| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 23, September 2023, pages 68-73
Quantitative analysis of phenylpropanoids in Rhodiola rosea from different producing areas
Limin Guoa, b, *, Chenyang Caia, b, Fei Zhangc, Rong Mac, Janar Jenisd
aInstitute of Agro-Products Storage and Processing, Xinjiang Academy of Agricultural Sciences, Urumqi 830091, China
bXinjiang Key Laboratory of Agro-Products Storage and Processing, Urumqi 830091, China
cSanjiang Zhonghui Biotechnology Co. Ltd., Huocheng, Yili Prefecture, Xinjiang, China
dResearch Center for Medicinal Plants, Al-Farabi Kazakh National University, 050040, Almaty, Kazakhstan
*Corresponding author: Limin Guo, Institute of Agro-Products Storage and Processing, Xinjiang Academy of Agricultural Sciences, Urumqi 830091, China. Tel: 086-139-9999-8509; E-mail: guolm_xj@163.com
DOI: 10.31665/JFB.2023.18355
Received: September 21, 2023
Revised received & accepted: September 28, 2023
| Abstract | ▴Top |
Rhodiola (Rhodiola rosea) grows worldwide, particularly in Europe, North America and several Asia countries like China and Kazakhstan. As a functional food and medicinal herb and broadly used in healthy foods and beverages, R. rosea possesses antioxidant, antifatigue, anti-ageing, inhibitory effects of inflammation, anticancer and other pharmacological properties. There are more than 90 species of Rhodiola distributed in Asia, Europe and North America and R. rosea is one of the major species. China is one of the most producing countries.Same as other plants, the bioactive components of R. rosea vary with growing location and conditions. Phenylpropanoids in R. rosea are a group of main bioactive phytochemicals and their contents are usually used as a quality indicator. Hence it is of great importance to characterize phenylpropanoids in R. rosea from different sources to ensure their effectiveness and efficacy. In this study, 18 R. rosea samples from Kazakhstan and 4 regions of China were collected and further characterized for their contents of rosavin, rosarin and rosin, the major biomarkers of R. rosea quality and pharmacological effects. ANOVA analysis showed that there were significant differences in the contents of three phenylpropanoids, particularly, rosavin among different regions. As an example, the content of total rosavins in R. rosea from Kazakhstan was more than three times that from Bazhou, Xinjiang province of China.
Keywords: Rhodiola rosea; Rosavin; Phenylpropanoid; Origin; Bioactivity
| 1. Introduction | ▴Top |
Rhodiola (Rhodiola rosea L, abbreviated as R. rosea) is a perennial herb or subshrub of the genus Rhodiola in the Crassulaceae family. There are chiefly six subtypes of Rhodiola rosea, namely, Rhodiola daflora, Rhodiola sakhara, Rhodiola rosea, Rhodiola angustifolia, Rhodiola longwhip and Rhodiola yunnanensis. R. rosea, also known as ‘rose Rhodiola’ is named “golden root” or “rose root” as well. It is widely grown in Europe, North America, the west and northeast of China, particularly in Xinjiang province. As early as the 19th century, R. rosea was used as a brain tonic in France. Recognized as an adaptogen and phytomedicine in 1985, R. rosea is listed in the Swedish Handbook of Medicines and Therapy (Lakemedelsboken 1997/98) as one of the most commonly used psychostimulants in officially registered herbal products (Olsson et al., 2009). In recent years, the extract of R. rosea has been used in beverages, food additives, health care and beauty products and medicine. R. rosea possesses antioxidant and anti-ageing (Samuel et al., 2013), antifatigue (Prechel et al., 2018), inhibition of radiation (Zhou et al., 2020), therapeutic potential for non-small cell lung cancer (Zhang et al., 2020), reducing stress (Croply et al., 2015), antihypoxia (Zhou et al., 2017) and other pharmacological effects. There are more than 90 species of Rhodiola in the world, distributed in Asia and North America. China is the main producing country for Rhodiola with more than 73 species, which mainly grow in the west of China, in particular, the region of Himalayas and Xinjiang province (Chen et al., 2021).
