| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 23, September 2023, pages 79-87
Antihyperglycemic and hypolipidemic properties of Acaudina leucoprocta peptides in type II diabetic mice
Yang Qina, #, Nannan Wanga, #, Jiaojiao Hana, b, *, Chenyang Lua, b, Jun Zhoua, b, Jiajie Xua, b, Xiurong Sua, b
aSchool of Marine Science, Ningbo University, Ningbo, Zhejiang, China
bState Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, Ningbo University, Ningbo, Zhejiang, China
#These authors contributed equally to this work
*Corresponding author: Jiaojiao Han, School of Marine Science, Ningbo University, 169 Qixing South Road, Ningbo, China. Tel/Fax: +86-574-87608368; E-mail: hanjiaojiao@nbu.edu.cn
DOI: 10.31665/JFB.2023.18357
Received: August 2, 2023
Revised received & accepted: September 26, 2023
| Abstract | ▴Top |
This study aimed to investigate the protective mechanism of Acaudina leucoprocta peptides (ALPs) in the kidney of type II diabetes mice (db/db mice). Serum lipid and glucose indexes were detected in diabetes mice after ALPs treatment. The two-dimensional gel electrophoresis was used to study the kidney protein of diabetic mice. The differential protein screening, GO function annotation, and metabolic pathways were used to determine the protective mechanism of ALPs in the kidney of diabetic mice. The symptoms of db/db mice were alleviated after 10 weeks of treatment with ALPs. The content of TC, TG, and LDL-C in the ALPs group was significantly decreased and the level of HDL-C was increased. After ALPs treatment, the urine glucose and fasting blood glucose of diabetes mice were significantly reduced. The expression of Haptoglobin was up-regulated, it plays a role in anti-inflammatory and immune regulation. And the expression of alpha-2-HS-glycoproteins was down-regulated, then the insulin signal pathway was restored to normal condition, to improve the symptoms of diabetes. This study provided a new strategy that will help treat diabetes.
Keywords: Diabetic mice; Acaudina leucoprocta peptides; Differential protein; Insulin signal pathway
| 1. Introduction | ▴Top |
Diabetes mellitus, one of the most serious diseases, has a significant impact on the health and life expectancy of patients as well as on the healthcare system in the modern world (Schmidt, 2018). It can be broadly categorized into type I and type II. The former develops as a result of insulin deficiency. However, the latter is due to insulin resistance. Type II diabetes mellitus (T2DM) is a heterogeneous, multifactorial disorder characterized by hyperglycemia and gradual decline in insulin action (insulin resistance), and followed by the inability of beta (β)-cells to compensate for insulin resistance (pancreatic β-cell dysfunction) (Nath et al., 2017). According to the latest survey, T2DM accounts for over 95% of all diabetes cases. This alarming scenario has fostered research into new anti-diabetic medication. Diabetic nephropathy (DN) is one of the common chronic complications of diabetes, with 20–40% of diabetic patients with nephropathy (Samsu, 2021). Hemodynamic abnormalities in the kidneys result in glomerular hyperfiltration, which can be detected at the onset of diabetes and may be a determinant of the development of DN. However, there are many problems with clinical drugs such as side effects and long-term safety (Hussein et al., 2004). Therefore, it is urgent to find new active substances with fewer side effects to relieve diabetic nephropathy from dietary supplements. It will help to prevent or delay the occurrence and development of diabetes-related health effects.
