| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 22, June 2023, pages 27-35
Study on antioxidant activity of Jujube seed oil
Fan Yang, Liang Bai*, Yong-Qing Tao
Tianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China
*Corresponding author: Liang Bai, Tianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China. E-mail: bailiang2022@tjcu.edu.cn
DOI: 10.31665/JFB.2023.18349
Received: March 16, 2023
Revised received & accepted: March 30, 2023
| Abstract | ▴Top |
Jujube seed oil is pressed by Ziziphi Spinosae Semen and widely cultivated in China with health-promoting property. Here, we focused on the components and antioxidant activity of Jujube seed oil. The pressed oil components were evaluated by GC-MS and its antioxidant activity was evaluated by free radical scavenging and Saccharomyces cerevisiae model. The results of GC-MS demonstrated that alkenes (e.g., d-limonene and α-farnesene) were the richest components of jujube seed oil. The experiments in vitro and in vivo suggested that jujube seed oil possessed good antioxidant capacity. The Jujube seed oil counteracted oxidative stress induced by lipid peroxidation and intracellular oxidation in Saccharomyces cerevisiae. Furthermore, experiments performed on mutant strains of redox molecules showed that the prevention of jujube seed oil against lipid peroxidation might be related to catalase encoded by the cytosolic catalase T (CTT1) gene. Taken together, our current data highlight the antioxidant capacity of jujube seed oil. Interestingly, the ctt1 activity is vital in the mechanism associated with the antioxidant of jujube seed oil.
Keywords: Jujube seed oil; Antioxidant; Gas chromatograph-mass spectrometer; Saccharomyces cerevisiae; Cytosolic catalase T
| 1. Introduction | ▴Top |
Ziziphus jujuba Mill. var. spinosa (Jujube) is widely cultivated in the world (Gao et al., 2013). The seeds of Ziziphi Spinosae Semen (Hu ex H. F. Chou) are an edible medicinal food reported by the Chinese Pharmacopoeia. Its health effects include nourishing the heart, benefiting the liver, and calming the nerves. Moreover, large amounts of research have demonstrated that jujube has antioxidant, anti-inflammatory, antihypertension, antiaging and anticancer and the potential for improving learning and memory, due to the abundant natural bioactive found in jujube (He et al., 2020; Xu et al., 2019).
Functional food oils contain a variety of active ingredients such as phytosterols, phospholipids, and polyunsaturated fatty acids (Wang et al., 2020). Pressed edible oil is a kind of functional food oil and commonly used for human daily consumption (Drozlowska et al., 2020). Specifically, pressed oil could replace daily used edible oil to reduce diet-induced obesity (Shen et al., 2020). The pressed jujube seed oil can be used in daily consumption, which is promising for healthy drinks and food development.
A group of unstable molecules, i.e. reactive oxygen species (ROS) are produced in the process of oxidation, including hydroxyl radical (OH−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and superoxide (O2−) (Yang and Lian, 2020). Under immune defense or pathological conditions, a relatively high level of ROS can cause oxidative damage or induce apoptosis. Various functions of ROS in biological systems may affect each lesion from different aspects, such as causing inflammation, nervous system diseases, diabetes, cardiovascular, and cerebrovascular diseases (Kaludercic and Di Lisa, 2020; Li et al., 2021; Moloney and Cotter, 2018).
