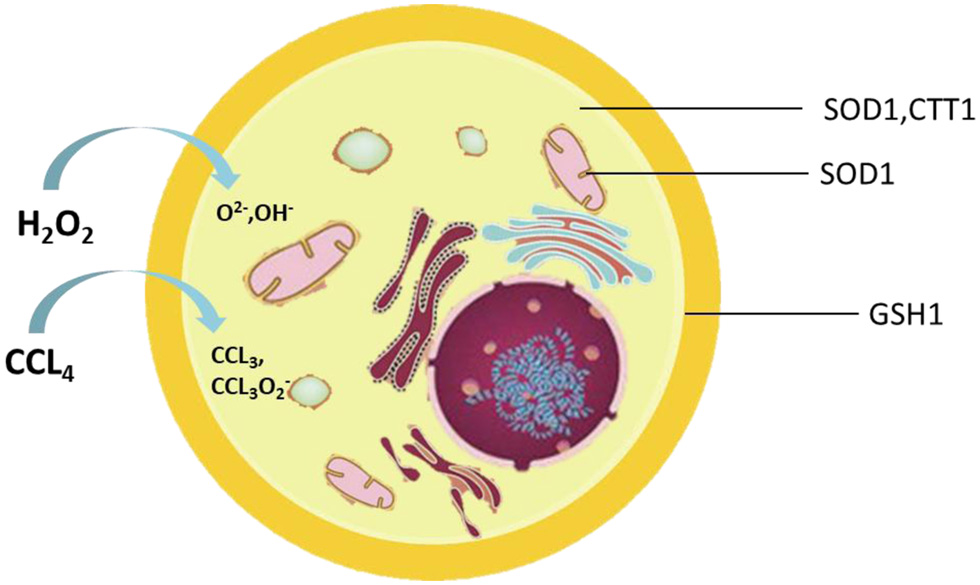
Figure 1. Location of enzymes associated with oxidative stress in the cell.