| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 23, September 2023, pages 35-45
The effects of Yam polysaccharides on acrylamide-induced RAW264.7 cell polarization
Aoni Zhang, Dongliang Jin, Ying Han, Jiankang Wang, Jing Wang*
School of Food Science and Engineering, Shaanxi University of Science & Technology, Xi’an 710021, China
*Corresponding author: Jing Wang, School of Food Science and Engineering, Shaanxi University of Science & Technology, Xi’an 710021, China. E-mail: wangjingliwang@126.com
DOI: 10.31665/JFB.2023.18352
Received: July 20, 2023
Revised received & accepted: September 16, 2023
| Abstract | ▴Top |
We previously found that acrylamide (AA) damaged RAW264.7 cells (a kind of macrophages) by up-regulating P2X7 protein level, and Yam polysaccharides (YPS) could protect RAW264.7 cells against AA injury. Macrophages polarization may generate various subtypes which are closely related with cell function. However, how AA and YPS influence RAW264.7 polarization is unknown. In this study, we studied the effects of AA and YPS on the polarization of RAW264.7 cells. The killing effect of RAW264.7 cell on tumor cells has also been evaluated. The results showed that AA caused cell polarization towards M1-like macrophages by elevating CD86 and COX-2 protein expression and decreasing CD206 protein expression. Inhibiting the expression of P2X7 protein could effectively interfere with AA-induced M1 polarization. YPS could modulate AA-induced M1 polarization by reducing the ratio of CD86/CD206 but not COX-2 protein expression. RAW264.7 cell medium treated with AA had no effect on HepG2 cancer cell viability, but RAW264.7 cell medium treated with YPS significantly inhibited HepG2 cell viability. These findings suggested that YPS could regulate AA-induced RAW264.7 polarization and improve cell function. The results will provide reference for further explaining the mechanism of YPS intervening in toxic AA.
Keywords: Acrylamide; Cell polarization; Yam Polysaccharides; RAW264.7 cell
| 1. Introduction | ▴Top |
Acrylamide (AA, 2-propenamide, C3H5NO) is a harmful substance commonly formed in thermally processed foods. Acrylamide has been frequently detected in various baked, fried, and roasted foods (Deribew and Woldegiorgis, 2021). Additionally, acrylamide as chemical raw material has also long been used in various branches of industry for oil exploitation, production of polyacrylamides, paper, and adhesives. Therefore, acrylamide pollution is very common in daily life, and acrylamide-polluted air, water and commodities, as well as dietary food are easy exposure pathway.
Acrylamide, after being absorbed and distributed into several vital organs, causes serious harm. It has been reported that acrylamide is responsible for causing neurotoxicity, reproductive toxicity and cardiac developmental toxicity (Aldawood et al., 2020; Eghan et al., 2022). Moreover, in vivo studies have recently revealed that the intake of acrylamide induced splenic damages in adult zebrafish, and caused reduction of natural killer (NK) cells in female BALB/c mice (Fang et al., 2014; Komoike et al., 2020). Additionally, our research indicated that acrylamide caused RAW264.7 cell disfunction and death due to excessive autophagy, pyroptosis and oxidative stress (Wang et al., 2023). These findings demonstrated that acrylamide can cause immunotoxicity. So further investigation of immunotoxicity of acrylamide as well as its immune mechanism is worth study. It is also necessary to take effective measures to intervene in toxic hazards of acrylamide.
It is recommended that multiple natural compounds have good bioactivity and can be used to intervene in the toxic acrylamide (Yan et al., 2023). Yam is a natural food resource with high value for nutrition and health care. Yam polysaccharides are important functional components of Yam with various biological activities. For example, Yam polysaccharides could promote the proliferation of human endometrial epithelial cells, inhibit the proliferation of Escherichia coli and scavenge free radicals (Ju et al., 2014; Yang et al., 2015). Yam polysaccharides could also exert hypoglycemic effect and regulate blood glucose and lipid metabolism in rats (Yu et al., 2020). The improvement of immunity by Yam polysaccharides has also attracted much attention. It is reported that Yam polysaccharides can enhance the phagocytic function of macrophages through TLR4-NF-κB signaling pathway (Li et al., 2017). Our research found that Yam polysaccharides were effective in inhibiting RAW264.7 cell (a kind of mononuclear macrophages) damage caused by acrylamide, promoting the proliferation and function of RAW264.7 cells (Wang et al., 2023). However, further research is still needed to better clarify the mechanism of Yam polysaccharides against acrylamide.
