic acid bacteria (LAB) in fermented foods possess bioactive peptides with antimicrobial activity and many food component peptides and are good candidates as food additives. The rising incidence of infections caused by human pathogens like Mycobacterium tuberculosis and Staphylococcus aureus have demonstrated the consequences of growing resistance to traditional antibiotics (Prestinaci et al., 2015). In some cases, the pathogen could not be killed by any antibiotics. As a potential alternative to conventional antibiotics, antimicrobial peptides are good candidates to overcome this issue.
Antimicrobial peptides can be toxic to humans, and many efforts have been made to make them less toxic while improving their potency to eliminate bacteria. Some strategies, such as chemical strategies, enhance peptide specificity and stability. To improve the stability of AMP, researchers have tried to modify and optimize the cyclization of peptides linking C and N termini to improve serum stability and microbicidal activity (Etayash et al., 2020).
Another approach to improve the stability of AMP is to replace natural amino acids with non-natural amino acids or D-amino acids to protect AMP from proteolytic enzyme degradation since the host protease can identify and hydrolyze the natural L-amino acids (Lu et al., 2020). Zhao et al. (2016), isolated a lysine-rich AMP from the venom of social wasp MPI, which exhibited activity against Gram-positive and Gram-negative bacteria.
The researchers have developed two peptides: one composed entirely of D-amino acids, known as D-MPI, and the second peptide sequence in which only the lysine residues were replaced with D-amino acids, referred to as D-lys-MPI to test the proteolytic activity of trypsin. This is because trypsin cleaves positively charged amino acids like lysine. Results showed that both peptides, D-MPI and D-lys-MPI, were resistant to trypsin digestion. Interestingly, only D-MPI was equal in terms of activity compared to MPI. Meanwhile, D-Lys-MPI was inert because introducing of a single D-amino acids destabilized the secondary structure (Zhao et al., 2016).
Improving the function of AMPs may be achieved by conjugating peptides to other active molecules, such as incorporating peptides into non-biological molecules such as polyethylene glycol (PEG) or biological molecules like sugar, lipids and proteins, hence taking advantage of both types of biological molecules to be combined and overcome their limitations (Chen and Lu, 2020; Erak et al., 2018; Wijesinghe et al., 2022; Zaman et al., 2019)
| 7. Limitations and challenges of the therapeutic use of bioactive peptides | Top |
In recent years, many bioactive peptides have been made to serve as antioxidants and antimicrobial peptides with limited success (Chalamaiah et al., 2019; Jakubczyk et al., 2020; Pei et al., 2022; Ulug et al., 2021). There are many reasons for this failure, but the main issue involves poor oral bioavailability and a short half-life in bloodstream stability observed for some bioactive peptides (Tan et al., 2018).
Bioactive or antimicrobial peptides, like dietary peptides, are susceptible to digestive enzymes in the gastrointestinal tract. Furthermore, even if peptides manage to pass through the stomach, their size often restricts their ability to permeate the intestines into the systemic circulation (Tan et al., 2018; Zhu et al., 2022, Fatoki et al., 2022).
The efficiency of the bioactive peptide depends on its ability to reach the organs where it would perform its function. Therefore, it is important to consider the in vivo differences between bioactive peptides. Peptides that are not broken down during gastrointestinal digestion, or their resulting fragments, must also navigate through additional breakdown by peptidases located at the brush border and/or on the cell membranes of the intestinal lining (Abeer et al., 2021). Before these peptides can enter the bloodstream through the cells of the intestinal wall, they face several barriers. There are four primary pathways for peptides to cross the intestinal barrier. These include the active transport via H+-coupled peptide transporters PepT1 and PepT2, sodium-coupled oligopeptide transport mechanisms SOPT1 and SOPT2, passive movement through the tight junctions between cells, and trans-cellular movement via endocytosis, which is influenced by the molecular size and characteristics like the hydrophobicity of the peptides (Zhang et al., 2021).
For hydrolysates or peptides to effectively perform their biological functions, it is crucial to assess their digestibility and the subsequent liberation of bioactive peptides using appropriate in vitro intestinal models and in vivo studies within the gastrointestinal (GI) tract. Employing in vitro techniques, such as the use of human intestinal Caco-2 epithelial cell monolayers, alongside in vivo models that measure permeability, helps in predicting the oral bioavailability of these compounds (Mukker et al., 2014; Kellett et al., 2018). The ability of the intestinal barrier to selectively allow these candidate peptides through depends on a comprehensive understanding of the peptides structural and chemical characteristics, their interactions within the GI tract, and a solid knowledge base regarding GI tract physiology (Vij et al., 2016). A few important properties of peptides, such as structural effects, hydrophobicity, size, and surface charge, affect the transport pathway.