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 22, June 2023, pages 27-35
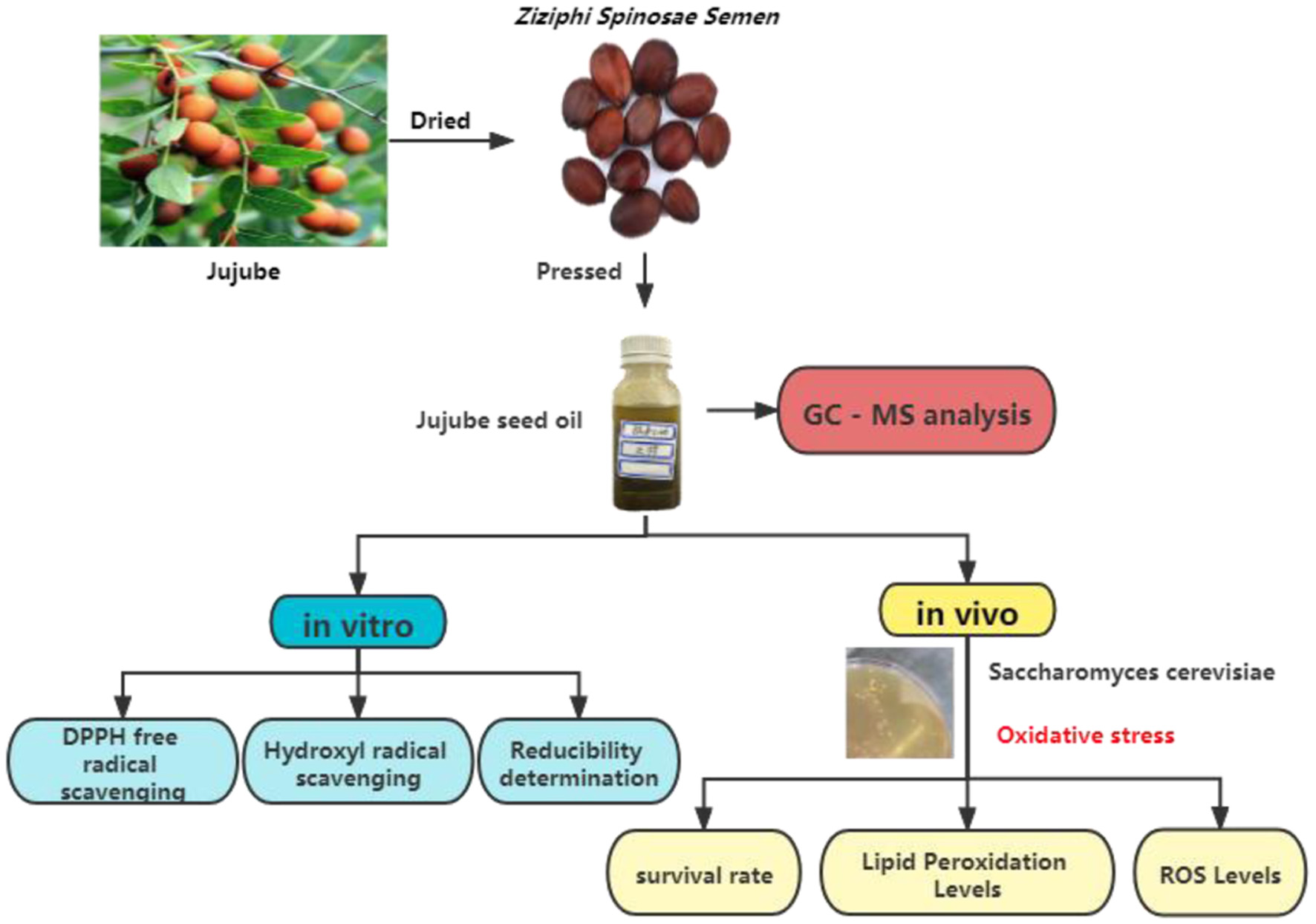