RAW264.7 cell is a mouse macrophage cell line. Macrophages, widely distributed in the whole tissue, are functionally heterogeneous and show remarkable phenotypic plasticity (polarization). According to the polarization phenotype classification of macrophages, there are two main macrophage phenotypes known as classically activated M1 and alternatively activated M2 macrophages. M1 phenotype macrophages also known as pro-inflammatory cells, support inflammatory processes and accelerate tissue damage, while M2 phenotype macrophages also known as anti-inflammatory cells, suppress inflammation and promote tissue repair. The imbalance between M1-and M2-polarized macrophages is one of the major reasons for pathophysiological changes (Shapouri Moghaddam et al., 2018). In fact, the potential of macrophage plasticity requires fine tuning and exactness at the molecular level for specific tasks. Macrophages can be specifically activated (polarized) by multiple biomolecules, such as endotoxin (M1), IFN-γ (M1), TNF-α (M1), TGF-β (M2), IL4 (M2), IL13 (M2), etc (Shapouri Moghaddam et al., 2018). Generally speaking, M1 type macrophages are characterized by expressing high level of surface markers such as CD86 and secreting pro-inflammatory factors. M2 macrophages express high level of surface markers including mannose receptor CD206, and secrete anti-inflammatory molecules for phagocytic debridement. Macrophages polarize into different subpopulations based on the surrounding cytokines and microenvironment (Gharavi et al., 2022; Minton, 2017). Thus, targeting macrophage polarization may be an effective way for regulating cell state and function.
Functional polysaccharides were widely reported to regulate immune function, which has attracted more and more concentration. Yam (Rhizoma dioscoreae) is a natural resource with high edible and health value. Yam polysaccharides (YPS) are important functional and bioactive component of yam and have been reported to have various activities including immunomodulation potential. Additionally, Yam polysaccharides has been reported to increase cell proliferation activity, enhance the phagocytic ability of macrophages and function of RAW 264. 7 macrophages (Li et al., 2017, Liu et al., 2022). We have previously found that acrylamide damaged macrophages by P2X7-mediated oxidative stress and YPS could effectively protect macrophages against injury caused by acrylamide. Based on our previous findings, this article further studied the effect of acrylamide on macrophages polarization, and the effect of YPS on macrophages polarization was also analyzed, to provide further evidence for elucidating the immunomodulatory mechanism of YPS. Due to the immunostimulatory and anti-inflammatory activities of active polysaccharides, multiple studies have developed polysaccharide-based materials into functional foods for health care or biomaterials for medical application (Li and Bratlie, 2021). Deeply elucidating the immune regulatory mechanisms of polysaccharides (derived from plants, animals, and microorganisms) also is of great significance for their rational utilization.
| 2. Materials and methods | ▴Top |
2.1. Chemicals and reagents
Acrylamide was from Amresco (USA). Yam polysaccharides (the content of polysaccharide and its derivatives determined by UV absorption spectrophotometry was 90.35%) was from Wuhan JONK Biotechnology Co., Ltd. (Hubei, China). Dulbecco’s modified Eagle’s medium (DMEM) was provided by Gibco (Invitrogen Corporation, USA). Fetal bovine serum (FBS) was from Zeta Life (USA). MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (USA). Assay kit for bichinconinic acid (BCA) protein, β-actin antibody (AF5003) and horseradish peroxidase (HRP)-labeled goat antirabbit IgG (A0208), PMSF and lysis buffer were obtained from Beyotime Institute of Biotechnology (Jiangsu, China). The products of the antibodies, such as mouse monoclonal antibody against P2X7 (sc-514962), COX-2 (sc-376861), GAPDH (glyceraldehyde 3-phosphate dehydrogenase) (sc-365062), and goat antimouse immunoglobulin G (IgG)-horseradish peroxidase (sc-2005) were obtained from Santa Cruz Biotechnology (USA). The antibodies of CD86 (bs-1035R) and CD206 (bsm-60761R) were from Beijing Bioss Biotechnology Co., Ltd (Beijing, China). The P2X7 receptor antagonist (A438079 hydrochloride) was from TargetMol. All other reagents were commercial products of the highest available purity grade.
2.2. Cell culture
RAW264.7 cells (Mouse Mononuclear Macrophages) and HepG2 cells (Human hepatocellular carcinoma) were purchased from National Collection of Authenticated Cell Cultures (Shanghai, China). Cells were cultured in DMEM supplemented with 10% of FBS and 1% of penicillin-streptomycin at 37 °C and in an atmosphere of 5% CO2.
2.3. Cell passage
When the cell adhesion reached about 80% of the bottom area of the culture dish, the cells were gently collected to a centrifuge tube with a sterile scraper. After centrifuged at 100 g for 3 minutes, cells were resuspended and transfer into two new culture dishes, and continued culture in an incubator (37 °C, 5% CO2).
2.4. Cell treatment
RAW264.7 cells (2 × 105/mL) were seeded in culture dish (60 mm) and left to adhere overnight, and then treated with either acrylamide, A 438079 (P2X7 inhibitor), or YPS. After 24 h of incubation, the protein levels of CD86, CD206 and COX-2 were detected by western blot.
2.5. Preparation of total cell extracts
After treatment, RAW264.7 cells were washed with PBS (phosphate buffered saline) and lysed with 100 μL of lysis buffer ((20 mM Tris, pH 7.5) (150 mM NaCl) (1% Triton X-100)), sodium pyrophosphate, β-glycerophosphate, EDTA, Na3VO4, leupeptin, pH 7.5) with 1% PMSF (100 μg/mL) on ice for 10 min. Cells were then collected and centrifugated for 10 min (15,000 g) at 4 °C, the supernatant was collected and the total protein concentration was measured and adjusted via bicinchoninic acid (BCA) protein assay(Thermo Fisher, Shanghai, China.)