Acaudina leucoprocta (A.leucoprocta) is a low-value sea cucumber, that belongs to the order Molpadiida and is widely distributed across the east sea of China (He et al., 2020). It is commonly known as ginseng, also known as white ginseng or sea eggplant. A.leucoprocta is rich in protein, polysaccharides, mineral elements, and trace elements, which be used as a good source for the preparation of peptides. Extensive research over the last 20 years has shown sea cucumber peptides have multiple biological activities, including antioxidant, anticancer, antimicrobial, and immunomodulatory properties (Bordbar et al., 2011). Previous studies showed that sea cucumber peptides could alleviate oxidative stress in neuroblastoma cells and improve survival in Caenorhabditis elegans exposed to neurotoxic paraquat (Lu et al., 2021). Sea cucumber peptides improved the mitochondrial capacity of mice and enhanced gluconeogenesis and fat catabolism during exercise for improved antifatigue properties (Yu et al., 2020). In addition, peptides from food sources have been discovered to impart their antidiabetic potentials through a decrease in blood glucose levels and an increase in insulin uptake. Milk peptides can significantly reduce the concentration of blood glucose, total lipids, triglyceride, and cholesterol in diabetes rats, and increase the concentration of globulin and high-density lipoprotein, to alleviate diabetes (El-Sayed and Awad, 2016). Peptides from salmon by-products such as skin and trimmings have improved insulin and lowered blood glucose in pancreatic BRIN-BD11 and GLUTag cells (Harnedy et al., 2018; Harnedy et al., 2018).
In this study, the optimized enzymatic hydrolysis conditions were used to hydrolyze A.leucoprocta. Then, a db/db mice model was used to study the regulatory effects of A.leucoprocta peptides (ALPs) on dyslipidemia and pathoglycemia in diabetic nephropathy. The biochemical indexes of blood glucose and blood lipid levels were measured, the find kidney protein expression was profiled using two-dimensional electrophoresis, and the differentially expressed protein, then the mechanism of the antidiabetic effect was clarified. We believe this study will provide a basis for the high-value development of ALPs and contribute to the development of new strategies for improving diabetes treatments.
| 2. Material and method | ▴Top |
2.1. Peptides identification
Hydrolysis of A.leucoprocta (purchased from Hepu Co., Ltd., Xiangshan, China) was performed under optimal conditions as described previously (Han et al., 2018). After 10 min inactivation with boiling water, it was cooled to room temperature. Then, ALPs were separated by ultrafiltration, and obtained by vacuum freeze-drying (Free Zone 2.5L, LABCONCO Co., Ltd, Kansas City, USA) for 48 h.
2.2. Ethics statement
All of the experimental procedures and animal care were performed by the Guide for the Care and Use of Laboratory Animals prepared by the Ningbo University Laboratory Animal Center (affiliated with the Zhejiang Laboratory Animal Common Service Platform), and the whole animal experiment was approved by the Ningbo University Laboratory Animal Center under permit number No. SYXK (ZHE 2008-0110).
2.3. Animal experiment design
After one week of acclimatization, ten 8-week-old male Db/m mice (20.34 ± 2.58 g) were used as the control group (control). Thirty 8-week-old male db/db mice with leptin knockout (20.46 ± 2.26 g, Shanghai Slack experimental animal limited liability company, SCXK 2007-0005, Shanghai, China) were randomly divided into model group, positive drug group (Metformin, 250 mg·kg−1·d−1) and experimental group (ALPs, 250 mg·kg−1·d−1). The control group and model group were gavaged with equal amounts of deionized water. During the experiment, all animals were given free access to water and food, and the room temperature was maintained at 23–25 °C, night and day intervals were set to 12 h. The entire experiment lasted for 10 weeks.
2.4. Effect of peptides on blood glucose and blood lipid
At the end of weeks 2, 4, 6, 8, and 10, urine samples were collected from each mouse using a metabolic cage after a 12 h fast. Urine samples were centrifuged at 9,000 g for 5 min. Then the concentration of urine glucose was measured using a urine glucose assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) according to the manufacturer’s instructions. At the same time, the blood sample was acquired from the inner canthus veins of the mice, and the serum was obtained by centrifugation at 3,500 g for 15 min. The fasting blood glucose concentration was measured by using a serum glucose assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). The levels of TC, TG, LDL-C, and HDL-C in serum were analyzed by commercial kits (Suzhou Comin Biotechnology Co., Ltd, Suzhou, Jiangsu, China). At the end of the experiment, the intraperitoneal glucose tolerance tests (IPGTT) were carried out after fasting for 12 h. Then the mice were injected intraperitoneally with glucose (0.5 g/kg). The blood glucose levels were measured at 0, 0.5, 1, and 2 h after glucose administration. Blood samples and tissues were collected and stored at −80 °C for analyses.