Study on antioxidant activity of Jujube seed oil
Figures

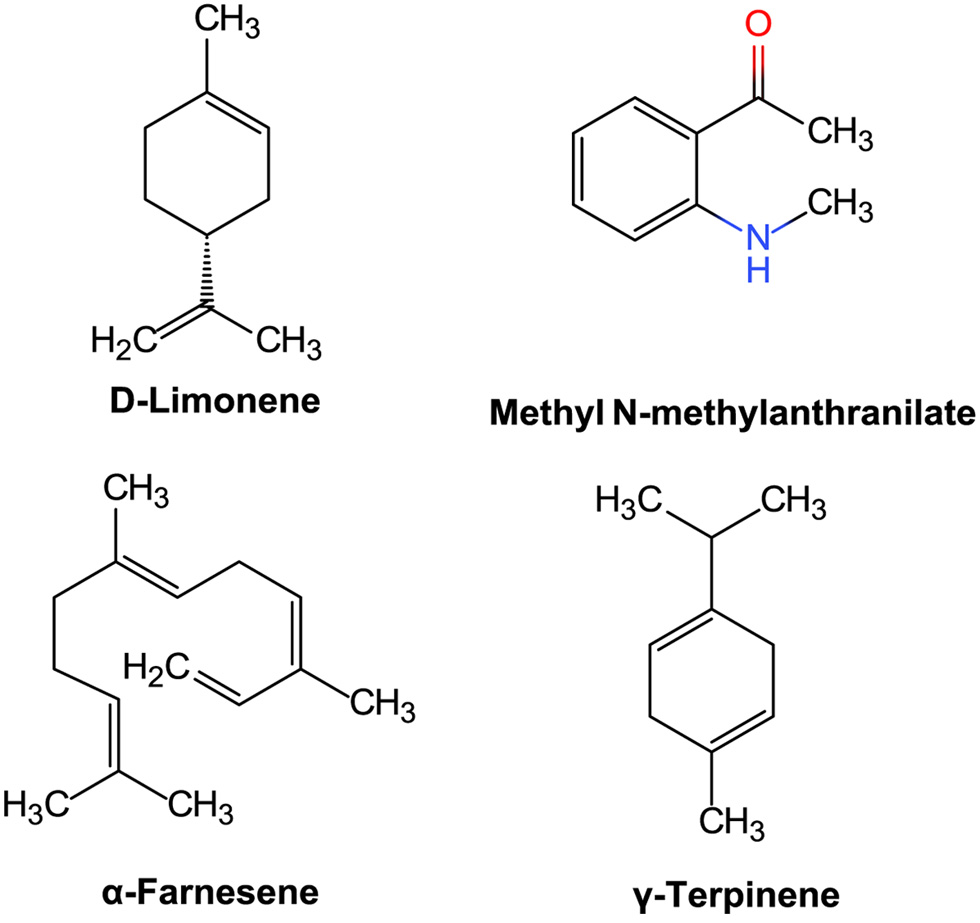
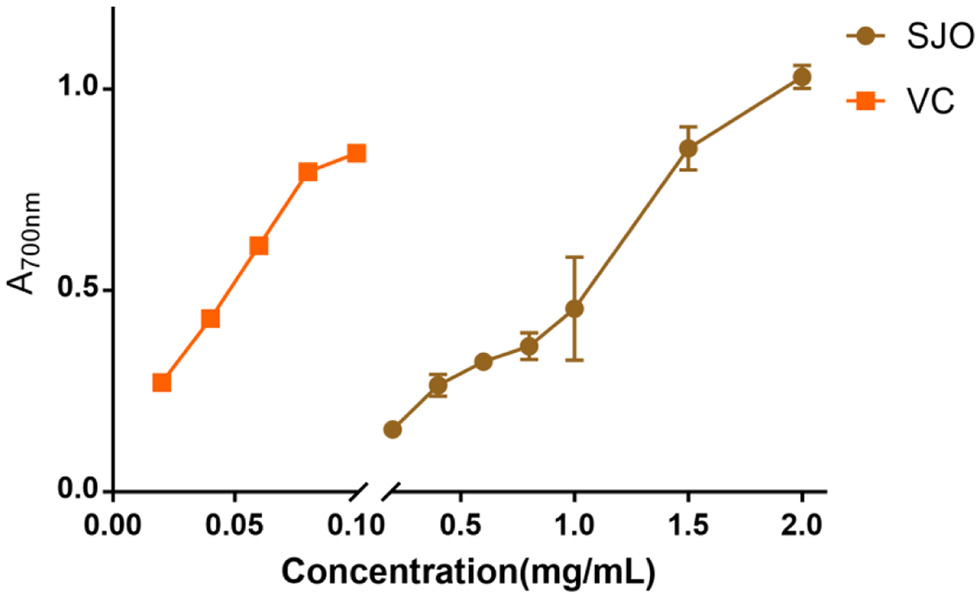