2.6. Western blot
The protein samples prepared were first separated by SDS-PAGE (Sodium dodecyl sulfate-polyacrylaminde gel electrophoresis) and then transferred onto PVDF (polyvinylidene difluoride) membranes (Millipore), followed by blocking with 5% of skim milk. After that, the membranes were washed three times with TBST (Tris-buffered saline with Tween 20), and incubated with the primary antibodies (1:1,000) at 4 °C overnight, followed by incubation with secondary antibody (1:2,000) at 25 °C for 3 h. All the bands were visualized with a chemiluminescent substrate (Enhanced chemiluminescence (ECL), Engreen Biosystem, Beijing, China) and exposed by a Molecular Imager ChemiDoc XRS System (Bio-Rad, Shanghai, China).
2.7. Tumor cell viability assay
RAW264.7 cells were seeded in 96-well plates at a density of 15 × 103/well, left to adhere overnight, and treated with acrylamide (4.0 mmol/L) or YPS (50 μg/mL) for 24 h. Then all culture medium were collected and used to incubate HepG2 cells for 24 h. The culture medium collected from RAW264.7 cells without acrylamide or YPS treatment was employed as a control group. At the end of the culture, each well was added to 100 μL of MTT solution (0.5 mg/mL) and then incubated for 4 h. The formazan crystals formed by live HepG2 cells were extracted with DMSO and the absorbance (490 nm) was recorded by a spectrophotometer (MK3, Thermo Fisher Scientific). The results were expressed as a percentage of viable HepG2 cells in comparison to the control (100%).
2.8. Statistical analysis
All data were representative of at least three independent experiments and presented as mean ± SD (standard deviation). Results were performed using SPSS 19.0 software and one-way ANOVA (analysis of variance) was used for comparisons of groups. The value of p < 0.05 was considered statistically significant.
| 3. Results | ▴Top |
3.1. The polarization of RAW 264.7 cell was affected by acrylamide
Polarization is the activation process of macrophages under stress, and the polarization of macrophages plays an important role in inflammatory response, and the repair of tissues and organs. M1 polarized cells was characterized by highly expressed CD86 and M2 polarized cells was characterized by CD206. This study analyzed the effect of AA on the polarization of RAW264.7 cells. The results in Figure 1 showed that AA up-regulated the expression of CD86 and down-regulated the expression of CD206. The ratio of CD86/CD206 in AA treatment significantly increased with the increase of AA concentration. The results suggested that AA could induce RAW264.7 cell polarization towards M1 type, and the degree of polarization is positively correlated with the concentration of AA (The original images are shown in Figure S1a and b).
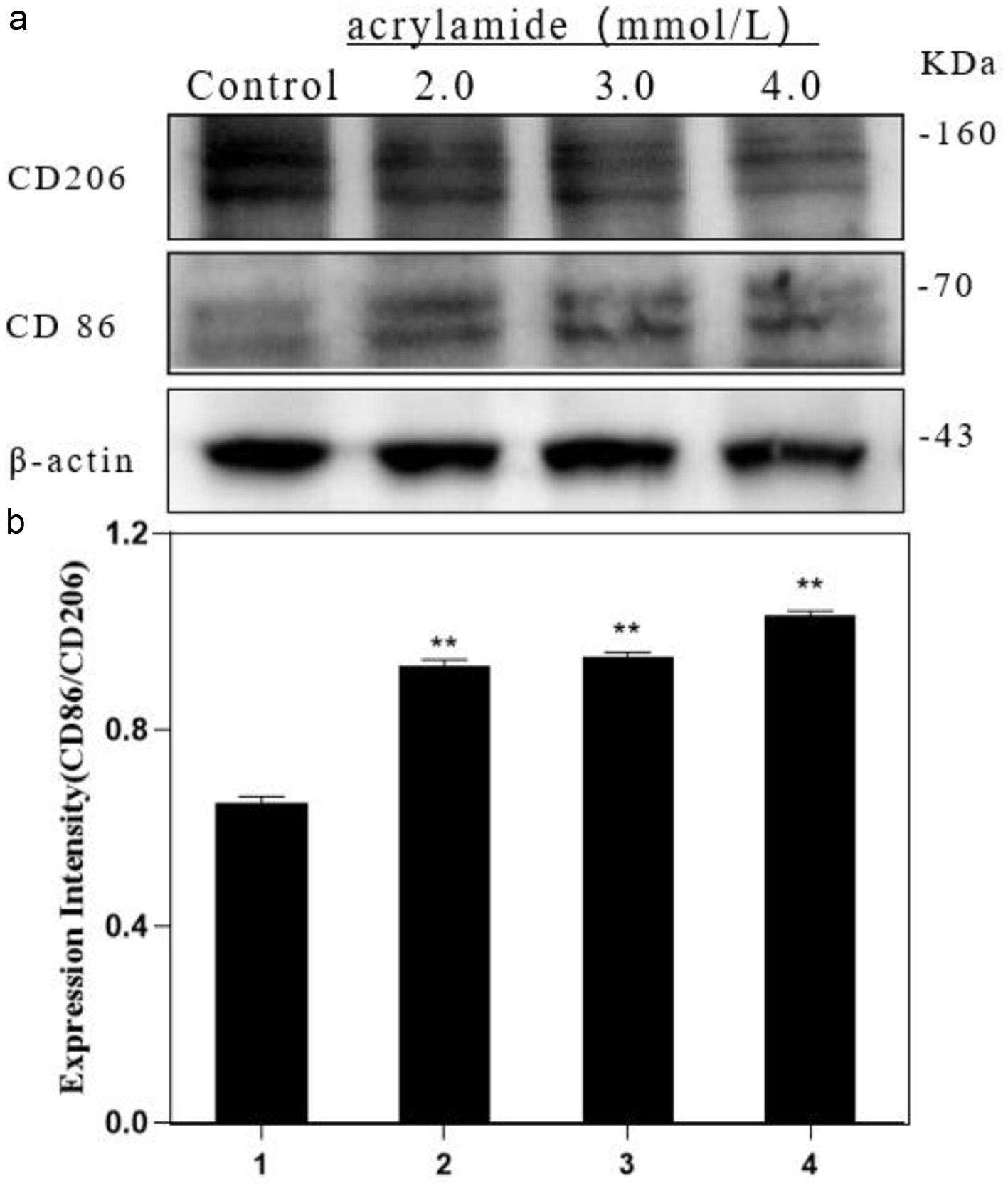 Click for large image | Figure 1. Effect of acrylamide on polarization of RAW264.7 cells. (a) Cells were treated with different concentrations of acrylamide for 24 h. Protein expressions were analyzed by western blot. (b) Bar chart of protein level. Lane1: Control, Lane2: 2.0 mmol/L AA, Lane3: 3.0 mmol/L AA, Lane4: 4.0 mmol/L AA. **p < 0.01, group with AA vs. group without AA. |
3.2. The protein level of COX-2 was elevated by acrylamide
