| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 22, June 2023, pages 36-42
Influence of self-assembly on the reactive sulfhydryl and antioxidant activity of aggregation-prone ovalbumin-derived peptides
Amanda Clairouxa, Chibuike C Udenigwea, b, *
aDepartment of Chemistry and Biomolecular Sciences, Faculty of Science, University of Ottawa, Ottawa, ON K1N 6N5, Canada
bSchool of Nutrition Sciences, Faculty of Health Sciences, University of Ottawa, Ottawa, ON K1H 8M5, Canada
*Corresponding author: Chibuike C. Udenigwe, School of Nutrition Sciences, Faculty of Health Sciences, University of Ottawa, Ottawa, ON K1H 8M5, Canada. E-mail: cudenigw@uottawa.ca
DOI: 10.31665/JFB.2023.18346
Received: March 22, 2023
Revised received & accepted: March 31, 2023
| Abstract | ▴Top |
Ovalbumin-derived peptides IFYCPIAIM, NIFYCPIAIM and YCPIAIMSA, containing a common region YCPIAIM, were previously identified as aggregation-prone peptides with variable fibril formation. In this study, we elucidated self-assembly mechanisms of the peptides, by determining the influence of self-assembly on sulfhydryl group accessibility. The free sulfhydryl group content and antioxidant capacity results demonstrate that the peptides assemble into β-sheets, possibly involving hydrogen bonding with the sulfhydryl groups. NIFYCPIAIM, IFYCPIAIM and YCPIAIMSA, in decreasing order, had the largest particle size, thioflavin T fluorescence, reactive sulfhydryl group content, and antioxidant activities. This demonstrates that the reactive sulfhydryl group content, which is influenced by the cysteine residue position relative to the N-terminal of the peptide, is dependent on fibrillation. Rheological studies further demonstrated the non-Newtonian shear-thinning behavior of the peptides. The results provide valuable insight on peptide self-assembly, which is imperative for future design of bioactive hydrogels with promising biomechanical properties for biomaterial applications.
Keywords: Peptide self-assembly; Sulfhydryl group; Antioxidants; Bioactive peptides; Biomechanical properties
| 1. Introduction | ▴Top |
Self-assembly is the ability of a molecule to associate through non-covalent interactions to form highly ordered three-dimensional structures. Self-assembly of peptide amphiphiles has been wildly studied due to the potential of producing functional nanostructures of biopharmaceutical or biomedical interest. The self-assembling peptides form secondary structure through intermolecular and intramolecular interactions (Acquah et al., 2018; Li et al., 2022). Aggregation into larger self-assembled structures occurs above the critical aggregation concentration. Vesicles, micelles, and fibrils are examples of self-assembled structures. The self-assembled biomaterials can be used for tissue engineering, drug delivery, and as antimicrobial agents. The self-assembling structure depends on the peptide sequence and length, making self-assembling peptides malleable for generating novel biomaterials (Edwards-Gayle and Hamley, 2017; Fichman and Gazit, 2014).
Hydrogels are three-dimensional cross-linked polymers with the ability to swell in water or gastrointestinal biological fluids and retain large quantities of fluid within their structure (Ho et al., 2022). Peptide hydrogels are formed through a fibrillar network of self-assembling peptides. Some hydrogels involve the formation of β-amyloid-like peptide fibrils that may elongate in three dimensions, leading to increased fibril thickness and length, which ultimately leads to fibrillar network formation. Peptide hydrogels have several potential applications, such as cell-culture scaffolds, controlled release drug-delivery systems, and wound-healing gels (Edwards-Gayle and Hamley, 2017; Fichman and Gazit, 2014).
Bioactive peptides exert a hormone-like effect or physiological benefits due to their native sequence (Chakrabarti et al., 2018). The development of food-derived bioactive peptides is propelled by the wide availability of their protein precursors, such as meat, milk, eggs, microalgae, and plants (Moughan et al., 2014). Bioactive peptides from egg white proteins have been demonstrated to exert some health-related beneficial physiological functions, such as anti-inflammatory, antioxidant, antimicrobial, and antihypertensive activities (Benedé and Molina, 2020; Chang et al., 2018; Rathnapala et al., 2021). As food-derived peptides are generally safe and often possess bioactivities, they represent an excellent material for the formation of peptide hydrogels. To date, the relationship between bioactivity and self-assembly of peptides is unclear, as bioactivity may be related to the peptide sequence or the properties of the fibrillar networks (Edwards-Gayle and Hamley, 2017).