The main active phytochemical ingredients in R. rosea are salidroside, p-tyrosol, rosavin, arbutin, gallic acid, kaempferol, and quercetin (Panossian et al., 2010). Three main phenylpropanoid glycosides in R. rosea, i.e. rosavin, rosarin and rosin, are considered to serve as quality indicators of R. rosea, and their total content is recorded as ‘total rosavins’ (Chiang et al., 2014). For instance, studies have shown that rosavin has effective anti-inflammation effect (Pooja et al., 2009; Andrey et al., 2017), and anti-pulmonary fibrosis (Prechel et al., 2018; Xin et al., 2014). Rosavin possesses anticancer (Skopinska-Rozewska, et al., 2008) and anti-depression effects (Kurkin et al., 2006), among others. The quality of an herb is almost always affected by various environmental factors, such as growing altitude, climate, soil conditions, and place of origin. Up to now, several studies have reported the differences of bioactive content in Rhodiola rosea from different origins, but have mainly focused on flavonoids and alcohols, not on phenylpropanoid glycosides. Prechel et al. (2018) analyzed salidroside, 6 phenylpropanoids (rosavin, rosin, rosarin, cinnamyl alcohol, salidroside and p-tyrosol)from European countries and other regions for R. rosea from six different origins with same or different altitudes, and found that the content of salidroside was different and related with different sources and altitudes. In another study, the comparison of six biocomponents in Rhodiola grandiflora from different origins showed that the contents of the analyzed components from different sources and varieties were significantly different. Therefore, same as the other species, the contents of bioactives in R. rosea from different origins and or different growing conditions vary from one another.
There are many studies on the optimization of extraction process and biological activity of salidroside and tyrosol in R. rosea, but the analysis of the major phenylpropanoids from R. rosea samples collected from China and other Asian countries has seldom been performed. This research was focused on the study of the total and individual contents of rosavirin in R. rosea from different production origins of China and Kazakhstan. Therefore, in this study, we aimed to analyze the content of phenylpropanoid compounds, i.e. rosavin, rosin and rosarin, which have been used as the indicator in determining the quality of R. rosea. The content of rosavirin in Rhodiola rosea from different origins was determined by high performance liquid chromatography (HPLC) method using the standards of rosavin, rosin and rosarin (Figure 1) to lay the foundation on the quality evaluation, targeted characterization and further research of Rhodiola rosea and development of its related products.
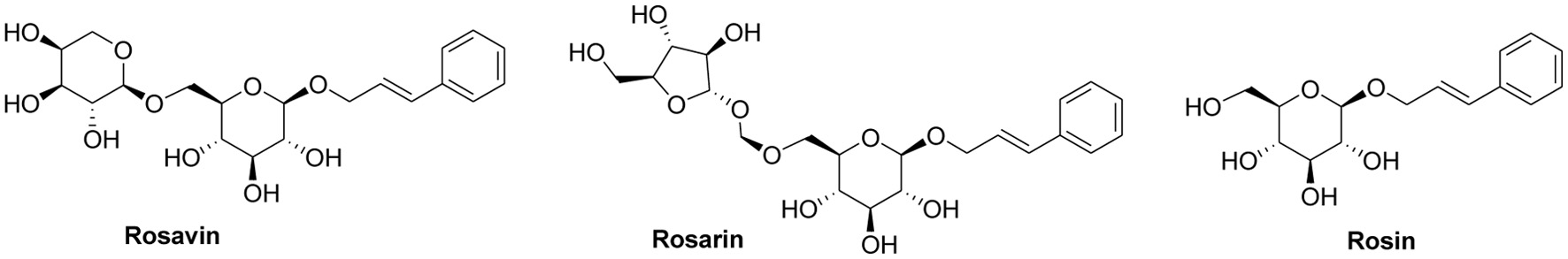 Click for large image | Figure 1. Structures of three phenylpropanoid compounds. |
| 2. Materials and methods | ▴Top |
2.1. HPLC system
An Essentia LC-16 HPLC (Shimadzu, Kyoto, Japan) was equipped with an autosampler, a solvent delivery pump system, a UV-vis monitor, and an analytical WondaSil C18-WR HPLC column (4.6 × 250 mm, 5 μm, Shimadzu). The mobile phase of an isocratic solvent system was consisted of methanol (35%) and 0.05% phosphoric acid aqueous solution. Flow rate was 0.8 mL/min; column temperature, 30 °C; monitoring wavelength, 250 nm; and injection volume 20 µL.
2.2. Reagents and materials
Methanol and phosphoric acid were LC grade and purchased from Sigma-Aldrich (Shanghai, China), HPLC grade water was prepared in house (water resistency, 18.2 MΩ). Other reagents were analytical grade and from Sigma-Aldirch unless specifically stated.