Cyclooxygenase-2 (COX-2) is an inducible isoform of cyclooxygenase, and is induced by various inflammatory mediators, such as endotoxins, mitogens, and cytokines, including TNF-α and interleukins (ILs). COX-2 is markedly elevated in pathologic processes such as inflammation and other stresses (Kaur and Singh, 2022). We found that AA could induce COX-2 protein expression, as indicated in Figure 2. The COX-2 levels of AA-induced groups all showed significant difference compared with the level of the group without AA. However, the expression level of COX-2 did not show a continuous upward trend with the increase of AA concentration. The expression level of COX-2 peaked at low concentrations of AA (2.0 mmol/L), and then began to decrease. The elevated level of COX-2 expression further proved that acrylamide promoted cells to polarize to inflammatory M1 type (The original image is shown in Figure S1c).
 Click for large image | Figure 2. Effects of acrylamide on COX-2 protein in RAW264.7 cells. (a) Cells were treated with different concentrations of acrylamide for 24 h. Protein expressions were analyzed by western blot. (b) Bar chart of protein level. Lane1: Control, Lane2: 2.0 mmol/L AA, Lane3: 3.0 mmol/L AA, Lane4: 4.0 mmol/L AA. *p < 0.05, group with AA vs. group without AA, **p < 0.01, group with AA vs. group without AA. |
3.3. Acrylamide-induced cell polarization was mediated by P2X7
We have previously found that acrylamide enhanced P2X7 protein expression to damage cell function. To further investigate whether AA induced cell polarization is mediated by P2X7, a P2X7 receptor inhibitor (10.0 μmol/L) was used for subsequent study. It is shown in Figure 3 that compared to AA (3.0 mmol/L) alone treatment, the inhibitor and AA co-treatment (3.0 mmol/L) caused a decrease in CD86 expression and an increase in CD206 expression, and the ratio of CD86/CD206 showed a significant decline. Additionally, the P2X7 inhibitor alone treatment regarding to the group without treatment reduced CD206 expression, resulting to an increase in the ratio of CD86/CD206. The results indicated that inhibiting P2X7 alone could affect the polarization of RAW264.7 cells. However, when RAW264.7 cells become polarized due to AA stimulation, inhibiting P2X7 could effectively modulate the polarization trend of RAW264.7 cells induced by AA (The original images are shown in Figure S2a and b).
 Click for large image | Figure 3. Effect of P2X7 receptor inhibitors on acrylamide-induced polarization of RAW264.7 cells. (a) Cells were treated with acrylamide and P2X7 inhibitor (A 438079) for 24 h. Protein expressions were analyzed by western blot. (b) Bar chart of protein level. Lane1: Control, Lane2: 3.0 mmol/L AA, Lane3: 3.0 mmol/L AA + 10.0 μmol/L A 438079, Lane4: 10.0 μmol/L A 438079. **p < 0.01 vs. Lane1, ##p < 0.01 vs. Lane2. |
3.4. Acrylamide-induced COX-2 expression was regulated by P2X7
COX-2 could be induced by acrylamide, and the effect of P2X7 on COX-2 expression was further investigated. As shown in Figure 4, the level of COX-2 expression by P2X7 inhibitor alone treatment was significantly lower than that of the group without treatment. Acrylamide (3.0 mmol/L) alone greatly stimulated COX-2 expression, whereas the P2X7 inhibitor could antagonize with AA and significantly prohibit COX-2 expression. These findings indicated that P2X7 could regulate COX-2 expression during acrylamide exposure (The original image is shown in Figure S2c).