As previously discussed, it is well-established that hydrolysates containing numerous short-chain peptides, particularly dipeptides and tripeptides, enhance absorption and are more effective than free amino acids or larger precursor peptides (Vij et al., 2016). When the molecular weight of a molecule exceeds 500 daltons (Da), its oral bioavailability declines. For instance, bioavailability was observed at 16.23% for casein-derived peptide fractions under 500 Da, whereas it dropped to 9.54% for those with a molecular weight between 500 and 1,000 Da (Singh et al., 2022). Additionally, the length of the peptide chain hints at the involvement of a transdermal transporter. Specifically, dipeptides and tripeptides are known as substrates for the PepT1 transporter, facilitating their transport across the cell membrane of enterocytes, utilizing an electrochemical protein gradient (Abeer et al., 2021). This transporter is located on the apical membrane of these intestinal cells. However, it was noticed that as the molecular weight of peptides increases, their ability to pass through the intestinal passage decreases (Wang and Li, 2017). Therefore, due to the incomplete bioavailability of a peptide after oral ingestion, a peptide with significant bioactivity observed in vitro may not necessarily translate to significant activity in vivo (Xue et al., 2021).
The process of gastrointestinal (GI) digestion has been studied merely for its capacity to transform food into nutrients, the energy source for our body. Only recently has the GI tract been considered a dynamic interface between the luminal environment and the internal environment. Interaction between nutrients and the intestinal barrier elicits the activation of multiple signalling pathways, including some involved in energy homeostasis regulation (Caron et al., 2017). With the exponential increase in the number of people affected by metabolic syndrome, alimentary proteins have become the subject of increasing interest since they reduce food intake, induce satiety by diseases, and increase energy expenditure. Yet, the underlying mechanisms are still not completely elucidated. The in vitro study of some mechanisms, notably the production and secretion of the GI hormones, highlighted the primary role of bioactive peptides originating from protein GI digestion (Snchez-Velzquez et al., 2021).
Nonetheless, alternative delivery methods can enhance the likelihood of peptide absorption and decrease the problem (Erdmann et al., 2008). Vilcacundo et al. (2017) purified concentrate of quinoa protein that was digested under in vitro gastrointestinal conditions. Pepsin completely hydrolyzed quinoa proteins at pH 1.2, 2.0 and 3.2. At high pH, only partial hydrolysis was observed. No intact proteins were seen during the duodenal phase, indicating their susceptibility to simulate digestive conditions in vitro (Vilcacundo et al., 2017; Aluko and Monu, 2003).
| 8. Other bioactivities: antihypertensive, anti-inflammatory, and opioid effects | Top |
Bioactive peptides can modulate the renin-angiotensin system (RAS) because they decrease the activities of renin and angiotensin-converting enzyme (ACE). These two main enzymes regulate mammalian blood pressure (Balgir, 2016). Antihypertensive peptides, known as ACE inhibitors, have been extracted from fish protein, milk and corn (Xue et al., 2021). Although milk proteins are the main antihypertensive peptides, other dietary sources, including soy and cereals, have recently been studied as antihypertensive peptides (Xue et al., 2021).
Various antihypertensive constituents from food sources, such as soybean, fish, milk, cereal, egg, vegetables, and fruits, have been characterized. They provide cardiovascular health by inhibiting ACE inhibitory, reducing free radical formation, inducing vasorelaxation, and lowering blood pressure and lipid levels (Huang et al., 2013; Wu and Ding, 2002). Among cereals, wheat gliadin hydrolysates can act as ACE inhibitors. The peptide IAP (Ile-Ala-Pro) prepared with acid protease significantly decreased blood pressure in spontaneously hypertensive rats (SHRs) with intra-peritoneal administration (Matsumura et al., 1993). Arginine-rich peptides from flaxseed protein isolate (FPI) obtained by enzymatic hydrolysis with trypsin and Pronase were observed to produce in vivo vasodilatory effects (Udenigwe et al., 2012). Cheonggukjang, an antihypertensive peptide, has been identified and characterized in a Korean soy product and obtained by fermentation with Bacillus subtilis CH-1023 (Korhonen and Pihlanto, 2003; Kim et al., 2021).