Saccharomyces cerevisiae (S. cerevisiae), a powerful model organism, is often used to study fundamental aspects of eukaryotic cell biology (Duina et al., 2014). Yeast genes have high homology with the genes involved in human genetic diseases. Understanding the physiological functions of these genes and their encoded proteins and interactions can greatly help us effectively prevent and treat diseases (Walker et al., 2004). Some previous studies have found that jujube seed has a certain antioxidant effect, but the key enzymes involved in this effect are still uncertain ( Li et al., 2021). Herein, we used different defective strains to preliminarily explore the antioxidant mechanism of jujube seed oil. In this study, the wild type of S. cerevisiae and its homologous gene-deficient strains SOD1, CTT1, GSH1, GTT1, and GTT2 (Figure 1) were used to test the antioxidant activity of jujube seed oil in vivo under H2O2 and CCl4 oxidative stress, to explore the antioxidant activity of jujube seed oil in yeast (Figure 2). We wish to lay a foundation for the development of jujube oil related functional foods and healthcare products.
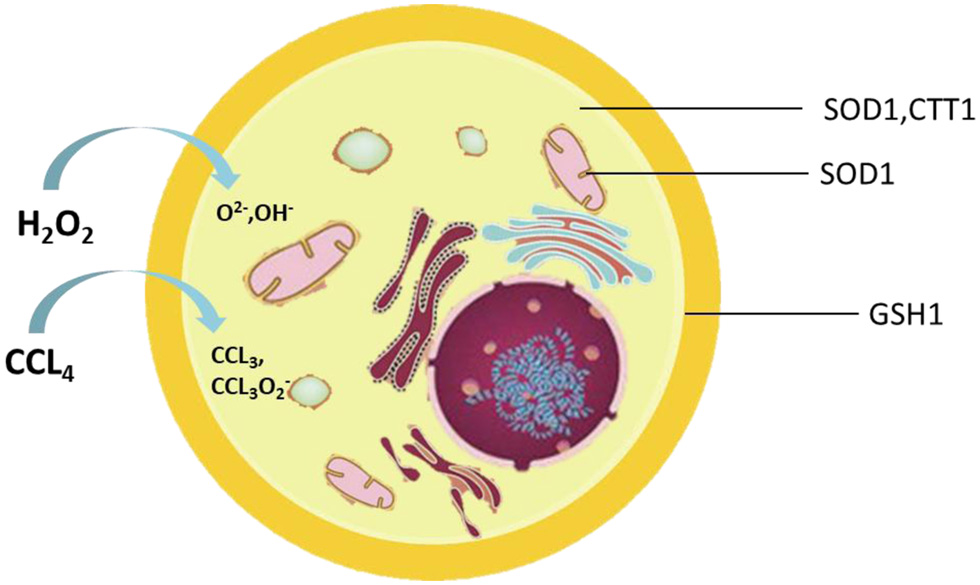 Click for large image | Figure 1. Location of enzymes associated with oxidative stress in the cell. |
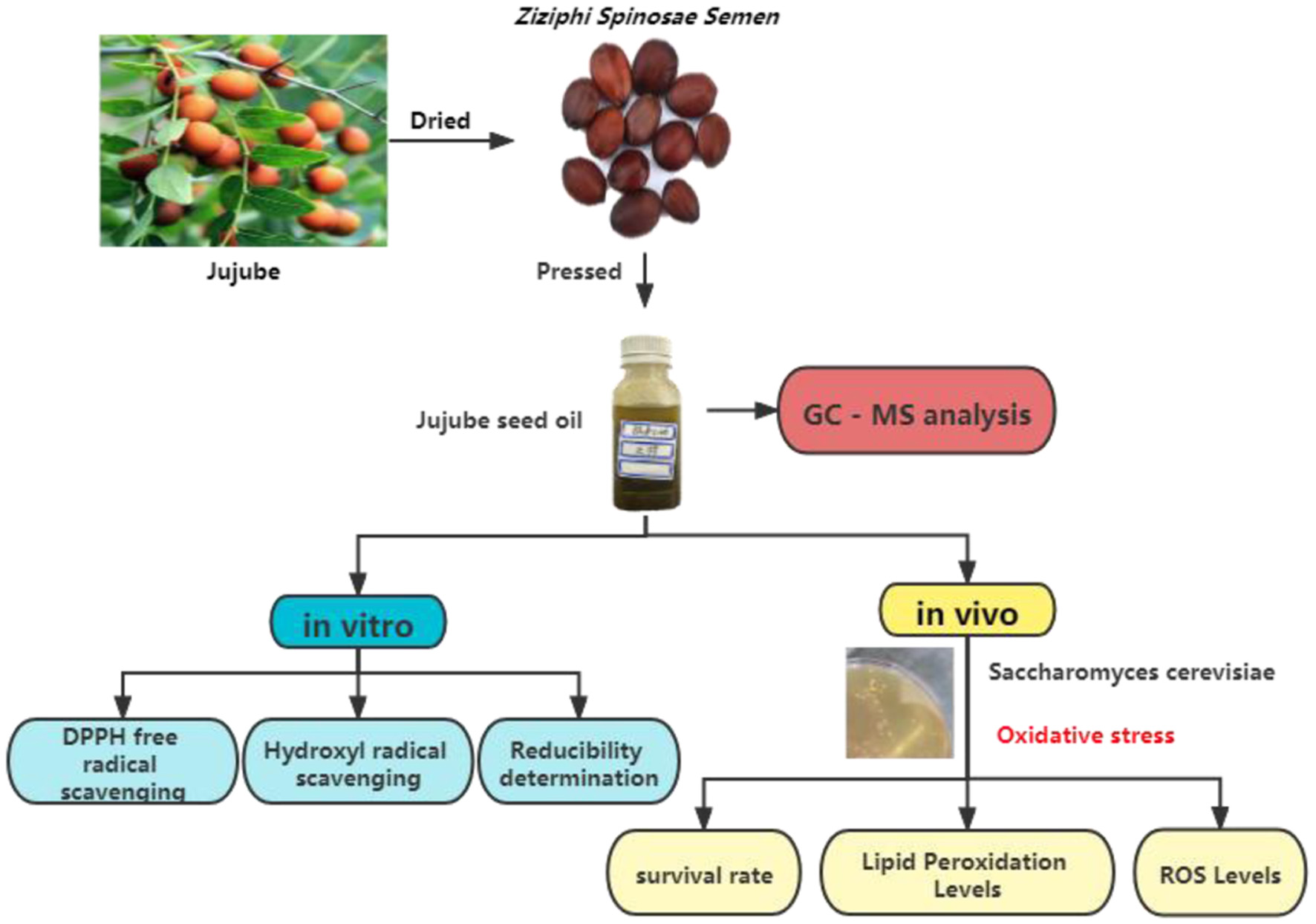 Click for large image | Figure 2. Technical route of this study. |
| 2. Materials and methods | ▴Top |
2.1. Strains, media, and growth conditions
Wild-type Saccharomyces cerevisiae BY4741 strain was stocked in our laboratory and its homologous redox gene-deficient strains, gtt1Δ, gtt2Δ, sod1Δ, ctt1Δ, and gsh1Δ, were gifted by Professor Marcos D Pereira of Universidade de Caxias do Sul. The strains were stored in a solid yeast medium (1% yeast extract, 2% glucose, 2% peptone, and 2% agar), and the yeast cells were cultured in liquid YPD (1% yeast extract, 2% glucose, 2% peptone) with orbital shaker (Aosheng Company, Hangzhou, Zhejiang Province, China) at 28 °C/160 rpm.
2.2. Sample source chemical reagents
Jujube seed oil was pressed from Hemu Liyuan Agricultural Technology Co., Ltd. (Baoding, Hebei province, China) and provided by Dr. Bo Zhang (Wang et al., 2020). Glass beads were washed with acid and purchased from Solarbio Technology Co., Ltd. (Beijing, China); 1,1-diphenyl-2-picrylhydrazyl (DPPH) was purchased from Zhongsheng Ruitai Technology Co., Ltd. (Beijing, China); ascorbic acid (VC), 2,3,4-dichloroacetic acid (analytical pure) and trichloroacetic acid (99%) were purchased from Kelong Chemical Reagent Factory (Chengdu, China); 6-di-tert-butyl-p-cresol (BHT), potassium ferricyanide, ferric chloride, salicylic acid, ferrous sulfate, ethylenediamine tetraacetic acid (EDTA), H2O2, CCl4, CdSO4, and other reagents were all analytical pure grade and also purchased from Kelong Chemical Reagent Factory. Dimethyl sulfoxide (DMSO), yeast extract, and peptone were purchased from Oxoid Company (Basingstoke, UK). Glucose was purchased from Amresco (Solon, OH, USA).
2.3. GC-MS Analysis
GC-MS analysis of the jujube seed oil was performed on a Trace1310, TSQ 8000 Evo GC-MS (Thermo Fisher Scientific Inc., MA, USA) equipped with a capillary column (Thermo TR-5MS, 30 m × 0.25 mm × 0.25μm). The temperature was programmed from 50 raised to 200 °C by a gradient of 3 °C/min, from 200 to 280 °C at a rate of 20 °C/min, and subsequently maintained at 10 min. The helium was the carrier gas at a flow rate of 1 mL/min. The shunt ratio was 60:1, and the solvent delay was 3 min. Ionization energy was 70 eV, ion source temperature 230 °C, interface temperature 250 °C, and mass scanning range was 50–300 m/z.
2.4. Determination of reducing power
A sample solution (2.0 mL) with different concentrations was added to a 2.0 mL of phosphate buffer (pH 6.6 0.2 mol/L) and potassium ferricyanide solution (0.01g/mL), mixed well, and then placed in a water bath at 50 °C for 20 min. Then 2.0 mL of trichloroacetic acid (0.1 g / mL) was added. after centrifugation, 2.5 mL of supernatant was mixed with 2.5 mL distilled water and 0.5 mL ferric chloride solution (0.1 g/mL). After standing for 10 min, the absorbance was measured at the wavelength of 700 nm. VC was used as a positive control for the measurement in triplicate.