Ovalbumin is the most abundant egg white protein, and several studies indicate its use as nanogels and nanocarriers (Hassan et al., 2011; Yu et al., 2006). This makes ovalbumin the most promising parent egg white protein for the discovery of peptide hydrogels. Our previous study reported the self-assembly and hydrogelation properties of aggregation-prone peptides from pepsin-generated egg white protein hydrolysates (Abioye et al., 2022). The six peptides spontaneously self-assembled in water at room temperature by the anti-solvent method, without physical triggers. The study demonstrated that the ovalbumin peptides formed hydrogels with promising biomechanical properties for biomaterial applications. Out of the six peptides, transmission electron microscopy demonstrated that IFYCPIAIM, NIFYCIPIAIM and YCPIAIMSA possessed similar fibril patterns. These peptides were characterized through the immediate formation of amorphous aggregates, followed by reassembly into fibrillar networks after 24 h. The role of amino acid sequence on peptide self-assembly is evident as the three peptides contain the shared sequence, YCPIAIM. Aggregation propensity is based on the position of specific amino acid residues relative to others. A region in the peptide sequence with five or more consecutive residues without a proline is termed an aggregation “Hot Spot”. The shared sequence of the three peptides is of interest to examine the effects of “Hot Spots” on peptide self-assembly. Furthermore, shared sequence of the three peptides contains a redox-active sulfhydryl group within its cysteine residue.
We propose three possible mechanisms of peptide self-assembly involving the cysteinyl sulfhydryl group. First, the peptides may assemble through hydrogen-bonding as β-sheets where the sulfhydryl groups participate in the β-sheet structure, leading to a compact structure. Second, the peptides may assemble as β-sheets, but the sulfhydryl groups form disulfide bonds. Third, the peptides may assemble as β-sheets, but the sulfhydryl groups remain accessible and do not participate in this structure. It is expected that these mechanisms determine the accessibility, reactivity, and bioactivity of the sulfhydryl group. Therefore, the objective of this study was to evaluate the influence of self-assembly on the reactive sulfhydryl group and antioxidant capacity of the six cysteine-containing ovalbumin peptides.
| 2. Materials and methods | ▴Top |
2.1. Materials
Reduced glutathione (GSH), tris(hydroxymethyl)aminomethane (Tris), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), potassium persulfate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), sodium phosphate, potassium ferricyanide, trichloroacetic acid (TCA), ferric chloride, thioflavin T (ThT), dimethyl sulfoxide (DMSO), glycine, urea, and 5,5′-dithiobis-[2-nitrobenzoic acid] (DNTB) were purchased from MiliporeSigma (Oakville, ON, Canada).
2.2. Peptide synthesis
Three ovalbumin peptides were synthesized and supplied as white powders by Shanghai Bootech BioScience and Technology Co., Ltd. (Shanghai, China) using the microwave fast peptide synthesizer. Purity was determined by the supplier to be >95% after observing the peptide peak by analytical high performance liquid chromatography and mass spectrometry analysis.
2.3. Peptide self-assembly study
Stock solutions of the peptides (20 mM) were prepared in DMSO, as previously reported (Abioye et al., 2022). Peptide solutions at varying concentrations (0.4–8 mM) were prepared by adding the stock solution into Milli-Q water, vortex mixing for 5 s, and leaving the mixture at room temperature for 24 h. The peptide solutions were vortexed before use.
2.4. Thioflavin T assay
Thioflavin T (ThT) fluorescence assay was used to determine the fibrillation of the ovalbumin peptides. Peptide solutions (160 μL of 400 μM) or Milli-Q water was mixed with 40 μL of 50 μM ThT. The solutions were placed on a black 96-well plate with clear bottom. The samples were shaken at 1440 rpm for 10 s using an orbital shaker. A single ThT fluorescence measurement was taken (430 nm excitation, 480 nm emission) at 25 °C. Baseline subtraction was done against the fluorescence of ThT in buffer.
2.5. Dynamic light scattering analysis
The average particle diameter and polydispersity index of the aqueous 400 μM peptide solutions were recorded after 24 h at 21 °C using Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK). Dynamic light scattering effect was analyzed in Milli-Q water (pH 7, refractive index 1.330, viscosity 0.8872 cP and dielectric constant 78.5) with Smoluchowski model at F(ka) 1.50 and backscattered angle of 173°.
2.6. Rheological measurements
Rheological properties of the peptides were determined using the Discovery Hybrid 502 Rheometer HR-2 (TA Instruments Ltd., New Castle, USA) containing a cone (2°, 40 mm diameter, 50 μm truncation gap) and a Peltier plate steel measuring system with a gap height set to 1 mm. The peptide solution (1.1 mL of 400 μM in Milli-Q water) was gently loaded onto the lower testing plate and equilibrated for 5 s at 25 °C. A flow sweep test was done by setting the shear rate from 5 × 10−2 s−1 to 1,000 s−1 to study the flow behavior.