2.5. Protein extraction and purification
The collected 1.0 g of the kidney tissue of the mice was chopped and cut into pieces in a pre-cooled mortar. Adding the liquid nitrogen to make them powder. Then they were suspended in 10 mL lysis buffer (8 mol/L urea, 2 mol/L thiourea, 40 mg/mL CHAPS, 10 mg/mL DTT, 2.5 mg/mL Tris). The powder was dissolved by adding the protease inhibitor (PMSF) and transferred into the EP tube and the proteins were extracted by ultrasonic disruption at 600 W for 10 min on ice. The lysates were then centrifuged at 12,000 g for 20 min at 4 °C. The supernatants were collected and combined with a 5:1 (v/v) ratio of acetone (containing 10% trichloroacetic acid) and incubated for 8 h at −20°C for protein precipitation. Then the samples were centrifuged at 12,000 g at 4 °C for 20 min. The supernatant was discarded and the protein was quantified by a non-interfering protein concentration kit (Sangon Biotech Co., Ltd, Shanghai, China) according to the manufacturer’s instructions.
2.6. Two-dimensional electrophoresis
According to a previous study, the total amount of protein between 200 μg and 250 μg was selected (Zhang et al., 2015). Add buffer (7 M urea, 3 M thiourea, 4% w/v CHAPS, 64 mM DTT, 0.5% v/v Bio-lyte) to make the total volume to 150 μL. First-dimension isoelectric focusing was carried out with rehydrating Immobiline DryStrips (7 cm, pH 4–7) with 150 μL protein solution overnight in a PROTEANIEF cell (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 20 °C with a maximum current setting of 50 mA/strip. The next was five steps of electrophoresis: step 1: 250 V for 1 h, linear; step 2: 500 V for 1 h, linear; step 3: 1,000 V for 1 h, linear; step 4: 4,000 V for 3 h, rapid; step 5: 4,000 V for 20,000 V/h, rapid.
Second dimension SDS-PAGE was performed by mounting the immobilized pH gradient (IPG) strips were equilibrated in 2.5 mL equilibration buffer (0.05 mol/L Tris-HCl (pH 8.8), 6 mol/L urea, 0.3 mL/mL glycerol,20 mg/mL SDS) containing 1% DTT for 15 min, then in equilibration buffer containing 2.5% iodoacetamide performed a second 15-min equilibration step. The equilibrated strips were loaded onto 12% SDS-polyacrylamide gels, then SDS-PAGE carried on using a PowerPac basic electrophoresis system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 80 V and 15 °C, with each sample repeated 3 times. After completion, the gels were rinsed in double distilled water (ddH2O) twice, and then the gels were incubated in Coomassie Brilliant Blue for 1 h at room temperature. Again rinsed twice in ddH2O and incubated in ddH2O for several hours on the shaker for the final rinse.
2.7. Image acquisition, protein digestion, and mass spectrometric analysis
The spot volume ratios that showed a statistically significant difference (with abundance variation greater than 1.5-fold and P<0.05) were processed for further analysis. According to previous studies, selected protein spots were excised from the preparative gels, and each gel was rehydrated with 20 μL of 0.01 mg/mL trypsin (Promega, Madison, WI, USA) (Li et al., 2023; Zhang et al., 2016). The peptides obtained after enzymatic digestion are freeze-dried. Afterward, the peptides were detected by Autoflex speed™ MALDI-TOF-MS/MS analyzer (Bruker Daltonics, Germany). The detection conditions were as follows: UV wavelength, 355 nm; recurrence rate, 200 Hz; accelerating voltage, 20,000 V; optimal mass resolution, 1,500 Da; mass of scanning range, 700–3,200 Da. The mass spectrometry was obtained using the default mode of the instrument, and the trypsin autocleavage peak was used as the internal standard to calibrate the mass spectrometer. The baseline peak was filtered and the signal peak was identified using flexAnalysis (Bruker Dalton) software. The MS data were analyzed with the NCBI protein sequence database using BioTools (Bruker Daltonics, Germany) via the Mascot search engine.