2.5. Ability tato scavenge DPPH radicals
Each of 2.0 mL sample solution at different concentrations was mixed with 2 mL of DPPH solution (1.0 × 10−4 mg/mL), then stored in darkness for 0.5 h. With absolute ethanol as a reference and at the wavelength of 517 nm, the absorbance A of 2 mL sample solution was measured, and the absorbance A0 of 2 mL of absolute ethanol were determined. The absorbance of the mixture of DPPH solution and 2 mL absolute ethanol at 517 nm wavelength was defined as A1. VC and BHT were used as the positive controls, and all experiments were carried out in triplicate. DPPH radical scavenging rate was calculated according to the following formula:
2.6. Ability to scavenge hydroxyl radical
Two mL ferrous sulfate solution (5 mmol/mL) and 2 mL of salicylic acid (5 mmol/mL) solution were mixed well followed by the addition of 2.0 mL each of different concentrations of sample solution, then mixed well. Finally, 2 mL H2O2 solution was added. After reaction for one h. at 37 °C, the absorbance A1 was measured at 517nm. The absorbance A0 was measured with water instead of the sample solution, and the absorbance A2 was determined with water instead of hydrogen peroxide solution. VC and BHT were used as the positive controls. Hydroxyl radical scavenging rate was calculated according to the following formula:
2.7. Toxicity determination
Yeast cells were inoculated in liquid medium overnight. Yeast cells were diluted appropriately. The sample solution was added and pretreated for 4 h, and then inoculated in a solid medium. The medium was incubated at 28 °C for 72 h and then the colony was counts. The toxicity was expressed as a percentage of survival.
2.8. Tolerance during oxidative stress
Yeast cells were inoculated in liquid medium for overnight culture. The yeast cells were diluted appropriately. The sample solution was added and pretreated for 4 h. Then the yeast cells were directly exposed to H2O2 (2.0 mM) and CCl4 (10 mM) for 1 h. The yeast cells were inoculated in solid medium. The medium was incubated at 28 °C for 72 h and then the colony was counted. The toxicity was expressed as a percentage of survival.
2.9. Determination of lipid peroxidation
The sample solution was added to the treated bacterial suspension and pretreated for 4 h, then yeast cells were directly exposed to H2O2 (2.0 mM) and CCl4 (10 mM) for 1 h. Cells were centrifuged at 6,000 rpm for 2 minutes and washed twice with distilled water, 0.5 mL of 10% trichloroacetic acid (w/v) was added to cell sediment with 1.5 g glass beads. Yeast cells were crushed and centrifuged at 2,000 rpm for 2 minutes. The supernatant was mixed with 0.1 mL EDTA (0.1 M) and 0.6 mL of 1% thiobarbituric acid (w/v). The reaction mixture was incubated in a boiling water bath for 15 min, and then ice bath cooling. The absorbance was measured at 532 nm.
2.10. Determination of intracellular oxidation
The oxidant-sensitive probe 2′7′-dichlorofluorescein diacetate was used to measure intracellular oxidation. The probe (5 mM) was added to the stressed cells and cultured at 28 °C, while shaking at a speed of 200 rpm for 15 min to allow the uptake of the probe. Cells were centrifuged at 6,000 rpm for 4 min and washed twice with water. Water (500 µL) and glass beads were added to the sediment. Yeast cells were crushed fully and centrifuged at 2,5000 rpm for 5 min. Supernatant was collected and diluted by 6-fold with water, and then the fluorescence measured at an excitation wavelength of 504 nm and an emission wavelength of 524 nm.
2.11. Statistical analysis
Statistical analysis was performed using SPSS statistical software ANOVA and Duncan test. The results indicated the mean ± standard deviation of at least three independent experiments. The latter denotes homogeneity between experimental groups at p < 0.05. Different letters indicate statistically different results of significance.
| 3. Results | ▴Top |
3.1. Composition of the essential oil
As shown in Figure 1, fifty components were identified in the jujube seed oil and more than half of were terpenoids, among which d-limonene (17.87%), methyl n-methylanthranilate (15.3%), α-farnesene (9.5%), and γ-terpinene (4.23%) accounted for the largest total amount (Figure 3). Terpenes are one of the largest group of natural products and have been considered potential candidates for functional food and drug development (Huang et al., 2012). Some studies found that d-limonene showed anti-oxidant activity (Anandakumar et al., 2021).and induced apoptosis and proliferation inhibition in gastric cancer cells and K562 cells (Gao et al., 2006; Lu et al., 2003). Interestingly, the composition percentage of the methyl n-methylanthranilate group was very high, accounting for 15.3%. Studies found that methyl n-methylanthranilate group of compounds isolated from mandarin and other citrus leaves were full of pungency, which could be used to explore new analgesics in medical therapy against pain (Correa et al., 2016). γ-Terpinene, terpinolene, carvone, thymol, and other compositions were also rich in the jujube seed oil and have demonstrated various biological and pharmacological activities (El Ouariachi et al., 2011). The unsaturated structures in the jujube oil terpenoids indicated that jujube seed oil has certain antioxidant biological activity. GC-MS results analysis were shown in Table 1.
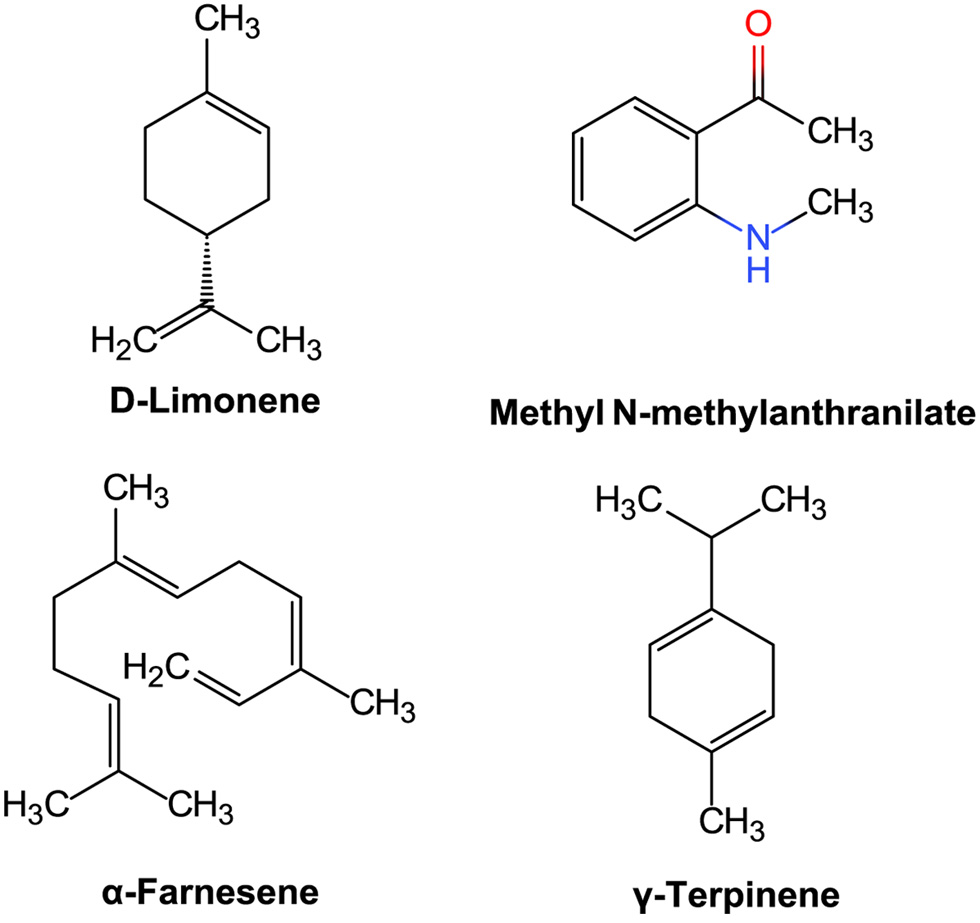 Click for large image | Figure 3. Structures of d-limonene, methyl N-methylanthranilate,α-farnesene, and γ-terpinene. |
 Click to view | Table 1. Chemical composition of the jujube seed oil |
3.2. Antioxidant properties in vitro
Reducing capacity is an important indicator of the ability of antioxidants, which can provide electrons to interrupt the chain reaction of free radicals and make them stable. Therefore, there is a relationship between reducing capacity and antioxidant capacity. The results of the reducing ability of jujube seed oil were shown in Figure 4, from which we could see the reducing power of VC and jujube seed oil concentration was dose-dependent. However, jujube seed oil indicated significantly lower reducing power than that VC at the same concentration.
