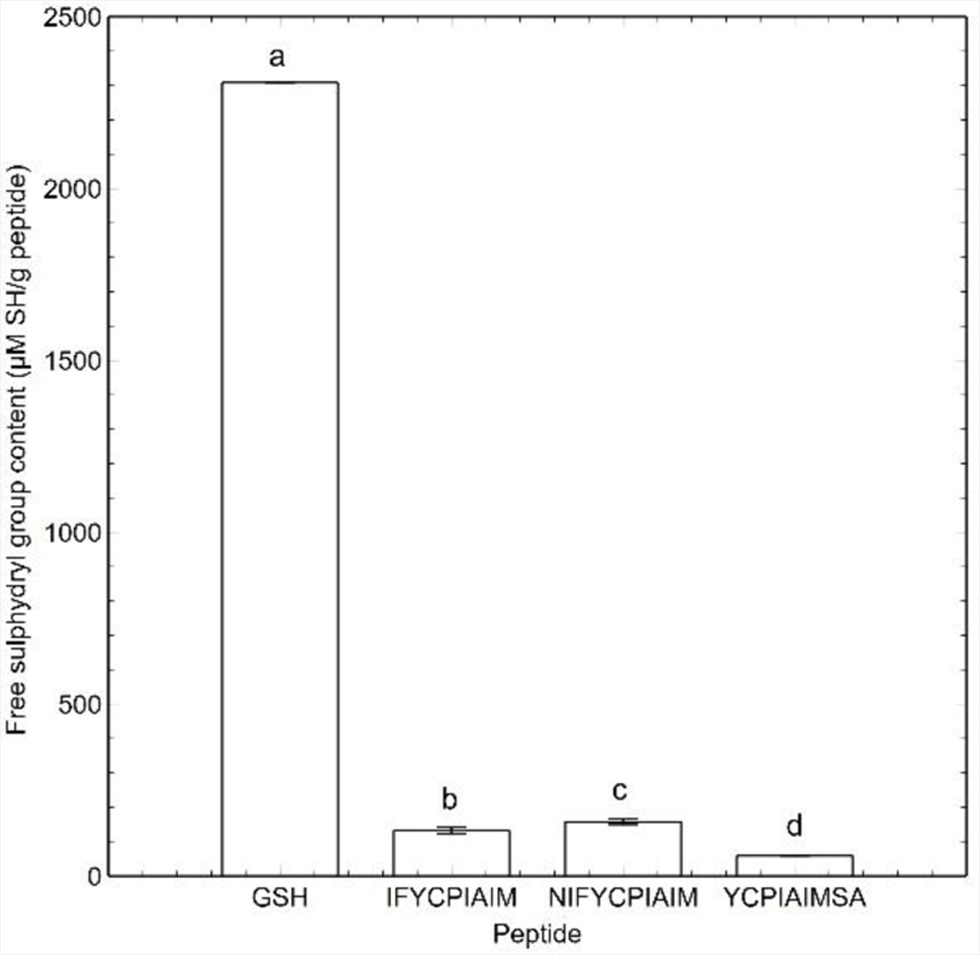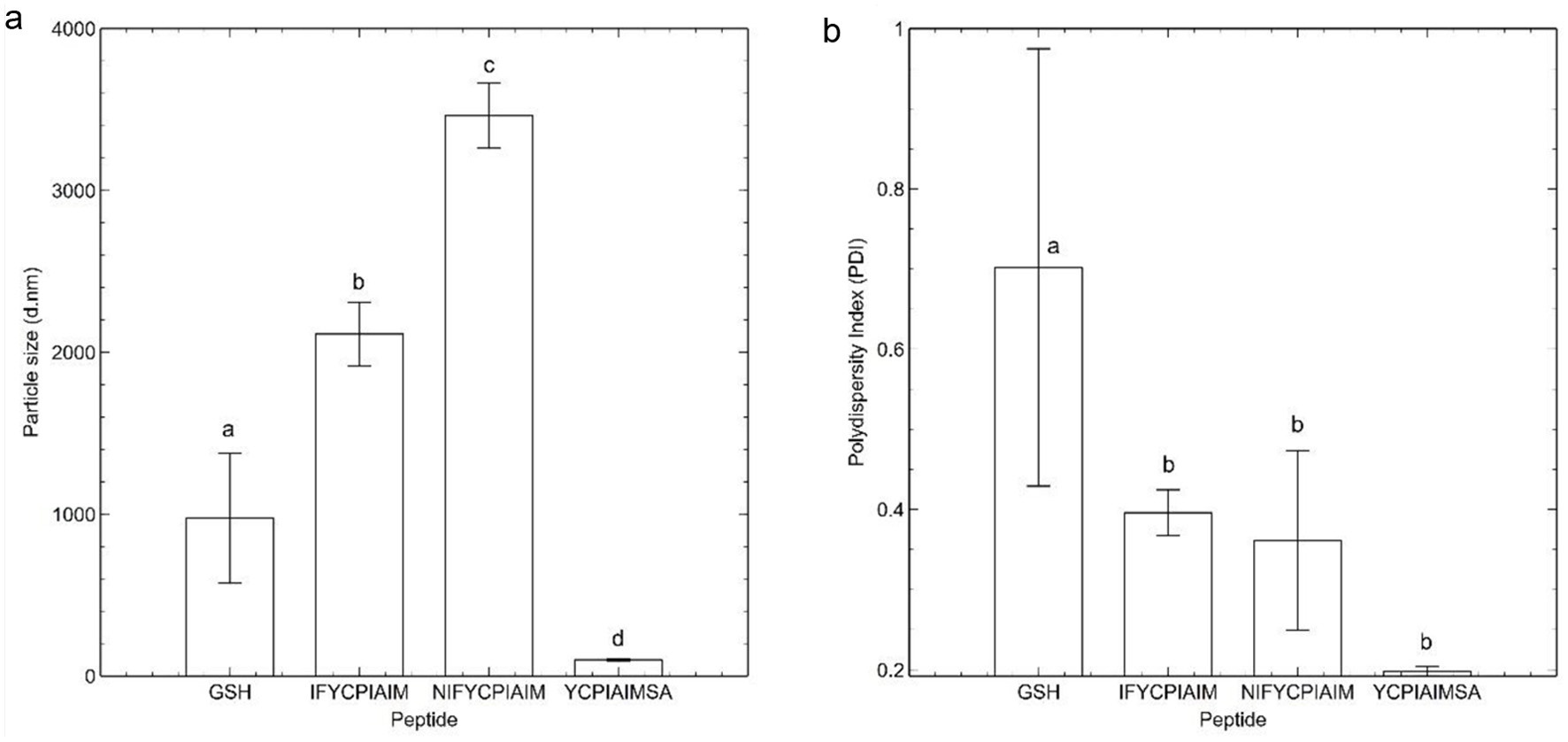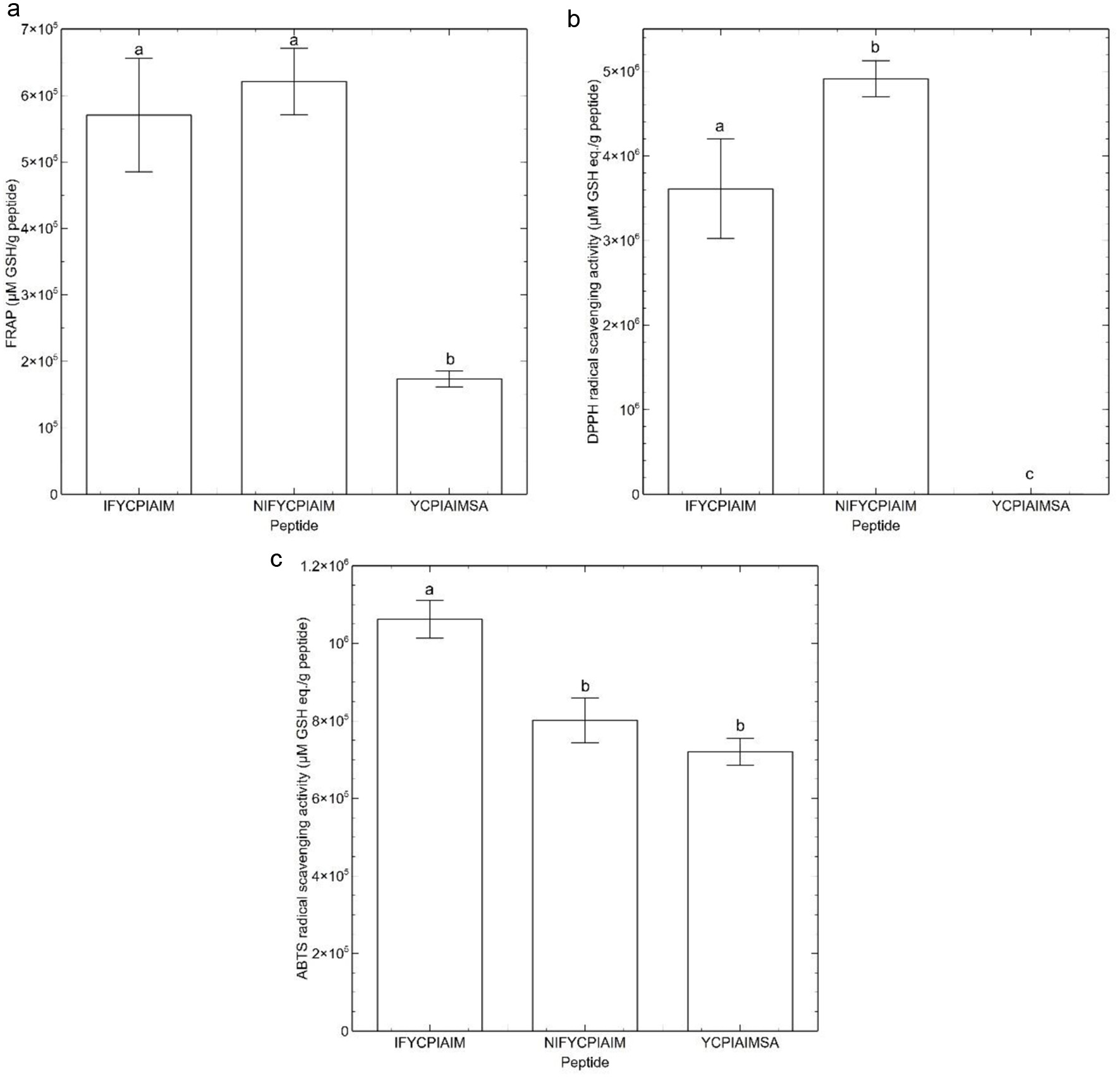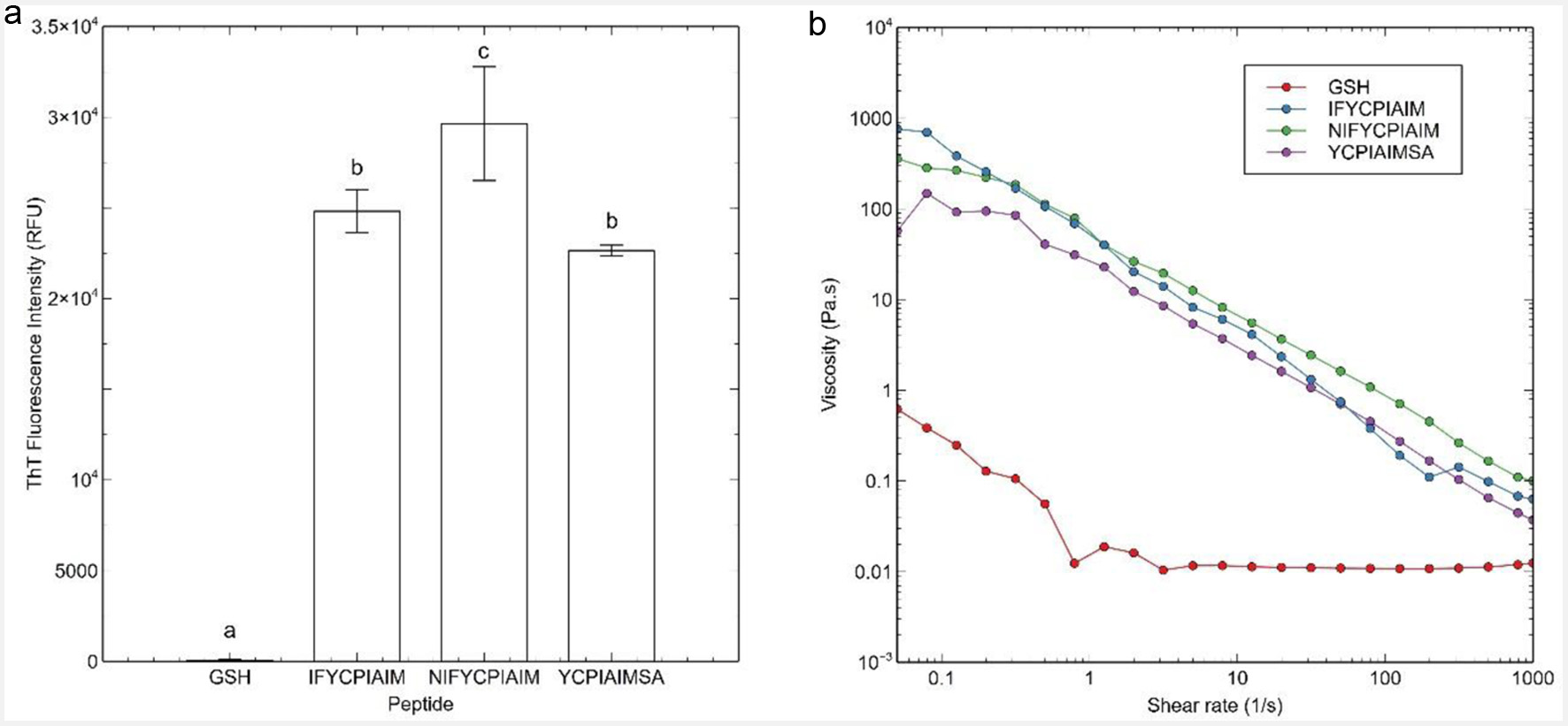
Figure 1. (a) Thioflavin T fluorescence data, and (b) flow sweep experiment of viscosity as a function of shear rate response of the ovalbumin-derived peptides and glutathione (GSH) at 400 μM after 24 h. Different lowercase letters represent significant differences at the p < 0.05 level.