2.8. Data analysis
All the data were recorded as mean ± standard deviation (M±SD) in each group. Statistical calculations by SPSS version 19.0 software (SPSS Inc., Chicago, USA) were carried out. One-way ANOVA was applied to determine differences between the results of samples. P<0.05 was considered a standard criterion of statistical significance.
| 3. Results | ▴Top |
3.1. ALPs significantly regulate blood lipid homeostasis in diabetes mice
Compared to the control group, serum TC (4.71 ± 0.25 mmol/L, P<0.05), TG (4.49 ± 0.23 mmol/L, P<0.05), and LDL-C (3.69 ± 0.54 mmol/L, P<0.05) levels increased significantly and serum HDL-C (1.11 ± 0.09 mmol/L, P<0.05) level decreased in model group. Compared with the model group, serum TC(3.24 ± 0.52 mmol/L, P<0.05; 3.39 ± 0.44 mmol/L, P<0.05), TG (3.03 ± 0.61 mmol/L, P<0.05; 3.14 ± 0.18 mmol/L, P<0.05) and LDL-C levels (2.01 ± 0.31 mmol/L, P<0.05; 2.63 ± 0.21 mmol/L, P<0.05) decreased, serum HDL-C levels (2.24 ± 0.1 mmol/L, P<0.05; 2.3 ± 0.17 mmol/L, P<0.05) were increased in metformin and ALPs group (Figure 1).
 Click for large image | Figure 1. Effect of ALPs on blood lipid in diabetes mice. (a) the content of TC in serum; (b) the content of TG in serum; (c) the content of LDL-C in serum; (d) the content of HDL-C in serum. Values are the mean±SD, n=10. In a, b, and c significant differences (P<0.05) were determined by ANOVA. |
3.2. ALPs significantly regulate blood glucose and urine glucose homeostasis in diabetes mice
As the experiment progressed, there was no significant change in the urine glucose of the control group mice. However, at the 2 weeks, there was no significant difference in urine glucose levels among the other three groups, but the levels of urine glucose were significantly higher than that in the control group. After 6 weeks of treatment, the urine glucose in the model group (45.76 ± 3.05 mmol/L, P<0.05) was significantly increased when compared to the control group, but there was no significant difference when compared to positive drugs (40.25 ± 1.08 mmol/L, P>0.05) and ALPs groups (36.97 ± 2.03 mmol/L, P>0.05). Starting from the 6th week, the urine glucose levels in the positive drug group and ALPs group mice gradually decreased. However, the urine glucose levels of the model group mice continued to increase. At the end of 10 weeks, the level of urine glucose in the model group had reached 64.25 ± 4.23 mmol/L. The urine glucose levels in the positive drug group (32.25 ± 3.15 mmol/L, P<0.05) and ALPs group (31.26 ± 2.97 mmol/L, P<0.05) were significantly decreased (Figure 2a).
 Click for large image | Figure 2. Effect of ALPs on glucose indexes in diabetes mice. (a) Fasting blood glucose; (b) urine glucose; (c) glucose tolerance. Values are the mean±SD, n=10. In a, b, and c significant differences (P<0.05) were determined by ANOVA. |
Like urine glucose, there has been no significant change in fasting blood glucose levels in the control group mice. After 2 weeks of treatment, there were no significant differences in the fasting blood glucose levels in the positive drugs group (17.34 ± 2.57 mmol/L, P>0.05), ALPs group (15.26 ± 1.08 mmol/L, P>0.05), and model group (13.57 ± 1.70 mmol/L, P>0.05). However, after 10 weeks of treatment, the fasting blood glucose levels in the positive drugs group (32.25 ± 3.15 mmol/L, P<0.05) and ALPs group (21.26 ± 2.97 mmol/L, P<0.05) were decreased when compared with that in model group (44.25 ± 4.23 mmol/L) (Figure 2b).