Three standard compounds, rosavin, rosin and rosarin, were purchased from China Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).Eighteen samples of R. rosea from two countries and five regions were purchased from the market from Huocheng County, Xinjiang Province, China (S1-S4), Kazakhstan (S5-S7), Hebei Province (S8-S11), Gongliu, Xinjiang province (S12-S15), and Bazhou, Xinjiang Province (S16-S18). The detailed description of the samples was listed in Table 1. All the samples were authentificated by botanist Mr. Zhou Meng.
 Click to view | Table 1. Samples of Rhodiola rosea from different regions |
2.3. Analyses of standard compounds
Each of the three standard compounds (rosavin, rosarin and rosin) was accurately weighed and transferred to a volumetric flask. The standard compounds were dissolved in 60% aqueous methanol to make standard solutions with a concentration of 0.15 mg/mL for each of rosavin, rosin and rosarin solutions, which was filtered on a 0.22 µm membrane filter and analyzed according to a previously established and validated HPLC analysis method of rosavin, rosarin and rosin (Cui, Guo, Wang, 2016).
2.4. Sample preparation and measurement of rosavin, rosin and rosarin
Each of the collected 18 of R. rosea samples was extracted with a mixed solvent of 40% water and 60% methanol. One gram of each sample was suspended on 10 mL of 60% aqueous methanol and the resultant mixture was sonicated for 2 h at room temperature. Upon filtration, the filtrate was transferred to a volumetric flask to prepare a solution with an accurate concentration. One milliliter of the filtrate was prepared for HPLC analysis after further filtration with a 0.22 µm membrane filter. The content of three major bioactives (rosavin, rosin and rosarin) in each of the 18 samples was then calculated by referencing the corresponding external standards (rosavin, rosin and rosarin). The detailed results are listed in Table 2. Each sample was prepared in triplicate and analyzed in parallel.
 Click to view | Table 2. Content (%) of three phenylpropanoids in Rhodiola samples from different regions |
2.5. Data anaylysis
Data were presented as the mean ± standard deviation of the mean of three or four independent experiments and analyzed. Statistical analysis was conducted by one-way analysis of variance (ANOVA).
| 3. Results and discussion | ▴Top |
3.1. Analyses of phenylpropanoids as external standards
Standard solutions of three phenylpropanoids, i.e. rosavin, rosin and rosarin, were prepared and analyzed with a validated HPLC method, previously established and re-validated with the newly prepared solutions of three phenylpropanoids. The HPLC method was used in the measurement of the newly collected 18 samples of R. rosea from different origins.
3.2. Measurement of three major phenylpropanoids in 18 samples of R. rosea from different origins
According to the validated HPLC method, the analysis of the individual contents of rosavin, rosin and rosarin in the sample solution was performed in triplicate (Table 2). First, the samples of R. rosea were extracted with methanol-water (60:40, v/v); the same solvent system as that in the preparation of the above three phenylpropanoids standard solutions. The extracted solutions were then filtered and transferred to volumetric flasks, yielding measured volumes and accurately calculated concentrations, which were then detected and analyzed with above established HPLC method. The results of the three phenylpropanoids in different origins of 18 samples of R. rosea were calculated based on the following formula using rosavin, rosin and rosarin as external standards.
(Cx, concentration of a detected phenylpropanoid in a sample of R. rosea; Cs, concentration of a standard phenylpropanoid; Ax, concentration of a detected phenylpropanoid in a sample; As, area of a pure phenylpropanoid as a standard)
As the usual quality indicators and main pharmacology index, phenylpropanoids are one of the major bioactive phytochemicals in R. rosea. From Table 2, we can see that R. rosea from Kazakhstan has the richest total rosavin content (1.521 ± 0.371) among the 18 samples analyzed, followed by that from Huocheng, Xinjiang (1.094 ± 0.181%), and Zhangjiakou, Hebei provinces (1.073 ± 0.156). The content of total rosavin from the two regions of Xinjiang, i.e. Bazhou and Gongliu, was the lowest with 0.413 ± 0.102 and 0.449 ± 0.211%, respectively (Table 2 and Figure 2). There were also big differences in the content of single phenylpropanoids in R. rosea among the five regions.