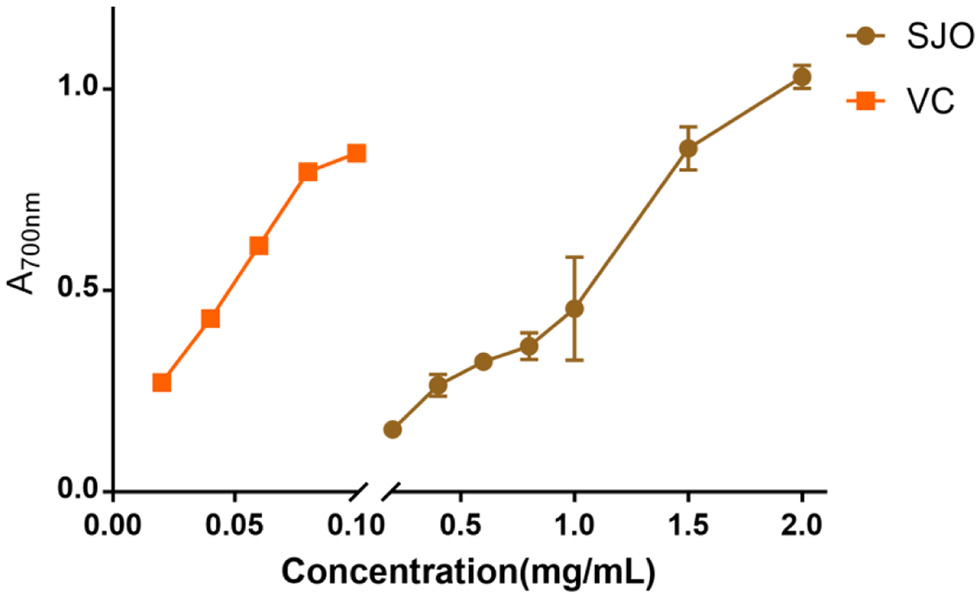 Click for large image | Figure 4. Reducing power of jujube seed oil. The concentration of SJO used was 0.2 mg/mL,0.4 mg/mL,0.6 mg/mL,1 mg/mL,1.5 mg/mL, and 2 mg/mL, repectively. The positive control group received 0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL,1 mg/mL, and 2 mg/mL of VC, respectfully. Data represent the mean ± SD of three independent trials. |
DPPH free radical scavenging is a relatively simple and convenient chemical method to evaluate antioxidant activity. DPPH forms purple and stable free radicals in organic solvents and reacts with antioxidants to generate colorless products, resulting in a decrease in the characteristic absorption peak and absorbance of the solution (Tsai and Lin, 2019). The scavenging ability of SJO, BHT, and VC on DPPH free radicals was shown in Figure 5. The results indicated that when the concentration of jujube seed oil increased, the scavenging rate of VC and BHT on DPPH free radical reached 97.75% ± 0.64, while the scavenging rate of jujube seed oil on DPPH free radical was only 30.74% ± 4.59. The results showed that the DPPH free radical scavenging ability of jujube seed oil was lower than that of VC and BHT.
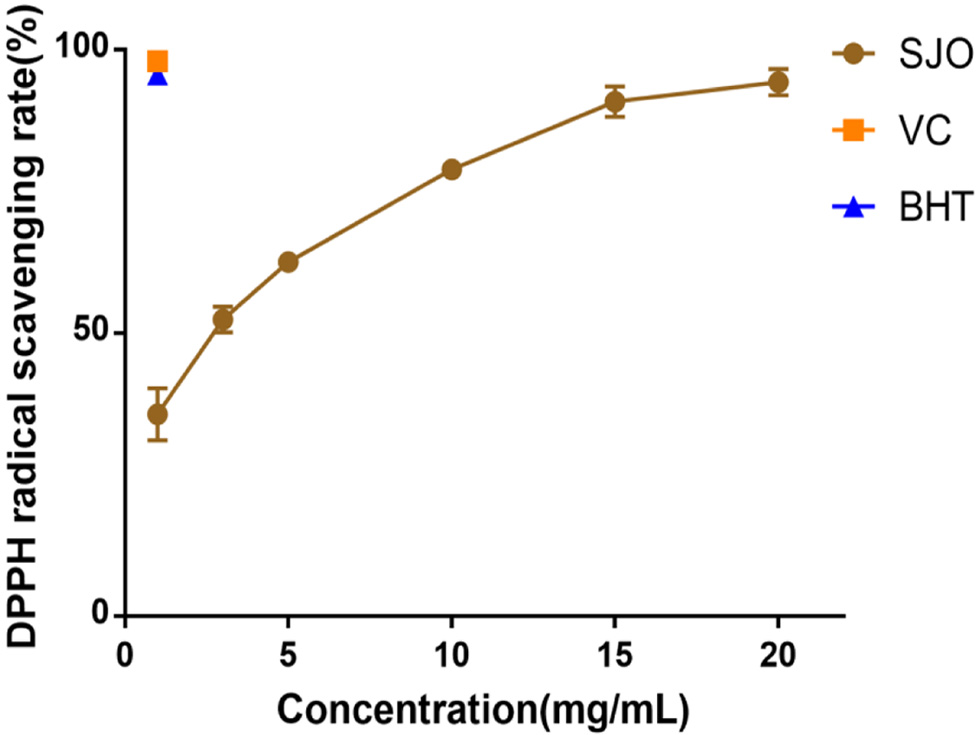 Click for large image | Figure 5. DPPH radical scavenging activity of SJO. The concentration of SJO used was 1 mg/mL,4 mg/mL,5 mg/mL, 10 mg/mL, 15 mg/mL, and 20 mg/mL, respectively. The positive control group received 1 mg/mL VC and 1 mg/mL BHT. Data represent the mean ± SD of three independent trials. |
H2O2 formed directly or through O2− disproportionation is freely permeable into lysosomal vacuoles where Fenton-type reactions can be carried out to produce OH−. The OH− produced in lysozyme may lead to lipid peroxidation, hence evaluating hydroxyl scavenging ability becomes an important indicator to evaluate antioxidant level (Brunk et al., 1992). The results indicated that jujube seed oil increased hydroxyl radical scavenging activity in a concentration-dependent (Figure 6). When the concentration was at 1 mg/mL, the scavenging ability of jujube seed oil to hydroxyl radical free radical was about 40% of VC and 50% of BHT. When the concentration of jujube seed oil was about 5 mg/mL, the scavenging ability of hydroxyl radical was almost equal to VC.