In the intraperitoneal glucose tolerance test (IPGTT) experiment, the levels of the blood glucose change followed a similar trend in four groups, and the highest content of blood glucose was at 0.5 h. At each moment, the blood glucose levels in the ALPs group (25.67 ± 3.01, 27.24 ± 2.76, 26.26 ± 1.87 and 23.96 ± 2.02 mmol/L at 0, 0.5, 1 and 2 h respectively) were significantly lower than those in the model group (32.17 ± 2.87, 42.69 ± 2.96, 38.26 ± 3.02 and 33.79 ± 3.17 mmol/L, P<0.05) (Figure 2c).
3.3. Screening for kidney differential proteins after ALPs treatment
Two-dimensional electrophoresis was used to compare the protein expression of db/db diabetic mice in the model group and the ALPs group. An average of 700 spots were detected in 2D-DIGE gels in the ALPs group. However, only 117 spots reproducibly matched all samples that were present, 70 spots were up-regulated proteins and 47 spots were down-regulated proteins. The protein spots were mainly concentrated in the molecular weight (MW) 10–130 kDa ranges and pI 4–7. The changes greater than 1.5 (P <0.05) were considered as the significant difference protein (Figure 3). The part of the results of mass spectrometry are shown in Table 1. After ALP treatment, the expression level of C13 (haptoglobin) significantly increased and the mass spectrometry score was relatively high. The expression level of D36 (alpha-2-HS-glycoprotein) significantly decreased.