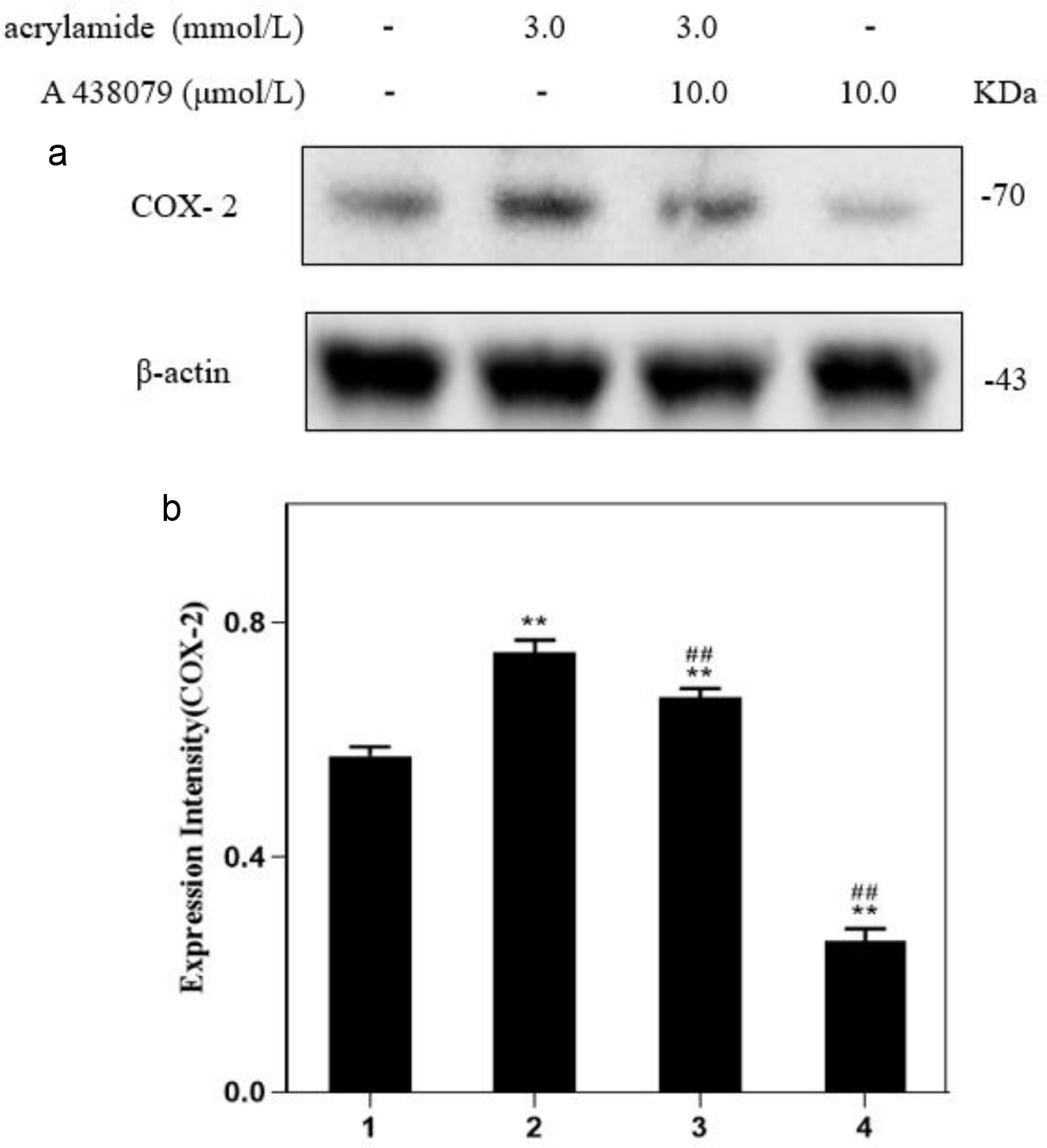 Click for large image | Figure 4. Effect of P2X7 receptor inhibitors on acrylamide-induced COX-2 protein in RAW264.7 cells. (a) Cells were treated with acrylamide and P2X7 inhibitor (A 438079) for 24 h. Protein expressions were analyzed by western blot. (b) Bar chart of protein level. Lane1: Control, Lane2: 3.0 mmol/L AA, Lane3: 3.0 mmol/L AA + 10.0 μmol/L A 438079, Lane4: 10.0 μmol/L A 438079. **p < 0.01 vs. Lane1, ##p < 0.01 vs. Lane2. |
3.5. Yam polysaccharides inhibited cell M1 polarization induced by acrylamide
It is previously demonstrated that Yam polysaccharides (YPS) could effectively protect cells against damage caused by acrylamide. The data in Figure 5 suggested that YPS also played a role in regulating cell polarization. Compared to AA (4.0 mmol/L) alone treatment, the YPS and AA co-treatment caused an increase in both CD86 and CD206 expression, but the ratio of CD86/CD206 significantly declined. Meanwhile, there were no obvious changes in CD86 and CD206 expression between the YPS alone treatment group and the group without treatment. However, YPS did not inhibit COX-2 expression induced by acrylamide, and there were no significant changes between AA alone treatment group and the group with YPS co-treatment (Figure 6). It is concluded that YPS could effectively regulate AA-induced polarization by altering CD86 and CD206 but could not block COX-2 expression (The original images are shown in Figure S3a–c).