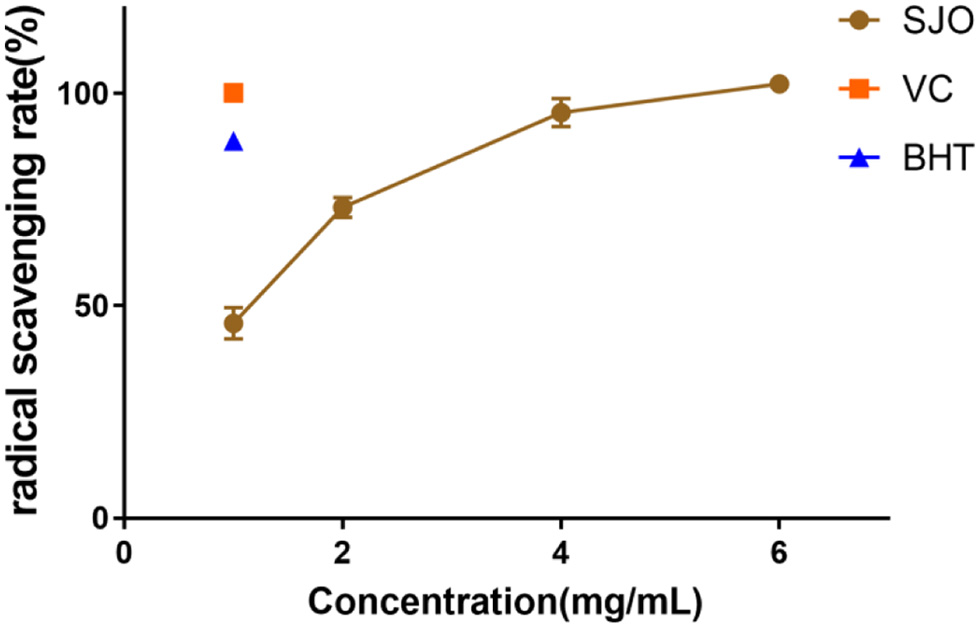 Click for large image | Figure 6. Hydroxyl radical scavenging activity of SJO. The concentration of SJO used was 1 mg/mL, 2 mg/mL, 4 mg/mL, and 6 mg/mL. The positive control group received 1 mg/mL VC and 1 mg/mL BHT. Data represent the mean ± SD of three independent trials. |
3.3. Antioxidant properties in vivo
S. cerevisiae acquire tolerance and cells continued to reach 100% when pre-adapted to different concentrations of jujube seed oil. The increased tolerance caused by jujube seed oil was similar in S. cerevisiae strains. The result indicated that different concentrations of jujube oil were nontoxic to all the S. cerevisiae strains, and jujube oil can be used to explore further antioxidants.
The antioxidant properties of SJO were assessed by exposing S. cerevisiae (Figure 7) either pre-adapted or untreated with 25 μg/mL SJO and 50 μg/mL SJO, to 10 mM CCl4, 2 mM H2O2. H2O2 was the most toxic to all yeast strains, and the survival rate of WT was lower than 50% under direct stress. H2O2 produces the most toxic and reactive hydroxyl radicals in cells. The organism has little resistance to excess hydroxyl radicals, and yeast cells have a low tolerance to H2O2 (Mariani et al., 2008). The survival rate of the WT strain exposed to all stresses was significantly ameliorated when pretreated with jujube seed oil, the jujube oil reliably protects WT yeast cells from oxidative damage. Some studies suggest that the treatment of mutant strains with the specific antioxidant system may be related to their protection mechanism (Gao et al., 2019; Wang et al., 2018). Therefore, we used mutants SOD1Δ, CTT1Δ, GSH1Δ, GTT1Δ, and GTT2Δ to determine whether superoxide dismutase, catalase, glutathione, and glutathione sulfur transferase were related to the mechanism of tolerance acquisition. Cells possess both enzymatic and non-enzymatic defense systems to protect and control the intracellular redox balance (Jamieson, 1998). The result from the exposure to H2O2 and CCl4 indicated that GSH1Δ, SOD1Δ, and GTT1Δ were unnecessary for this adaptive treatment. It is worth noting that the protection of jujube seed oil against H2O2 seemed to require glutathione sulfur transferase, since the GTT2Δ strain could not be tolerated after adaptation to jujube seed oil. GTT2Δ also seems to be involved in the protective mechanism of jujube seed oil. In addition, the protective effect of the CTT1Δ strain on jujube seed oil was not obvious, and it is worth paying attention that catalyse might also play a role in the protective mechanism of jujube seed oil against oxidative stress.