2.7. Free sulfhydryl group analysis
The peptide solution (140 μL) was mixed with 140 μL of 0.1 M Tris-glycine buffer (pH 8.0) containing 7.5 M urea at 40 °C for 30 min. Thereafter, 7 μL of DTNB (4 mg/mL, in Tris-glycine buffer) was added to the mixture and left at room temperature for 10 min. The final concentration of peptide was 400 μM. Absorbance was then measured at 412 nm in a 96-well round bottom plate. Reduced glutathione (GSH; 2%, v/v) was used as a positive control, while 2% (v/v) DMSO was used as a negative control. Free sulfhydryl group content was determined as follows (Udenigwe et al., 2016):
2.8. ABTS radical scavenging assay
ABTS stock solutions included 7.4 mM ABTS solution and 2.6 mM potassium persulphate solution. The working solution was prepared by mixing the two stock solutions in equal amounts for 12 h at room temperature in the dark. Afterwards, the ABTS solution was mixed with methanol until the absorbance reached at 734 nm was 1.1 ± 0.2 units. Fresh working solutions were prepared for each assay. In a 96-well round bottom plate, 190 μL of working ABTS solution was pipetted into 10 μL of peptide solutions, for a final concentration of 400 μM. The samples were incubated at room temperature in the dark for 10 min, and the absorbance was measured at 734 nm. A standard curve of 0–2% (v/v) GSH in 2% (v/v) DMSO was used as positive control, while 2% (v/v) DMSO was used as a negative control. The ABTS radical scavenging activity (%) was calculated as follows (Girgih et al., 2011):
A0 is the absorbance of the negative control and is the absorbance of the sample assay. ABTS radical scavenging activity was then represented as micromolar equivalents of GSH/g peptide.
2.9. DPPH radical scavenging assay
DPPH solution (0.15 mM in methanol) was kept in the dark prior to use. Peptide solutions and DPPH were mixed in equal amounts in a 96-well round bottom plate, to a final peptide concentration of 400 μM. The samples were kept in the dark for 30 min at room temperature and the absorbance determined at 517 nm. A standard curve of 0–2% (v/v) GSH in 2% (v/v) DMSO was used as positive control, while 2% (v/v) DMSO was used as a negative control. DPPH scavenging activity was calculated using eq. 2 and was then represented as micromolar equivalents of GSH/g peptide (Girgih et al., 2011).
2.10. Ferric reducing antioxidant power (FRAP)
Peptide solutions (100 μL), 100 μL of 0.2 M sodium phosphate buffer (pH 6.6) and 100 μL 1% potassium ferricyanide were mixed and left at 50 °C for 20 min. The final concentration of the peptides was 400 μM. After incubation, 100 μL of 10% (w/v) aqueous TCA was added. Ferric chloride (50 μL of 0.1%) was diluted in 200 μL water and 250 μL aliquots of peptide/TCA were added into the ferric chloride solutions and incubated at room temperature for 10 min. The samples were centrifuged at 1,000 ×g for 5 min at 4 °C, and the absorbances of the supernatants were read in a 96-well round bottom plate at 700 nm. A standard curve of 0–2% (v/v) GSH in 2% (v/v) DMSO was used as positive control, while 2% (v/v) DMSO was used as a negative control. Ferric reducing antioxidant power was represented as micromolar equivalents of GSH/g peptide (Udenigwe et al., 2016).
2.11. Statistical analysis
Statistical analysis was performed using one-way analysis of variance with jamovi program (Version 2.3; The jamovi project). Significant values between the mean values were defined at p < 0.05 using post-hoc Tukey test.
| 3. Results and discussion | ▴Top |
3.1. Self-assembly of the ovalbumin peptides
Our previous study reported the self-assembly and hydrogelation of six aggregation-prone regions of ovalbumin at 40 μM (Abioye et al., 2022). Three of the peptides demonstrated more self-assembly into fibrils at 400 μM compared to 40 μM, based on ThT fluorescence results (Figure 1a). In addition, transmission electron microscopy at 40 μM showed the absence of fibrillation at 0 h and the presence of extensive fibrillation at 24 h. Finally, the particle sizes of the self-assembled IFYCPIAIM and YCPIAIMSA remained the same after 24 h (120.1 and 53.8 nm), while that of NIFYCPIAIM increased to 5611.3 nm after 24 h (Abioye et al., 2022). This led to further study of the three self-assembling peptides at 400 μM after 24 h.