 Click for large image | Figure 5. Effects of YPS on acrylamide-induced polarization of RAW264.7 cells. (a) Cells were treated with YPS and acrylamide for 24 h. Protein expressions were analyzed by western blot. (b) Bar chart of protein level. Lane1: Control, Lane2: 50.0 μg/mL YPS, Lane3: 4.0 mmol/L AA + 50.0 μg/mL YPS, Lane4: 4.0 mmol/L AA. **p < 0.01 vs. Lane1, ##p < 0.01 vs. Lane4. |
 Click for large image | Figure 6. Effects of YPS on acrylamide-induced COX-2 protein in RAW264.7 cells. (a) Cells were treated with YPS and acrylamide for 24 h. Protein expressions were analyzed by western blot. (b) Bar chart of protein level. Lane1: Control, Lane2: 50.0 μg/mL YPS, Lane3: 4.0 mmol/L AA + 50.0 μg/mL YPS, Lane4: 4.0 mmol/L AA. **p < 0.01 vs. Lane1, ##p < 0.01 vs. Lane4. |
3.6. Yam polysaccharides improved the killing power of RAW 264.7 cell against cancer cells
Macrophages are widely distributed innate immune cells. Activated M1 macrophages could cause adaptive immune response and clear tumor cells by releasing high levels of cytokines. Therefore, RAW264.7 cells were first cultured with YPS and then the culture solutions were collected for subsequent culture of cancer cells (HepG2), to analyze whether RAW264.7 have an impact on the cell viability of HepG2 cells. The results suggested that RAW 264.7 culture medium untreated did not reduce the viability of HepG2 cells, whereas culture medium treated with different concentrations of YPS could significantly decrease HepG2 cell viability (Figure 7). Additionally, the viability of HepG2 cells did not change in RAW 264.7 culture medium pre-treated with acrylamide. However, HepG2 cell viability showed a downtrend when cells were incubated in RAW264.7 culture medium pre-treated with acrylamide and YPS (Figure 8). It is indicated that YPS could enhance the killing power of macrophages against cancer cells.
 Click for large image | Figure 7. The cell viability of HepG2 cell cultured in RAW264.7 cell medium treated with YPS. Lane1: Control, Lane2: 50.0 μg/mL YPS, Lane3: 100.0 μg/mL YPS, Lane4: 150.0 μg/mL YPS. Lane5: 200.0 μg/mL YPS. Lane6: 400.0 μg/mL YPS. **p < 0.01 vs. Lane1. |
 Click for large image | Figure 8. The cell viability of HepG2 cell cultured in RAW264.7 cell medium treated with YPS and AA. Lane1: Control, Lane2: 1.0mmol/LAA, Lane3: 1.0 mmol/L AA + 50.0 μg/mL YPS, Lane4: 1.0 mmol/L AA + 100.0 μg/mL YPS. Lane5: 1.0 mmol/L AA + 150.0 μg/mL YPS. Lane6: 1.0 mmol/L AA + 200.0 μg/mL YPS. **p < 0.01 vs. Lane1, ##p < 0.01 vs. Lane2. |
| 4. Discussion | ▴Top |
AA has been reported to inhibit the humoral immune and cellular immune systems of mice. Macrophages with diverse forms and wide distribution are important in the human immune system, participating in both innate and specific immune regulation. Macrophages play an important role in immune regulation, anti-infection, anti-inflammation, and tumor intervention. The phagocytosis of macrophages is the most basic defense mechanism in innate immunity. For example, in specific immune response, most thymus-dependent antigens must be phagocytosed by macrophages before presented to T lymphocytes. Macrophages also release a variety of cytokines to participate in the regulation of immunity. In the non-specific immune process, monocyte and macrophages exert non-specific phagocytic effect on pathogens. Activated macrophages can also recognize, kill, and promptly eliminate tumor or mutated cells in the body. Macrophages have various immune functions such as sterilization, phagocytosis, antigen presentation, and secretion of cytokines (Duque and Descoteaux, 2014; Mamilos et al., 2023). Diversity and plasticity are important hallmarks of monocyte macrophage cell lines. Under different conditions and stimuli, macrophage can polarize into different phenotypes and perform different functions. It is considered that M1 type macrophages have strong antimicrobial and anti-tumor activities, and mediate ROS production to cause tissue damage. M1 type macrophages exert strong capability of antigen presentation, and promote the inflammatory response and the immune response of T helper cells (Th cells). M2 type macrophages have strong phagocytic ability, clear debris and apoptotic cells, promote tissue repair and wound healing, and have characteristics of promoting angiogenesis and fibrosis (Shapouri Moghaddam et al., 2018; Tang et al., 2022).