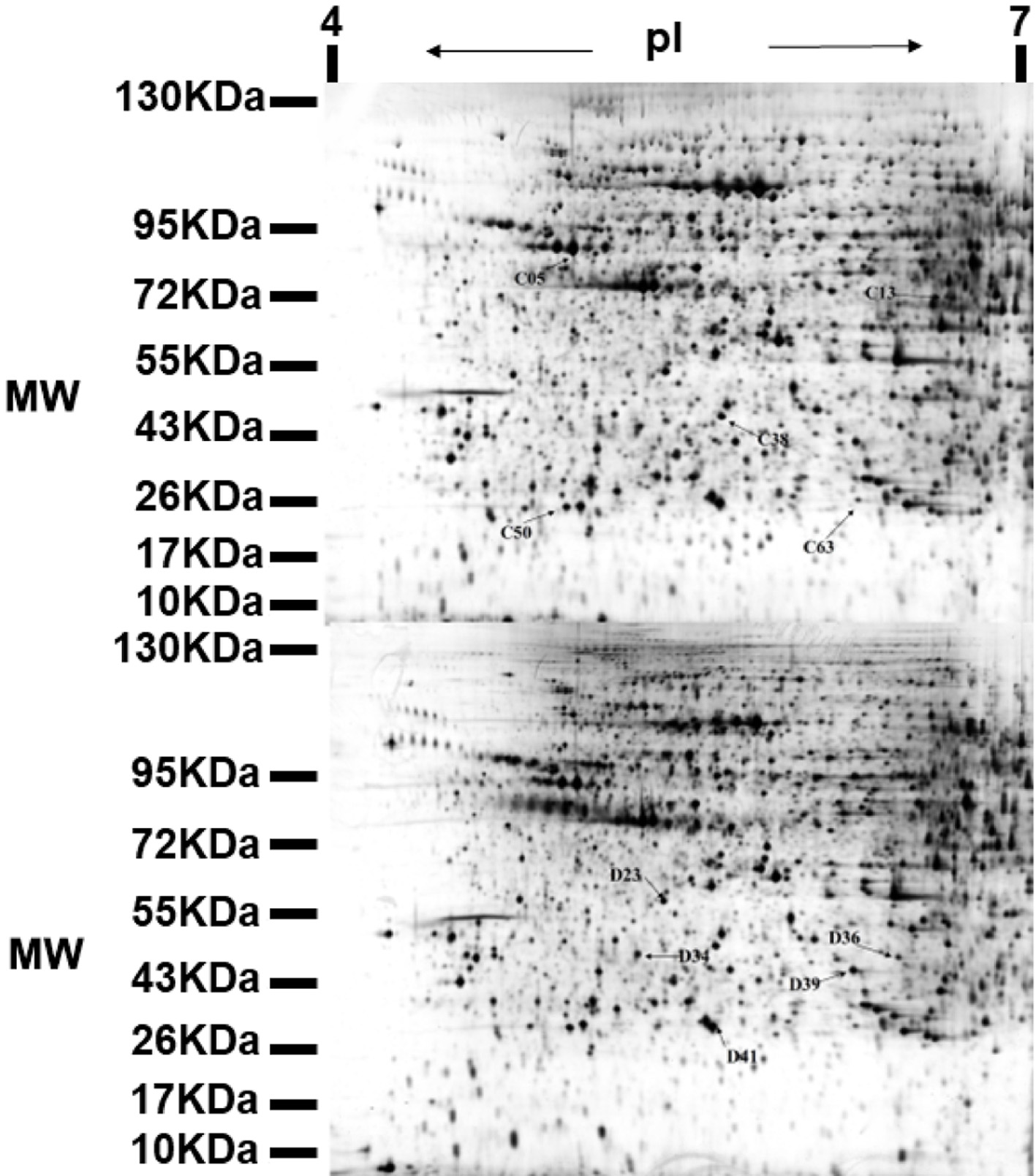 Click for large image | Figure 3. Representative 2-DE map obtained from diabetic mouse kidney after ALPs treatment. C denotes the up-regulated proteins in the ALPs group when compared with the model group; D denotes the down-regulated proteins in the ALPs group when compared with the model group. |
 Click to view | Table 1. Differentially expressed proteins in different groups as identified by MALDI-TOF-MS/MS. C indicates proteins up-regulated in the ALPs group, and D indicates proteins down-regulated in the ALPs group |
3.4. Differential protein functional analysis
Gene ontology (GO; http://www.geneontology.org/) annotations were performed with the sequences identified by MS/MS using BLASTx (NCBI database). GO analysis showed that the biological processes involved in differentially expressed proteins were mainly related to protein transport, response to calcium ions, and axonogenesis. There were some interactions between the protein transport process and diabetes, these processes can often control key proteins in biological processes of the role, thus affecting the entire biological process. The molecular functional processes of differential protein enrichment were mainly related to energy metabolism, ATP binding, and kinase activity. There was a wide range of roles between diabetes and many genes, which contain coding sequences of key metabolic enzymes that control the expression of key enzymes in the metabolic pathway, affecting the entire metabolism. The differential proteins-enriched cellular components were associated with mitochondrion, cytosol, and cytoplasm (Figure 4).