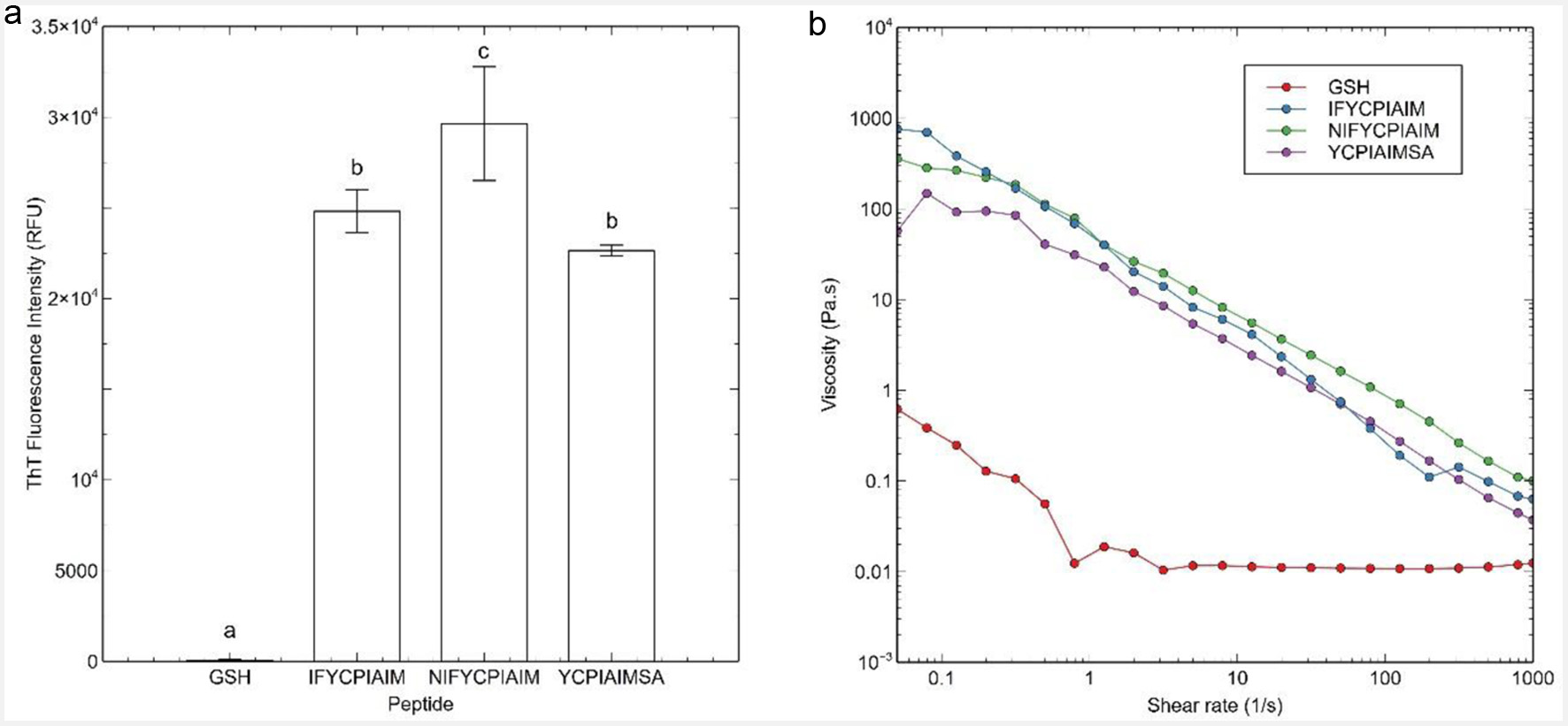 Click for large image | Figure 1. (a) Thioflavin T fluorescence data, and (b) flow sweep experiment of viscosity as a function of shear rate response of the ovalbumin-derived peptides and glutathione (GSH) at 400 μM after 24 h. Different lowercase letters represent significant differences at the p < 0.05 level. |
The three peptides produced mostly a β-sheet structure after self-assembly at 40 μM, based on circular dichroism (Abioye et al., 2022). ThT assay is also commonly used to measure the concentration of β-amyloid-like fibrils present in solution. Based on ThT assay, peptides IFYCPIAIM, NIFYCPIAIM and YCPIAIMSA had high relative fluorescence while the negative control, reduced glutathione, had a very low ThT fluorescence (Figure 1a). The large ThT fluorescence of the three peptides, compared to reduced glutathione, indicates that the peptides self-assembled into fibrils at 400 μM after 24 h.
Biomechanical properties of the self-assembling peptides were evaluated through a flow sweep. IFYCPIAIM, NIFYCPIAIM and YCPIAIMSA were observed to be shear-thinning fluids (Figure 1b). Shear-thinning behavior confirms that self-assembly and fibrillation occurred at 400 μM after 24 h, as hydrogels are generally non-Newtonian fluids with shear-thinning behavior (Stojkov et al., 2021).
3.2. Effect of self-assembly on free sulfhydryl group (FSG)
The three ovalbumin peptides contain the common sequence YCPIAIM, which contains a sulfhydryl group. The thiols can interact during self-assembly to form disulfide bonds. Alternatively, these thiols can also remain accessible, thus participating as reducing agents against free radicals and reactive oxygen species. The possible self-assembly mechanism was evaluated based on the influence of self-assembly on sulfhydryl group accessibility.