It is also known that antihypertensive drugs cause negative side effects in patients and could reduce compliance with prescribed drug treatment for example, using Aliskiren, a recently approved renin-inhibitory antihypertensive drug, has been associated with gastrointestinal disorders (Iijima et al., 2022). Therefore, increased interest has been shown in developing natural compounds that are less likely to induce negative side effects (Aluko, 2015; Udenigwe and Aluko, 2012).
Inflammation is a biological response by which the immune system defends the body from harmful pathogens, cell injury, toxic substances, and irritation (Abril et al., 2022; Snchez-Velzquez et al., 2021). Peptides from different food sources have been reported to have a neutralizing effect on the inflammatory process. Bioactive peptides extracted from fish and shellfish proteins have shown relevant anti-inflammatory effects. Gao et al. (2020) identified peptide sequences with potential anti-inflammatory activity derived from sturgeon muscle protein in the lipopolysaccharide (LPS)-induced RAW264.7 cell inflammatory model. LC-MS/MS identified fourteen novel peptides by mass spectrometry, and from that three peptides were synthesized (KIWHHTF, VHYAGTVDY, and HLDDALRGQE) for further studies. These synthetic peptides decreased the release of inflammatory mediators and inflammatory cytokines (NO, IL-6, and IL-1), while significantly increasing superoxide dismutase (SOD) activity in the cell model. Salmon by-products have been reported to be important sources with anti-inflammatory properties. An enzymatic hydrolysate of salmon (Salmo salar) skin exhibited anti-inflammatory activity (Liu and Bo, 2022). Milk is another food source of bioactive peptides. Previous studies isolated the milk casein-derived peptide QEPVL (Gln-Glu-Pro-Val-Leu) from fermented milk. The results showed that QEPVL significantly activated lymphocytes in vitro and in vivo (Jiehui et al., 2014).
Opioid peptides derived from food proteins have affinities to bind to opiate receptors and express opiate activity, which in turn can be reversed by an opioid antagonist, such as naloxone (Froehlich, 1997; Kitts and Weiler, 2003). Naloxone crosses the blood-brain barrier and blocks opioid activity, thus being a helpful tool for determining the specific effects of agonist opioid peptides (Tyagi et al., 2020; Liu and Udenigwe, 2019). Food-derived opioid peptides resist further hydrolysis by intestinal brush border enzymes and directly affect specific gastrointestinal target receptors (Kostyra et al., 2004). Peptides with opioid activity have been generated by in vitro digestion of casein and separated using milk protein hydrolysates by solvent extraction and chromatography technique. Depending on the source of the - or -casein, they are classified as - or -casomorphins (Kostyra et al., 2004; Aslam et al., 2020). Bioactive peptides that have opioid activity have also been separated from plant protein hydrolysate, such as wheat gluten is a well-known source of opioid peptides among food proteins (Zaky et al., 2022).
| 9. Conclusions | Top |
The isolation and purification of bioactive peptides are very important for exploring their physicochemical properties and evaluating their in vitro and in vivo bioactivities. Bioactive peptides can be separated from a protein hydrolysate mixture by several approaches, mainly different chromatography and membrane-based separation techniques. For example, Enzymatic hydrolysis is a common method for procuring proteins and hydrolysates/peptides from various food sources, especially by using digestive enzymes. The microbial fermentation role in fermented products is not only due to their physiological effect but also their technological importance in developing texture and taste.
Bioactive peptides hold promise as potential food ingredients and pharmaceutical ingredients aimed at addressing or preventing certain medical conditions and lifestyle-related diseases like obesity, type II diabetes, and hypertension. Regardless of the significant progress in the isolation and purification of bioactive peptides from different natural sources and the characterization of their bioactivities, there are still several obstacles to overcome, particularly from the production prospect of large scale production without losing activity. Thus, this review article has made an attempt to shed light on a few factors that may contribute to the translational gap in bioactive peptide antioxidant and antimicrobial activities. While it is certain that bioactive peptides will hold an important place as food additives, in the fields of pharmaceuticals and healthcare, we are optimistic that future translational research will accelerate the integration of these advancements, incorporating these bioactive peptide therapeutics into general use as health promoting food ingredients.
Acknowledgments
We are grateful to the Natural Science and Engineering Research Council (NSERC) of Canada for financial support in the form of a Discovery Grant to FS (RGPIN-2016-04468).
| References | Top |