 Click for large image | Figure 2. Comparison of rosavin, rosarin and rosin, and total rosavins in R. rosea collected from two countries and five regions. (Hebei and Xinjiang are two provinces of China). |
The content of rosavin in R. rosea from Kazakhstan was the highest among the 18 samples from 5 regions, with an average content of 1.018 ± 0.338%. It should be noted that there was one sample that had higher content of rosavin (1.38%) than the other two samples in Kazakhstan. It could be postulated that the three samples from Kazakhstan could be from different areas or have other significant environmental changes. The specific reason for the differences is currently being investigated. The content of rosavin from Huocheng, Xinjiang was the second highest with an average content of 0.797 ± 0.145%, and followed by R. rosea sample from Zhangjiakou, Hebei province (0.585 ± 0.114%). The other two regions, meaning Bazhou and Gongliu of Xinjiang offered the lowest content of rosavin among the 18 samples tested, with a respective content of 0.305 ± 0.144 and 0.300 ± 0.075%.
The content of rosarin in 18 R. rosea samples also showed differences. The content of rosarin was almost the same between R. rosea samples from Kazakhstan (0.331 ± 0.068%) and Zhangjiakou, Hebei province (0.367 ± 0.052%). The rosarin content of 0.217 ± 0.034% in Huocheng, Xinjiang samples was average among the 18 samples tested. However, Bazhou and Gongliu of Xinjiang province exhibited the lowest rosarin content with 0.069 ± 0.023 and 0.088 ± 0.046%, respectively, two to three times less than rosarin, indicating that Bazhou and Gongliu regions in Xinjiang provide lower rosarin content of R. rosea.
The trend for the content of rosin among the 5 regions was the same as the total amount of rosavins, rosavin and rosarin. Rosin content in Zhangjiakou, Hebei province (0.122 ± 0.041%) and Kazakhstan (0.175 ± 0.038%) were 2-3 times higher than that in Huocheng (0.068 ± 0.030%), Bazhou (0.044 ± 0.006%) and Gongliu (0.057 ± 0.028%) of Xinjiang province.
Overall, the three detected phenylpropanoids, rosavin, rosarin and rosin in R. rosea from five regions, Kazakhstan, Huocheng, Xinjiang and Zhangjiakou, Hebei province, China provided the most content of total rosavins and rosarin, whereas Kazakhstan and Huocheng, Xinjiang had rich content of two individuals, rosavin and rosin. Two regions in Xinjiang province, Bazhou and Gongliu, showed the lowest content in each of the above category: total rosavins, rosavin, rosarin and rosin, indicating the quality of R. rosea from these two regions (Figure 2).
3.3. Analyses of content differences of rosavin, rosin and rosin in R. rosea from different origins
Rosavin, rosin and rosin are the major phenylpropanoids that are regarded as the quality indicators of R. rosea in evaluation or quality control. Hence this study used these three phenylpropanoids or also called ‘total rosavins’ to investigate the content of the three phenylpropanoids in R. rosea from five regions. The different content of total rosavins and each of the three individual phenylpropanoids in R. rosea from five regions have been compared in the previous section. Herein we compare the content variation of the total and individual three phenylpropanoids among the samples including intra-group and inter-group variation, mean square and significance (Table 3).
 Click to view | Table 3. Analysis of variation (ANOVA) for rosavin, rosin and rosin in R. rosea from different origins |
ANOVA analysis in Table 3 showed that the p values of total rosavins in the three groups (intragroup, intergroup and total variation) were all less than 0.05, indicating that it is significant among the three groups. The mean square values of rosarin and rosavin in inter-groups were 0.002 and 0.031, respectfully, demonstrating the significance of the corresponding groups.
| 4. Conclusion | ▴Top |
Rhodiola rosea is a nutritional food and medicinal harb. Its phenylpropanoids are one of the main bioactives, which are the biomarkers for quality index and monitoring. Yet, growing in different regions in different environments such as temperature, altitudes and soil conditions, the content and amounts of bioactives in R. rosea and other plants are different, which often affecting the bioactivity and efficacy. Hence it is of great significance to characterize and compare the content of phenylpropanoids in R. rosea from different origins. In this study, results from the analysis and comparison of three phenylpropanoids, i.e. rosavin, rosarin and rosin, demonstrated that R. rosea produced in Kazakhstan, Huocheng of Xinjiang and Zhangjiakou of Hebei provinces, China have more content of total and also individual phenylpropanoids than Gongliu and Bazhou in Xinjiang province, China. This study provides scientific evidence for quality control of R. rosea.
This research was funded by the regional collaborative innovation project of Xinjiang Province - Joint Research Center for Quality Evaluation of Natural Plant Medicinal Resources and Key Technologies of Green Processing in China and Kazakhstan (Grant No.2020E01048)
| References | ▴Top |