The accessibility of the sulfhydryl groups of the self-assembling peptides was determined as the free sulfhydryl group content (FSG). As shown in Figure 2, IFYCPIAIM, NIFYCPIAIM and YCPIAIMSA contained over 90% less FSG (131.52 ± 9.94, 157.40 ± 8.02 and 52.81 ± 0.80 μM SH/g, respectively) compared to equal amount of GSH (2308.22 μM SH/g). Moreover, IFYCPIAIM and NIFYCPIAIM have 123 and 167% higher FSG compared to YCPIAIMSA (Figure 2). As GSH and the ovalbumin peptides each contain a cysteine residue with a free sulfhydryl group, the decrease in FSG suggests that the sulfhydryl groups in the self-assembling peptides are either sterically or chemically inaccessible. Lower FSG could reflect the formation of disulfide bonds in the self-assembling peptides. However, it is likely that the sulfhydryl groups of the peptides remain free but are sterically inaccessible due to their participation in hydrogen bonding during self-assembly.
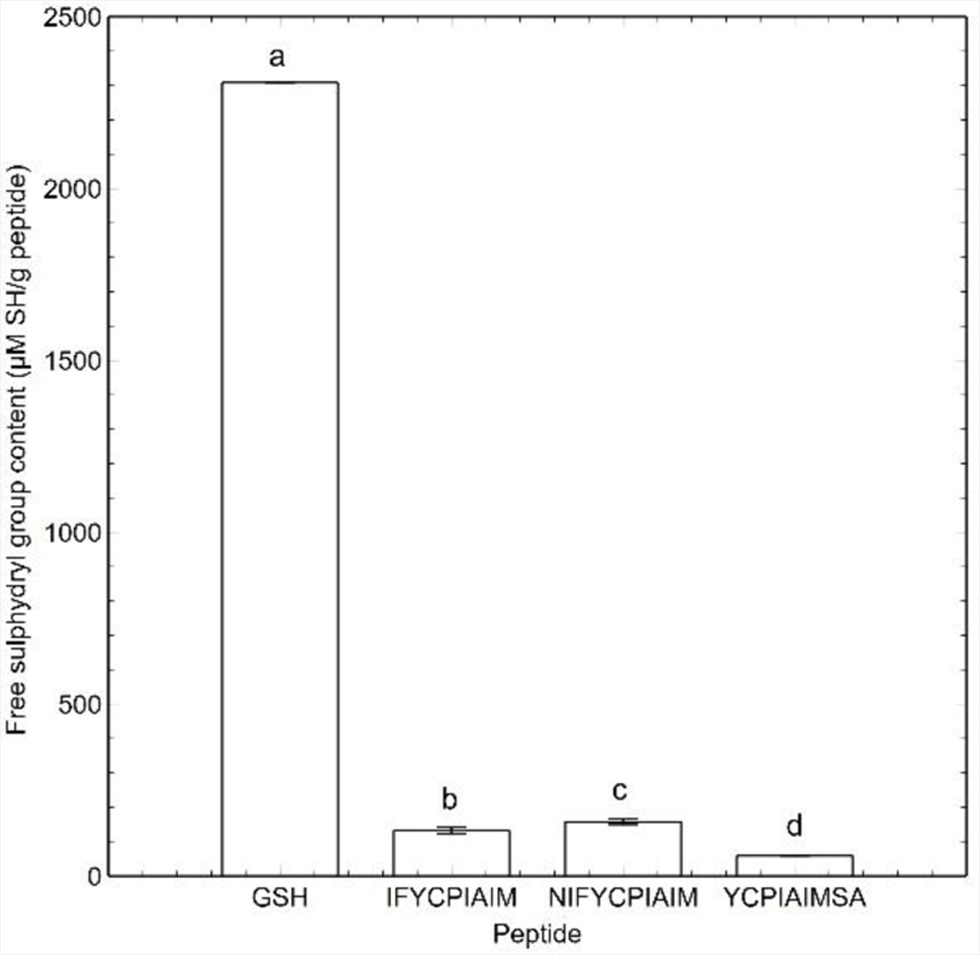 Click for large image | Figure 2. Free sulfhydryl group content of self-assembling ovalbumin peptides and reduced glutathione at 400 μM. Different lowercase letters represent significant differences at the p < 0.05 level. |
Furthermore, FSG follows the position of the cysteine residue in the sequence of the self-assembling peptides. The FSG seems to increase the farther the sulfhydryl group is located from the N-terminus. If the sulfhydryl groups are indeed sterically inaccessible, this trend suggests that the closer the sulfhydryl group is to the middle of the peptide sequence, the less likely it participates in self-assembly.