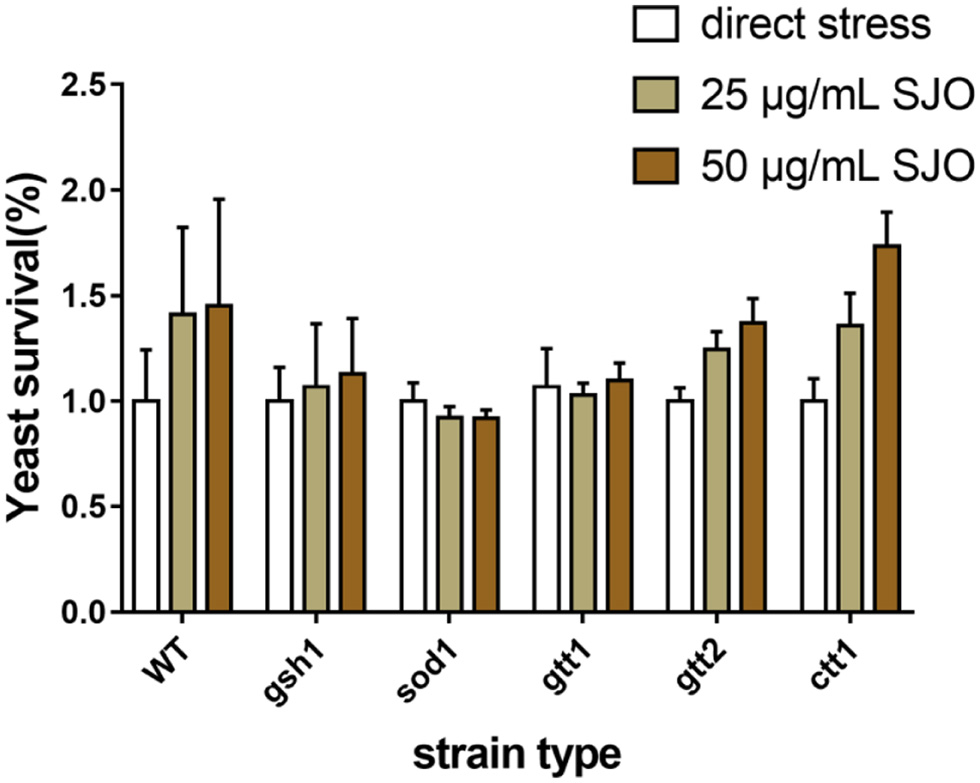 Click for large image | Figure 7. The survival rate of S. cerevisiae cells is directly exposed to increasing SJO concentrations. The concentration of SJO used was 25 μg/mL and 50 μg/mL. Data represent the mean ± SD of three independent trials. |
Membrane lipid peroxidation level is one of the important indicators to evaluate oxidation. The first line of defense against free radicals entering the cell is the biofilm, preventing membrane peroxidation. Oxidation products of unsaturated fatty acids such as malondialdehyde react with TBA to produce colored compounds, thus assessing the level of membrane lipid peroxidation. Our results confirmed that H2O2 and CCl4 increased oxidative stress in S. cerevisiae and the oxidative stress increased the membrane peroxidation levels in WT, GSH1, and GTT1. It can be seen from Figure 8 that H2O2 caused the most severe damage to lipid peroxidation of all yeast cells. The research result showed that jujube seed oil can protect GSH1 and GTT1 cells well and reduce the level of membrane lipid peroxidation under hydrogen peroxide stress. Indeed, it could be related to the superexpression of other compensational antioxidant systems (Franca et al., 2005). By contrast, there was no significant difference in membrane peroxidation level of the mutants of SOD1, GTT2, and CTT1 type under the stress of H2O2, which suggested that the protective mechanism of jujube seed oil on S. cerevisiae might be related to superoxide dismutase and catalase. GSH1, GTT1 and CTT1 mutants exposed to CCl4 reduced the level of membrane lipid peroxidation after pretreatment with jujube seed oil, suggesting that jujube seed oil did not show a specific relationship with glutathione and catalase in membrane lipid peroxidation mechanism of S. cerevisiae. In general, jujube seed oil inhibited membrane lipid peroxidation, and the mechanism may be related to superoxide dismutase and catalase in S. cerevisiae.
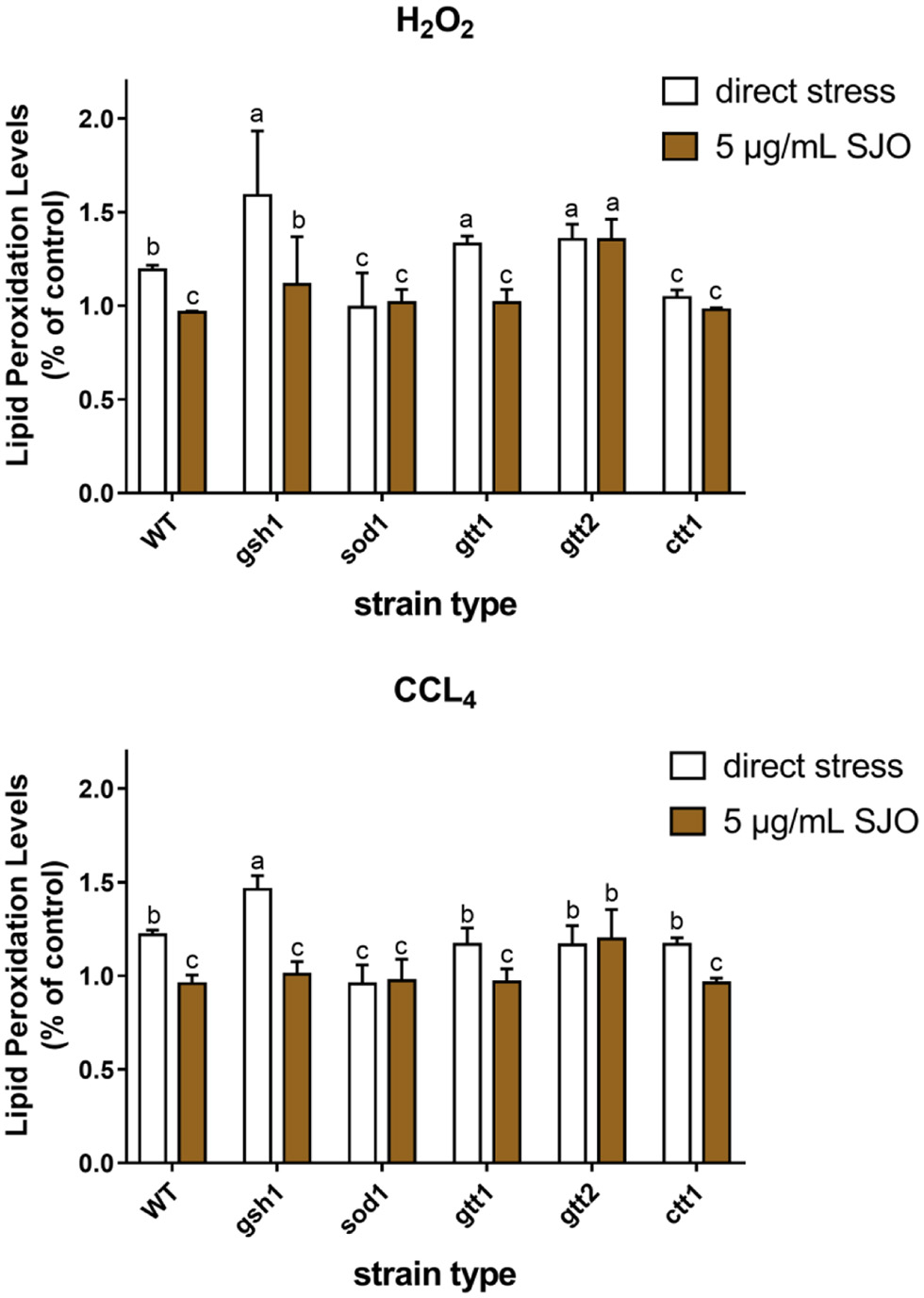 Click for large image | Figure 8. Effect of 2 mM H2O2, 10 mM CCL4 on lipid peroxidation level of S. cerevisiae cells (WT, ctt1Δ, sod1Δ, gtt1Δ, gtt2Δ, and gsh1Δ) and the membrane protective effect of SJO. The concentration of SJO used was 5 μg/mL. Lipid peroxidation levels of non-stressed cells without SJO treatment were used as a control. Data represent the mean ± SD of three independent trials. In each oxidative stress group, the statistically different results were denoted by different letters, p < 0.05. |
The previous results have shown that sour Jujube seed flavonoids extract exerts ameliorative antioxidant capacity and reduced the accumulation of ROS (Yang et al., 2019). Our result has confirmed that jujube seed oil has a certain ability to scavenge free radicals and inhibit membrane peroxidation. Free radicals penetrate through biofilms and then attack organelles. Therefore, the protective effect of jujube seed oil on cells under attack can be further explored by detecting ROS levels. ROS is a partial reduction or excitation form of atmospheric oxygen. As a signal factor in cells, ROS is often considered a harmful accessory of aerobic metabolism (Mittler, 2017). Adaptation responses to redox stress may involve mitochondrial channels, whose activation leads to intra- and inter-mitochondrial redox-environment change leading to ROS release (Zorov et al., 2014). In this study, intracellular oxidation levels of yeast cells were measured by fluorescent probes. Once the 2′,7′-dichloroacetate fluorescent probes entered the cells, they were attacked by ROS and produced compounds with higher fluorescence intensity. This probe has been widely used to evaluate the enhancement of ROS after oxidative stress.