 Click for large image | Figure 4. The GO cluster analysis of different expression proteins in ALPs vs. model group. The numbers in parentheses indicate the number of genes. GO:0005739 mitochondrion, GO:0005829 cytosol, GO:0005737 cytoplasm, GO:0005615 extracellular space, GO:0005813 centrosome, GO:0016324 apical plasma membrane, GO:0005634 nucleus, GO:0043234 protein complex, GO:0005524 ATP binding, GO:0016301 kinase activity, GO:0005525 GTP binding, GO:0005509 calcium ion binding, GO:0005200 structural constituent of the cytoskeleton, GO:0046982 protein heterodimerization activity, GO:0015031 protein transport, GO:0051592 response to calcium ion, GO:0007409 axonogenesis, GO:0030336 negative regulation of cell migration, GO:0006508 proteolysis, GO:0051592 response to calcium ion, GO:0008584 male gonad development, GO:0006886 intracellular protein transport. |
3.5. Differential protein metabolic pathway analysis
In addition, the homologous sequences involved in metabolic pathways were mapped according to the Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) and used to putatively map these homologous sequences to specific biochemical pathways. Differential protein KEGG analysis found that these proteins are involved in a total of 18 metabolic pathways, including metabolic pathways, insulin resistance, phagosome, Hippo signaling pathway, and so on (Figure 5).
 Click for large image | Figure 5. The KEGG pathway analysis of the different expression proteins. map01100 Metabolic pathways; map04931 Insulin resistance; map04145 Phagosome; map04390 Hippo signaling pathway; map05132 Salmonella infection; map05416 Viral myocarditis; map05414 Dilated cardiomyopathy; map05410 Hypertrophic cardiomyopathy (HCM); map04810 Regulation of actin cytoskeleton; map05412 Arrhythmogenic right ventricular cardiomyopathy (ARVC); map05100 Bacterial invasion of epithelial cells; map04910 Insulin signaling pathway; map04612 Antigen processing and presentation; map04151 PI3K-Akt signaling pathway; map04020 Calcium signaling pathway; map05164 Influenza A-Mus musculus; map04520 Adherens junction; map04670 Leukocyte transendothelial migration. |
| 4. Discussion | ▴Top |
Bioactive peptides have received increasing attention due to their good therapeutic effects, minimal side effects, and stability compared to proteins (Hayes, 2021). Bioactive peptides not only provide nutrients to the body, but also have various functions, such as antioxidant, anti-radiation, anti-aging, hypoglycemic, antibacterial, and anti-inflammatory, participating in immune regulation of the body, and can also lower blood pressure and blood glucose levels in the body (Dong et al., 2018). Decreased serum high-density lipoprotein cholesterol (HDL-C) and increased low-density lipoprotein cholesterol (LDL-C), serum total cholesterol (TC), and triglyceride (TG) are considered to be significant risk factors for hyperlipidemia (Elahi et al., 2009). The levels of TC, TG, and LDL-C in the ALP group were significantly lower, and the HDL-C level was significantly higher in the ALPs group when compared to the model group, which indicated that the ALPs could regulate the dyslipidemia in diabetic mice.
Haptoglobin (Hp), as an acute-phase protein, is considered to have anti-inflammatory and anti-oxidative properties (Langlois and Delanghe, 1996). It has been reported that Hp is involved in modulating the immune response, autoimmune diseases, and major inflammatory disorders (Quaye et al., 2006). Type II diabetic patients exhibit higher serum levels of pro-inflammatory cytokines and acute-phase reactants (King, 2008). Hp has a certain regulation role in inflammation, so it has a potential role in type 2DM pathogenesis. Diabetes nephropathy is accompanied by renal inflammation. In order to alleviate renal inflammation, blood accumulates in the kidney and the content of Hp in the kidney increases (di Masi et al., 2020). In immune modulation, cells of both arms of the immune system, upon contact with danger signals, stimulate the generation of anti-inflammatory and pro-inflammatory factors to repair injured tissue or eliminate infection, while ensuring that local cellular damage is minimized (Matzinger, 2002; Pavarini et al., 2006; Shi et al., 2003). Hp on the immune function is not limited to lymphocytes, other immune cells also have an effect.