3.3. Particle characteristics of the self-assembled peptides
The particle size is a measure of fibril size of the self-assembling peptides; hence, particle size increases as fibrillation progresses. The three self-assembling peptides had varying particle sizes, with IFYCPIAIM and NIFYCPIAIM showing 20- and 33.5-fold higher particle size compared to YCPIAIMSA (Figure 3a). Notably, the particle size follows the same trend as the FSG. An increase in particle size is expected with the formation of disulfide bonds. This would be applicable for peptides that contain the lowest FSG, such as YCPIAIMSA. However, YCPIAIMSA contained the lowest particle size. This suggests that disulfide bonds did not participate in the self-assembly process and the FSG is more reflective on the steric accessibility of the sulfhydryl groups. A plausible mechanism is that the self-assembling peptides form hydrogen bonds involving their sulfhydryl groups, which leads to a decrease in space between the peptides, leading to smaller particle sizes. In addition, the ThT fluorescence results (Figure 1a) follow the same trend as the FSG, with IFYCPIAIM and NIFYCPIAIM showing 10 and 31% higher fluorescence intensity compared to YCPIAIMSA. Although the sulfhydryl group is not necessarily involved in fibrillation, the FSG may have been affected due to proximity of the self-assembling peptides.
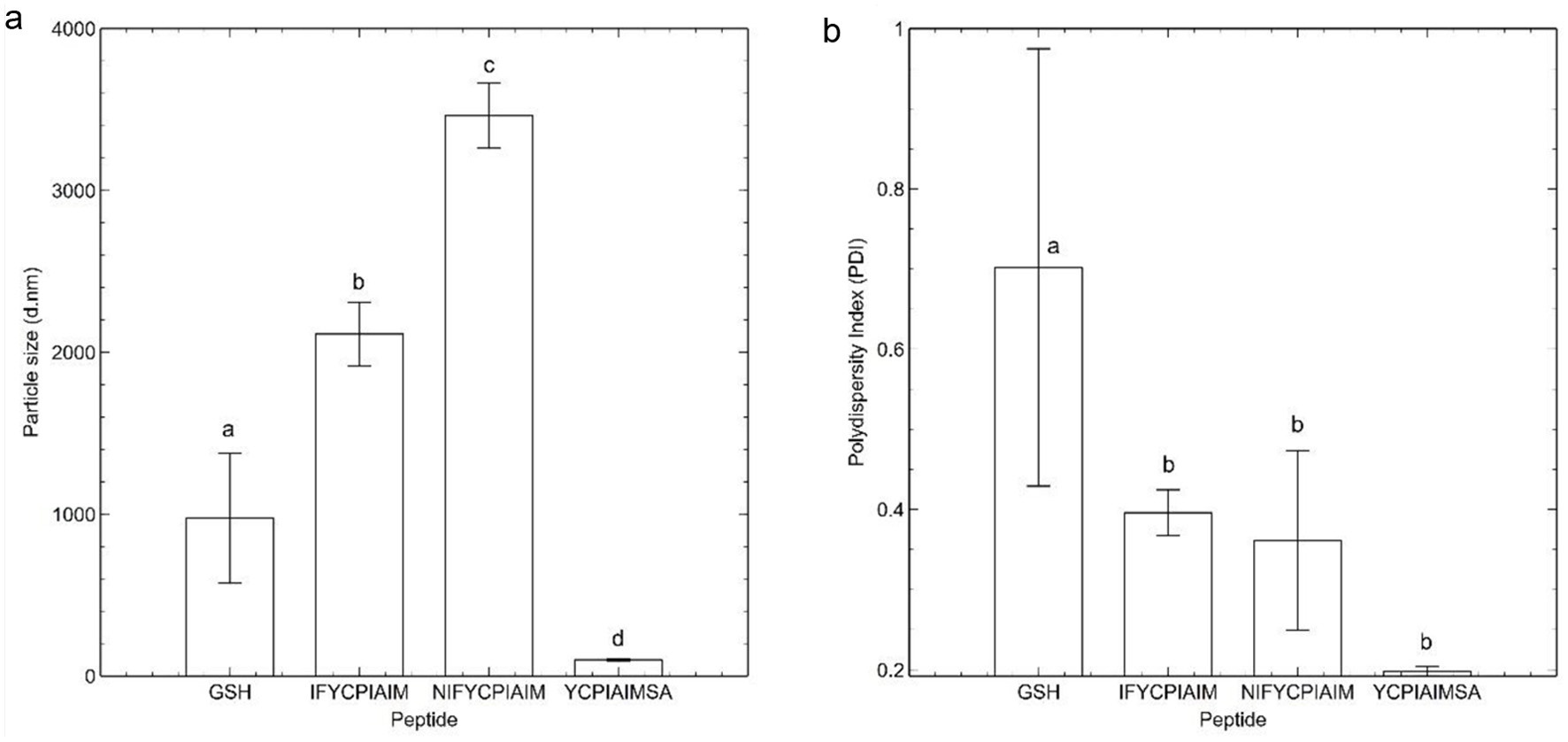 Click for large image | Figure 3. Particle characteristics of self-assembling ovalbumin peptides and reduced glutathione at 400 μM. (a) Average particle size (nm) and (b) polydispersity index of the peptide fibrils. Different lowercase letters represent significant differences at the p < 0.05 level. |
The polydispersity index (PDI) is a measure of the size distribution of fibrils in solution. The measured PDI of IFYCPIAIM, NIFYCPIAIM and YCPIAIMSA were 0.396 ± 0.028, 0.3613 ± 0.1121 and 0.198 ± 0.006, respectively (Figure 3b). IFYCPIAIM and NIFYCPIAIM had 100 and 82% higher PDI compared to YCPIAIMSA. PDI value < 0.3 is indicative of homogenous size distributions. Thus, the low PDI values of the self-assembled peptides especially YCPIAIMSA, compared to that of glutathione, suggests the homogeneity and structural stability of the fibrils after 24 h at room temperature. The polydispersity index did not follow any pattern with the FSG or particle size.