Figures 8–10 show that both H2O2 and CCl4 can increase the intracellular ROS level, while jujube seed oil can reduce the ROS level of S. cerevisiae under the oxidative stress of H2O2 and CCl4. Similar to the previous results, H2O2 stress on yeast cells still showed higher ROS levels. However, the high ROS levels stimulated by both stressors are reduced after treatment with jujube seed oil, and this remission was more pronounced in CCl4. The augment in ROS level of WT, GSH1, and SOD1 has reduced when pretreatment by jujube seed oil, but the increase in ROS level of GTT1, GTT2, and CTT1 was no significant change. And jujube seed oil alleviates oxidative damage in CCl4 stress and may have a close correlation with superoxide dismutase and catalase.
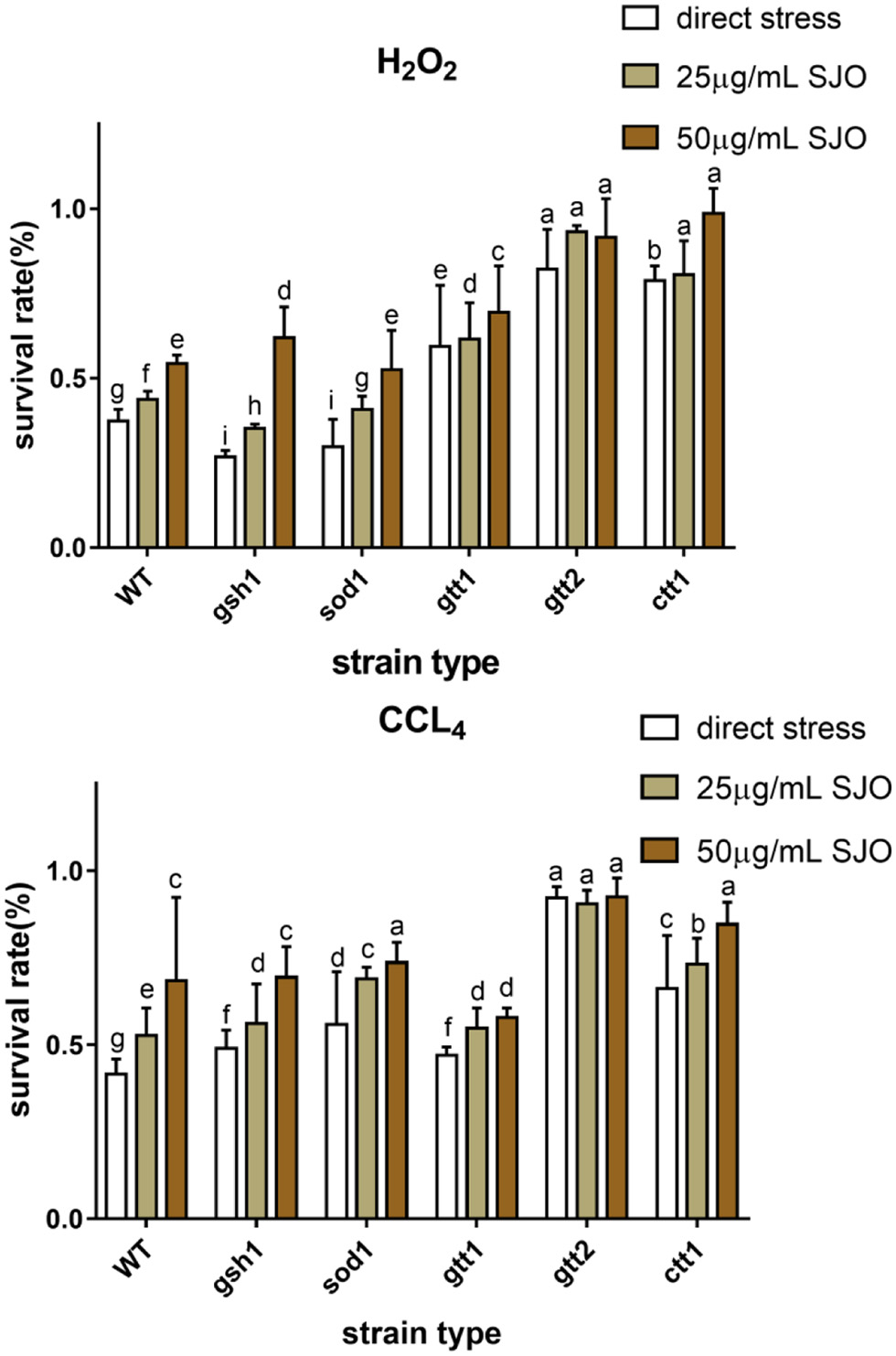 Click for large image | Figure 9. Effect of 2 mM H2O2, 10 mM CCl4 on survival of S. cerevisiae cells (WT, ctt1Δ, sod1Δ, gtt1Δ, gtt2Δ, and gsh1Δ) and the antioxidant effect of SJO. The concentration of SJO used was 25 μg/mL and 50 μg/mL. Data represent the mean ± SD of three independent trials. In each oxidative stress group, the statistically different results were denoted by different letters, p < 0.05. |
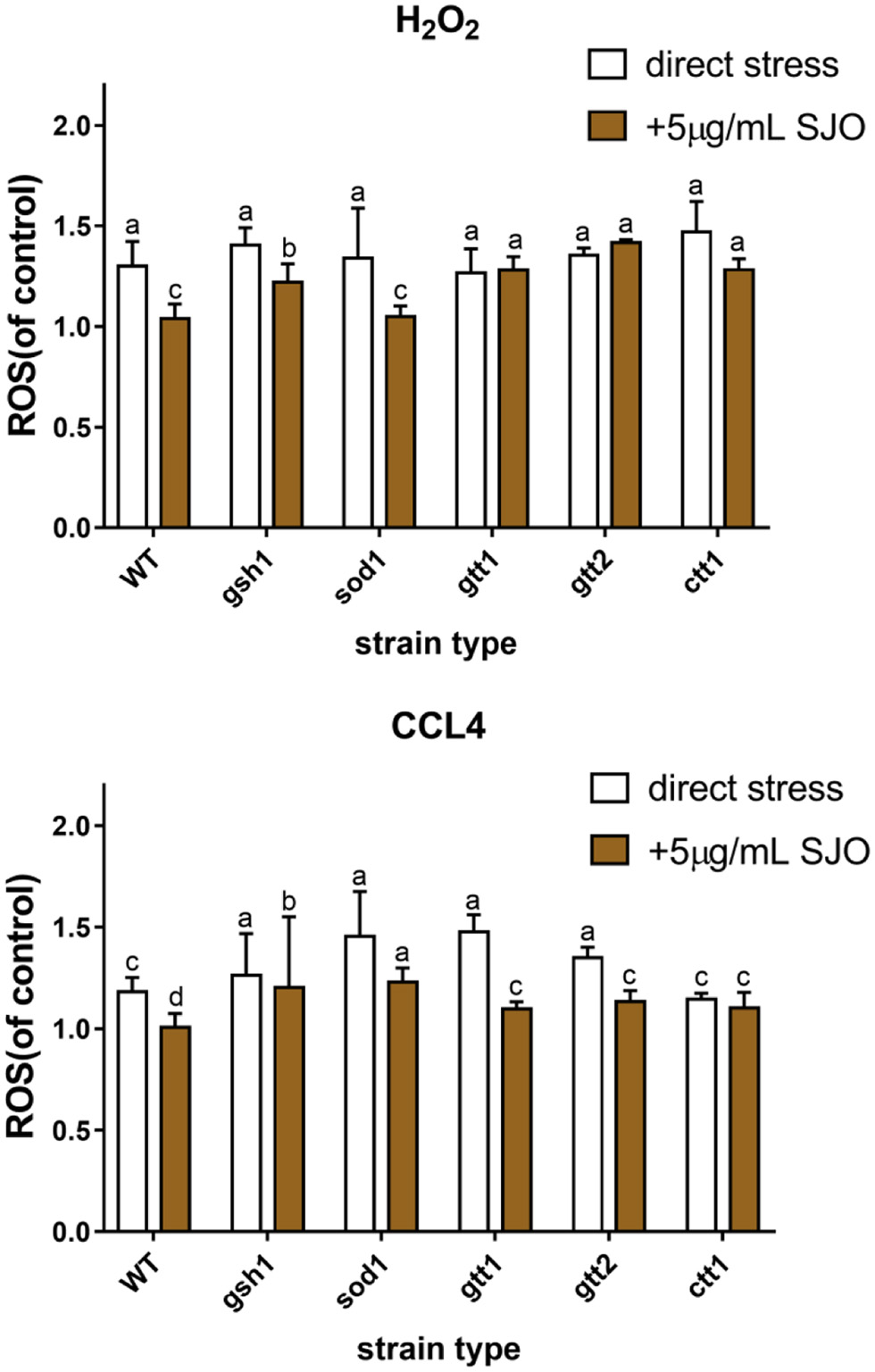 Click for large image | Figure 10. Effect of 2 mM H2O2, 10 mM CCL4 on ROS level of S. cerevisiae (WT, ctt1Δ, sod1Δ, gtt1Δ, gtt2Δ, and gsh1Δ) and the ROS effect of SJO. The concentration of SJO used was 5 μg/mL. ROS levels of non-stressed cells without SJO treatment were used as a control. Data represent the mean ± SD of three independent trials. In each oxidative stress group, the statistically different results were denoted by different letters, p < 0.05. |
It is interesting to note that tolerance test, membrane lipid peroxidation level, and ROS showed that the protective effect of jujube seed oil on GTT was not significant under the stress of oxidizer, which may be related to the sensitivity of mutants lacking GTT1 and GTT2 to a series of oxidants and xenobiotics (Collinson and Grant, 2003). Other studies have shown that the mechanism of GTT1 and GTT2 on H2O2 stress was time-dependent (Mariani et al., 2008). In addition, the CTT1 mutants in the results also draw our attention. The ROS and membrane peroxidation levels of the strain exposed to H2O2 were lower than those of WT, and there was no significant difference between the strain pretreated with jujube seed oil and the strain untreated. Catalases efficiently scavenge H2O2 and protect cells against H2O2 stress (Martins and English, 2014). Therefore, it can be inferred that the antioxidant protection of jujube seed oil on S. cerevisiae is closely related to catalase, but its further protective mechanism needs to be studied.