Insulin is an important hormone regulating energy metabolism, glucose metabolism, and lipid metabolism, when the body’s glucose is in excess, insulin inhibits liver glucose production and promotes glucose decomposition into non-sugar substances, maintained in a narrow range. This breakdown of homeostasis may lead to insulin resistance (IR) (Deborah et al., 2016). To regulate the normal level of blood sugar, the body secretes too much insulin, resulting in a series of pathological changes in the body (Park et al., 2003). Alpha-2-HS-glycoprotein, also known as fetuin-A, is an abundant plasma protein synthesized predominantly in the liver (Brown et al., 1992). alpha-2-HS-glycoprote has multiple biological functions including the inhibition of insulin receptor auto-phosphorylation (Auberger et al., 1989; Mathews et al., 2000) and the regulation of calcium homeostasis (Heiss et al., 2003). Mathews et al (2000) found that alpha-2-HS-glycoprotein is a specific inhibitor of insulin receptor auto-phosphorylation and interacts with the insulin receptor (Saroha et al., 2012). They also found that alpha-2-HS-glycoprotein -null mice exhibit significantly enhanced insulin sensitivity and were resistant to weight gain in a high-fat diet (Mathews et al., 2002). Recently, it showed that serum alpha-2-HS-glycoprote levels are related to homeostasis model assessment (HOMA) of insulin resistance in non-diabetic humans (Mori et al., 2006). These confirmed that alpha-2-HS-glycoprote plays a physiological role in the regulation of insulin signaling and energy homeostasis (Inoue et al., 2008). It inhibits insulin-dependent insulin receptor auto-phosphorylation and receptor tyrosine kinase activity in the liver and skeletal muscle and further inhibits insulin receptor substrate-1 (IRS-1) and phosphatidylinositol-3-kinase (PI) -3K), resulting in a variety of biological effects mediated by such as glycogen synthesis, glucose transport, anti-lipolysis and other inhibited. The results of this study showed that the expression of alpha-2-HS-glycoproteins in the diabetic mice fed with the Acaudina molpadioidea peptides decreased, the auto-phosphorylation of insulin receptor and tyrosine kinase activity returned to normal and the insulin signaling pathway returned to normal (Figure 6).
 Click for large image | Figure 6. Effects of alpha-2-HS-glycoprotein on insulin signal pathway. (a)The pathogenesis of type II diabetes. when the expression of alpha-2-HS glycoprotein increases, it will inhibit insulin receptor auto-phosphorylation, insulin and insulin receptor is no longer binding, but also inhibit the downstream tyrosine kinase activity, then glycogen synthesis and glucose transport are inhibited, resulting in insulin resistance. (b) The expression of alpha-2-HS-glycoprotein in diabetic mice after feeding the ALPs is down-regulated, the insulin and insulin receptors are normally bound, the autophosphorylation of the receptor activates the intrinsic tyrosine kinase activity, further leading to the downstream signal Cascade cascade reaction occurs, the insulin signaling pathway returned to normal, resulting in insulin-mediated glucose and lipid metabolism. |
| 5. Conclusion | ▴Top |
Compared to the diabetes mice, the content of TC, TG, and LDL-C in serum was decreased, the levels of HDL-C were increased and the disorder of blood lipid was improved in diabetic mice after feeding the ALPs. Simultaneously regulating and reducing urine glucose and fasting blood glucose levels. The expression of Haptoglobin was up-regulated, it plays a role in anti-inflammatory and immune regulation. And the expression of alpha-2-HS-glycoproteins was down-regulated, then the insulin signal pathway was restored to normal condition, to improve the symptoms of diabetes.
Acknowledgments
This work was sponsored by the Natural Science Foundation of Zhejiang Province (LQ22D060002 and LTGC23C190001), Fund of State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products (ZS20190105), General Project of Zhejiang Provincial Department of Education (Y202146257), and K.C. Wong Magna Fund of Ningbo University.
Conflict of interest
The authors declare no competing financial interests.
Qin Yang and Jiaojiao Han: conceptualization, methodology, writing-original draft, writing-review & editing. Qin Yang and Chenyang Lu: data curation, validation. Jun Zhou: project administration, supervision, investigation. Xiurong Su: project administration, supervision.
| References | ▴Top |