3.4. Antioxidant activities of the self-assembled peptides
The sulfhydryl group of the shared sequence YCPIAIM can participate in antioxidant reactions. Antioxidant assays were performed to confirm the influence of peptide self-assembly on sulfhydryl accessibility and reactivity. The antioxidant assays also served to determine if the fibrils formed by the self-assembling peptides possess antioxidant activity. Hydrogels with antioxidant activity can be used for biomedical applications, such as wound-healing gels (Ho et al., 2022; Xiong et al., 2022). FRAP and DPPH radical scavenging assays were used to assess the reducing capacity of the self-assembled peptides. Free sulfhydryl groups of cysteinyl residues of peptides can donate electrons to free radicals to exhibit antioxidant activity. As shown in Figure 4a, the three self-assembled peptides had ferric reducing capacity. However, YCPIAIMSA showed the lowest FRAP, which was 2.3- and 2.6-folds lower than the FRAP observed for IFYCPIAIM and NIFYCIPIAIM, respectively.
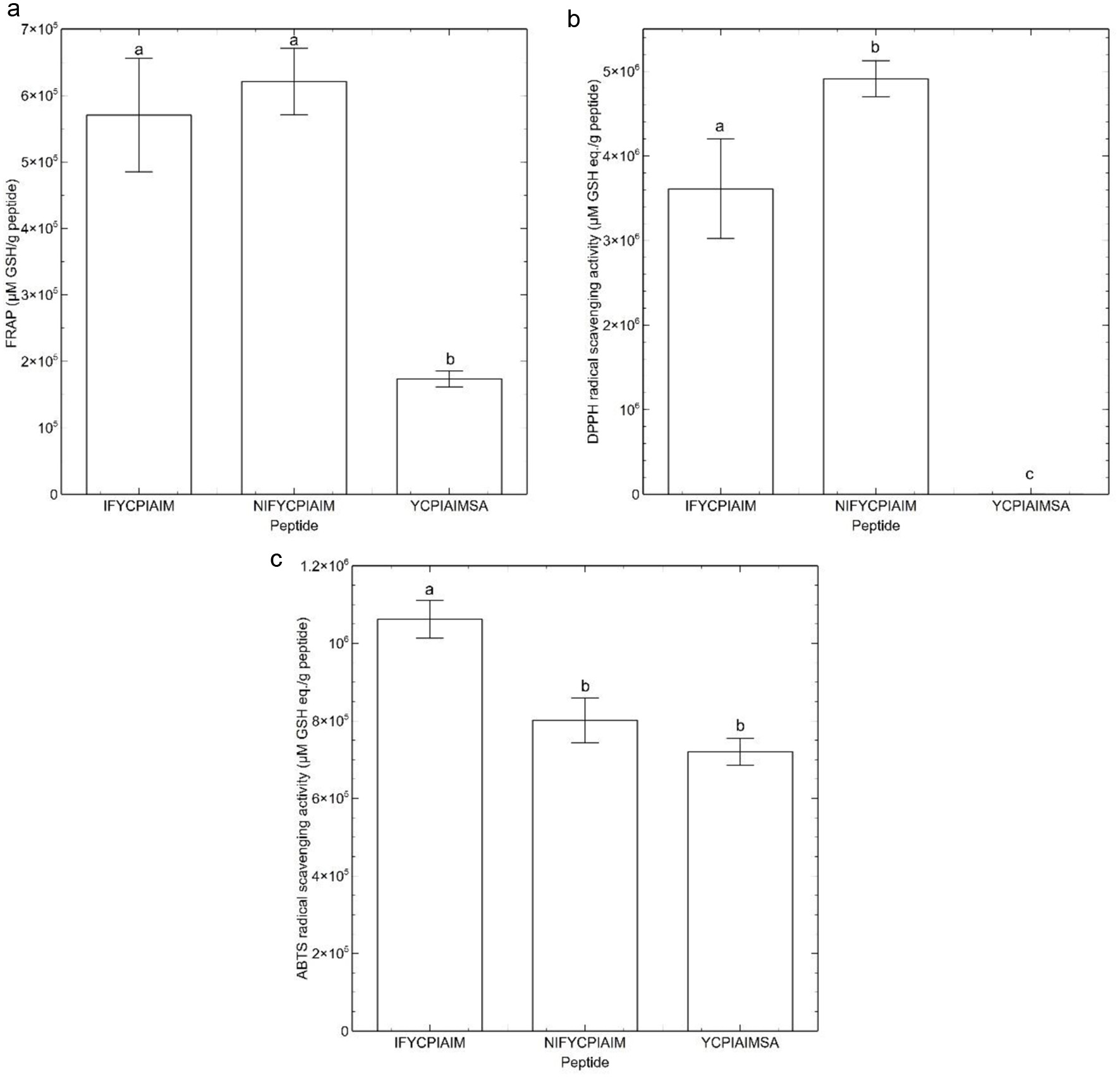 Click for large image | Figure 4. Antioxidant assays of the self-assembling peptides. (a) Ferric Reducing Antioxidant Power (FRAP) (b) DPPH radical scavenging activity and (c) ABTS radical scavenging activity. Different lowercase letters represent significant differences at the p < 0.05 level. |
In contrast, DPPH radical scavenging activity based on glutathione equivalent was not apparent for YCPIAIMSA (Figure 4b) but it still scavenged ∼30% DPPH radical at 400 μM. The other two peptides had significantly higher DPPH scavenging activity with NIFYCIPIAIM exhibiting 36% higher activity than IFYCPIAIM (Figure 4b). Notably, the antioxidant activity of the self-assembled ovalbumin peptides followed the same trend as the FSG. Higher FSG indicates the presence of more electrons available to reduce Fe3+ or DPPH•+. This confirms that the sulfhydryl groups of IFYCPIAIM and NIFYCPIAIM may participate in hydrogen bonding but are still available to play antioxidative role. Although a strong redox-active residue, the sulfhydryl mechanism does not preclude the potential involvement of other residues in the overall antioxidant activity of the peptides.