In our previous research, RAW264.7 cell (mouse mononuclear macrophage) was used as a cell model to explore the toxic mechanism of AA damaging immune cells. It is demonstrated that AA could reduce phagocytic activity, inhibit NO production, induce ROS production, and cause programmed cell death such as autophagy and pyroptosis in RAW264.7 cells. YPS could effectively alleviate cell injury and inhibit cell death induced by AA (Wang et al., 2023). However, the polarization which is critical and variable for macrophages has not been elucidated. Our previous research also found that AA promoted the release of TNF-α and the protein expression of iNOS. TNF-α and iNOS are related to inflammatory responses. Additionally, it is reported that M1 phenotype macrophages stimulate reactive oxygen species (ROS) to mediate tissue regeneration, and wound healing. These results indicated that the polarization of RAW264.7 cells may also be influenced by acrylamide. Therefore, the effect of acrylamide and YPS on polarization of RAW264.7 cells were explored in this study. As expected, acrylamide could regulate RAW264.7 cell polarization, and stimulate cell polarization towards M1 phenotype. COX-2 can be rapidly and robustly induced in response to a wide range of pro-inflammatory cytokines and mediators. COX-2 expression was also induced by acrylamide. These findings were consistent with previous results of elevated levels of TNF-α, ROS and iNOS in AA exposure groups.
P2X7 receptor is a transmembrane protein which is mainly expressed in lymphocytes, macrophages, monocyte and microglia. P2X7 plays a key role in innate and adaptive immunity. Many evidences indicate that P2X7 is of great significance in the pathogenesis of immune diseases such as systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, etc. P2X7 can also inhibit intracellular bacterial infections by regulating the immune response. These characteristics make P2X7 a highly attractive target for immune regulation (Cao et al., 2019). A number of studies have shown that P2X7 can regulate the function of various macrophages. For example, P2X7 can regulate the ischemic/hypoxic and inflammatory responses of mice peritoneal macrophages and human macrophages (Faria, 2022; Vargas-Martínez et al., 2020). The expression of P2X7 is highly co-localized with Tumor-associated macrophage (TAMs) (Qin et al., 2020). P2X7 can activate Kupffer cells (resident macrophages in the liver) and play various roles in liver inflammatory response (Toki et al., 2015). P2X7 can also significantly activate inflammatory bodies and induce cell pyroptosis (Sun et al., 2023). Our previous results showed that P2X7 was stimulated by AA, mediating oxidative stress and cell death. Moreover, in this study we found that P2X7 could also mediate AA-induced cell M1 polarization by restricting CD86 expression and promoting CD206 expression. Additionally, P2X7 was effective in blocking AA-induced COX-2 expression. It has been reported that inhibiting P2X7 receptor promotes microglial M2 polarization and suppresses chronic compression injury-induced neuropathic pain (Gui et al., 2020). It is also reported that P2X7 was highly expressed in TAMs and P2X7 deficiency restricted the progression of lung carcinogenesis by reversing M2-like TAM polarization (Qin et al., 2020). Our data were consistent with the reports of microglial polarization that suppression P2X7 could induce M2 polarization. However, the data were contrary to the reports of TAM polarization that P2X7 deficiency impaired the M2 polarization. Therefore, P2X7 plays a vital role in cell polarization, but its regulation on polarization may be variable depending on cell type and stimuli conditions. Unexpectedly, YPS had little inhibitory effect on AA-induced P2X7 although YPS exerted inhibitory effect on AA-induced damage, according to our previous findings. YPS did not inhibit P2X7 expression induced by AA, and YPS alone stimulated P2X7 expression (Wang et al., 2023). It is speculated that the effect of YPS may be through other potential targets such as Toll-like receptor 4 (TLR4) (Li et al., 2017) or more complex signaling pathways. These possibilities still need further research in-depth.
Interestingly, the impact of YPS on cell polarization seems more complicated. YPS alone did not obviously alter cell polarization. However, YPS when cells were simultaneously exposed to AA could promote AA-induced CD86 and CD206 expression. Based on the research results available, when attacked by external AA, cells may easily undergo oxidative stress and M1 polarization. YPS could inhibit the harm of AA by suppressing oxidative stress and inflammation and modulating polarization, ensuring cell survival and function. In particular, YPS upregulated the expressions of both CD86 and CD206 rather than suppressing CD86 expression (M1 polarization index). However, the ratio of CD86 and CD206 level was reduced, indicating that the M1 polarization become slow and M2 polarization become activated. The cells have already begun polarization transformation. In fact, macrophage polarization is a constantly dynamic process in which M1 and M2 macrophage can be transformed under certain conditions. This transformation enables macrophages to maintain homeostasis when microenvironment changes occur (Shapouri Moghaddam et al., 2018; Xu et al., 2013). On the whole, although YPS could not directly limit M1 polarization during the stimulation process of AA, it indeed had a certain impact on the polarization trend of RAW264.7 cells, thereby affecting cell function and homeostasis. According to literature, M1 polarization was stimulated by various polysaccharides including Poria cocos Polysaccharide PCP-1C, Dendrobium officinale polysaccharide, polysaccharides of Brassica rapa L.,Apple polysaccharide,Astragalus polysaccharide RAP, Tremella fuciformis polysaccharide (Guo et al., 2022; Hu et al., 2023; Sun et al., 2020; Wang et al., 2022a; Wei et al., 2019; Xie et al., 2023). However, it is also reported that polysaccharide from Large Yellow Tea (LYP-S3) and Laminaria japonica could promote the M2 polarization of macrophages (Li et al., 2022b; Wang et al., 2022b). Additionally, Ganoderma atrum polysaccharide (PSG-1) can restrict extreme M1 and M2 polarization induced by stimuli, thereby to achieve balance between M1and M2 polarization (Li et al., 2022a). The effect of YPS on polarization seems to be more similar to Ganoderma atrum polysaccharide (PSG-1), which can modulate extreme polarization behavior. Anyway, these results showed that polysaccharide had effects on the behavior modification of macrophage polarization.