| 4. Discussion | ▴Top |
GC-MS analysis showed that the main components of jujube seed oil were d-limonene(17.87%), Methyl N-methylanthranilate (15.3%), α-farnesene (9.5%) and γ-terpinene (4.23%). These compounds have biological and pharmacological activities and have potential research value in the future. In vitro, the antioxidant activity test showed that the scavenging rate of DPPH free radical of jujube seed oil was slightly lower than that of BHT and VC, and the reducing ability of jujube seed oil was also slightly lower than that of BHT and VC, but with the increase of mass concentration of jujube seed oil, its reducing ability reached the level of VC.
Based on these results we could conclude that jujube seed oil is a promising antioxidant product showing scavenging free radical and reductive ability in vitro. Then yeast cells were induced to produce a series of oxidative stress reactions by adding oxidants and different oxidants show different mechanisms such as H2O2 form free radical O2−, OH− and CCl4 attacking cell form CCl3− and CCl3O2−, these toxicants, chemicals or biological reactions formed free radical continuous interaction with biological systems cause cumulative damage to DNA, protein, lipid, carbohydrates, and membrane (Ingawale et al., 2014; Sies, 2017). By analyzing the survival rate, lipid peroxidation, and intracellular oxidation levels of different genotypic yeast under different stress conditions, we can infer the antioxidant system that yeast depends on under different stress conditions. After the yeast cells were pretreated with jujube seed oil, the mutants showed different antioxidant activity under the same stress condition. By analyzing the survival rate, lipid peroxidation, and intracellular oxidation levels of different genotypic yeast under different stress conditions, we can infer the antioxidant system that yeast depends on under different stress conditions.
After the yeast cells were pretreated with jujube seed oil, different mutants showed different antioxidant activities under the same stress conditions, to analyze the antioxidant system which jujube seed oil depended on for its antioxidant effect in yeast cells. Taken together, we identified that the antioxidant genes CTT1 and SOD1 form a tight relationship regarding the antioxidant mechanisms of jujube seed oil through the yeast model. These results provide some references for the further development of jujube seed in food, medicine, and human health.
| References | ▴Top |