For the ABTS radical scavenging assay, a decreased absorbance indicates radical scavenging activity. As shown in Figure 4c, YCPIAIMSA had the lowest ABTS radical scavenging activity followed by NIFYCIPIAIM and then IFYCPIAIM. The ABTS radical scavenging activities do not correlate with the FSG or the other antioxidant activities. It is likely that, in addition to the sulfhydryl mechanism, trapping of the ABTS cation radical occurred at the phenylalanine and tyrosine residues of the peptides. Therefore, radical trapping and reducing activity may have jointly contributed to the ABTS radical scavenging activities. It is more likely that radical trapping occurred for ABTS cation rather than DPPH as the free radical in the latter is surrounded by three phenyl groups, making it more difficult to access by the peptide fibrils due to steric effect. The ABTS cation, however, contains an accessible free radical to facilitate the formation of covalent bonds with the antioxidant peptides.
| 4. Conclusion | ▴Top |
In this study, we investigated the possible self-assembly mechanisms of three ovalbumin-derived peptides, by determining the influence of self-assembly on sulfhydryl group accessibility and reactivity. Peptide NIFYCPIAIM followed by IFYCPIAIM possessed the highest free sulfhydryl group content. Notably, the amino acid sequence of the peptides may have influenced the free sulfhydryl group content, particle size, and antioxidant capacities of the fibrils. Specifically, peptides with cysteine residue located nearer to the N-terminal of the peptide sequence had less fibrillation. Furthermore, antioxidant assays demonstrated that the three self-assembling ovalbumin peptides exhibited antioxidant capacities as fibrils. This finding could be further explored to evaluate if the peptides can be applied as antioxidant wound-healing hydrogels. To elucidate the relationship between bioactivity and self-assembly of the peptides, our findings indicated that antioxidant activity may be related to the peptide sequence or the physicochemical properties of the fibrillar networks. Taken together, findings from this study are essential to understand the self-assembly mechanisms towards the design of bioactive peptide hydrogels with promising biomechanical properties for biomaterial applications.
Acknowledgments
Authors acknowledge the financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC), reference number RGPIN-2018-06839, and the University Research Chairs Program of the University of Ottawa, Canada.
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
| References | ▴Top |