YPS could regulate the homeostasis and function of cells, ensuring macrophages more conducive to perform functions, such as the ability to modulate immune responses and kill cancer cells. We found that RAW264.7 culture medium treated with YPS were more effective in inhibiting the growth of HepG2 cells (liver cancer cell line). AA-treated RAW264.7 cells experienced pro-inflammatory reactions with a polarization trend towards M1, but the culture medium from AA-treated RAW264.7 cells hardly kill cancer cells. Meanwhile, RAW264.7 culture medium from YPS and AA co-treatment exerted inhibitory effect on HepG2 cell viability. Although M1 macrophage is the recognized anti-tumor phenotype while M2 is the recognized tumor promoting phenotype, the overall ability of the RAW264.7 cells after AA exposure to kill cancer cells still decreased, even though RAW264.7 cells polarized to the so-called anti-tumor M1 type. This may because that from the existing results, the RAW264.7 polarization is simultaneously accompanied by decreased phagocytic activity and NO secretion, excessive oxidative stress damage, and enhanced programmed cell death (Wang et al., 2023). Excessive inflammatory stimulation may also lead to impaired macrophage function. Therefore, only relying on polarization induction may have limited effectiveness in prohibiting cancer cell growth. When YPS intervened in AA-induced injury, cellular inflammation decreased and polarization patterns were adjusted, which probably indicated that extreme M1 polarization accompanied by low vitality might not effectively control tumors. YPS can maintain a stable degree of polarization of macrophages when stimulated by AA, rather than excessively deviating from the steady state. Moreover, YPS also effectively restored the phagocytic activity of RAW264.7, promoted proliferation, and facilitated cell survival. These conditions may together help RAW264.7 cells release cytokines and other substances that can inhibit cancer cell proliferation. For the treatment of RAW264.7 macrophages with YPS alone, although the polarization did not change significantly, the previous experiments have shown that the phagocytic and proliferative activity of RAW264.7 increased significantly, thereby promoting RAW264.7 cells to release some cytokines that are harmful to the survival of HepG2 cells. It is important to note that the above explanations are only our speculations based on existing results and literature reports. In addition, there are limitations in this study since RAW264.7 cells are not induced tumor-associated macrophages (TAMs) which are closely related with tumor tissues in vivo. The specific and complex mechanisms of YPS as well as acrylamide still require deeper research.
In conclusion, acrylamide can induce M1 polarization in RAW264.7 cells through up-regulating CD86 and COX-2 and down-regulating CD206. Acrylamide-induced cell polarization was mediated by P2X7. Yam polysaccharides could modulate acrylamide-induced RAW264.7 polarization and promote the ability of RAW264.7 cells to kill HepG2 cells.
| Supplementary material | ▴Top |
Figure S1. Effect of acrylamide on polarization of RAW264.7 cells. (a) Effects of acrylamide on CD 86 protein in RAW264.7 cells. (b) Effects of acrylamide on CD 206 protein in RAW264.7 cells. (c) Effects of acrylamide on COX-2 protein in RAW264.7 cells. Cells were treated with different concentrations of acrylamide for 24 h. Protein expressions were analyzed by western blot.
Figure S2. Effect of P2X7 receptor inhibitors on acrylamide-induced polarization of RAW264.7 cells. (a) Effect of P2X7 receptor inhibitors on acrylamide-induced CD 86 protein in RAW264.7 cells. (b) Effect of P2X7 receptor inhibitors on acrylamide-induced CD 206 protein in RAW264.7 cells. (c) Effect of P2X7 receptor inhibitors on acrylamide-induced COX-2 protein in RAW264.7 cells. Cells were treated with acrylamide and P2X7 inhibitor (A 438079) for 24 h. Protein expressions were analyzed by western blot.
Figure S3. Effects of YPS on acrylamide-induced polarization of RAW264.7 cells. (a) Effects of YPS on acrylamide-induced CD 86 protein in RAW264.7 cells. (b) Effects of YPS on acrylamide-induced CD 206 protein in RAW264.7 cells. (c) Effects of YPS on acrylamide-induced COX-2 protein in RAW264.7 cells. Cells were treated with YPS and acrylamide for 24 h. Protein expressions were analyzed by western blot.
Acknowledgments
This work was supported by National innovation and entrepreneurship training program for college students (202110708045), National Natural Science Foundation of China (21707086), the Scientific Research Program Funded by Shaanxi Provincial Education Department (22JK0293), XI’AN Science and Technology Bureau (22NYYF048), Enterprise cooperation projects (210210176), National Foreign Expert Project Funded by the Ministry of Science and Technology of the People’s Republic of China (G2022041012L), the Science and Technology plan of the Weiyang District in Shaanxi Province (202313).
Conflict of interest
The authors declare that there is no conflict of interest.
| References | ▴Top |