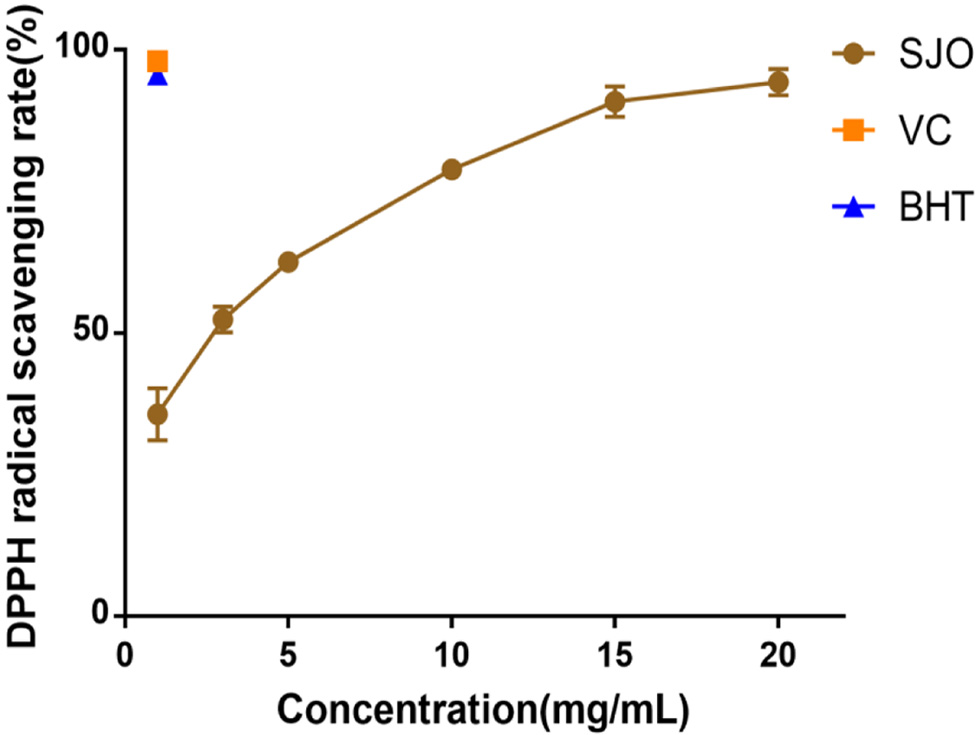
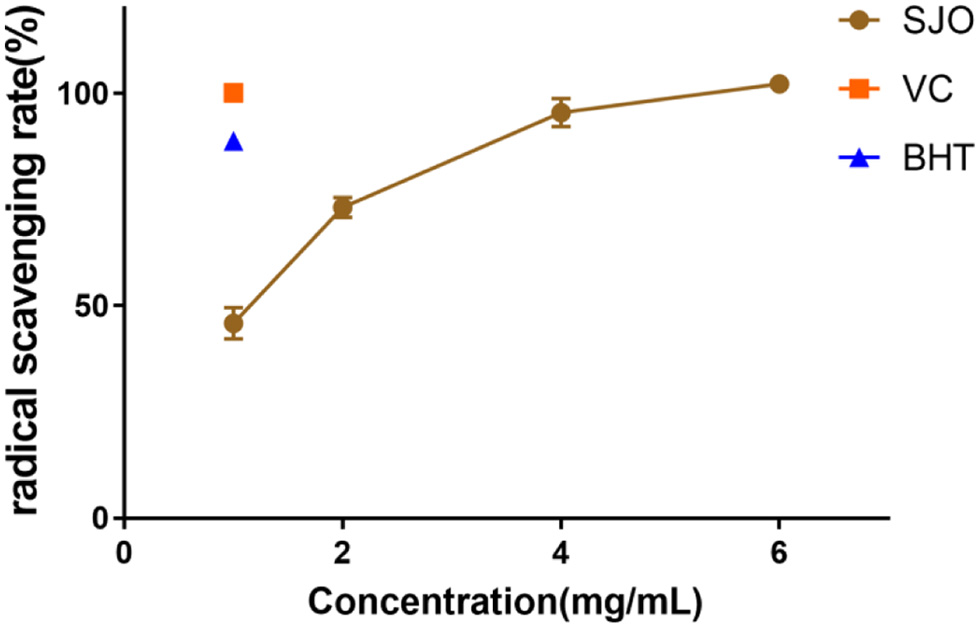
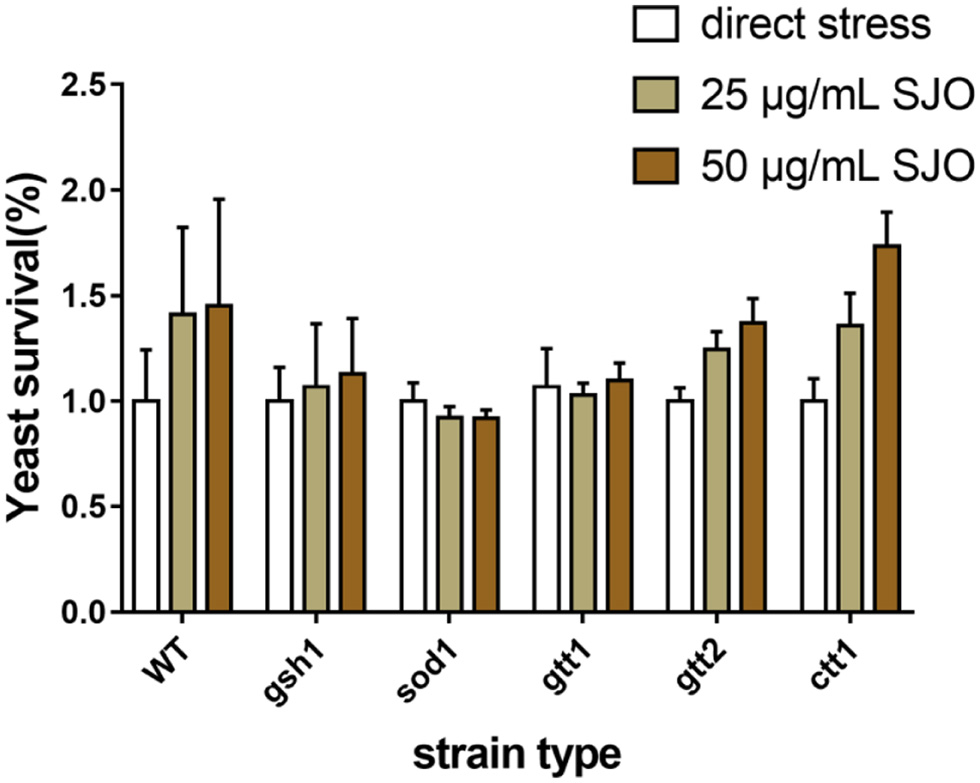
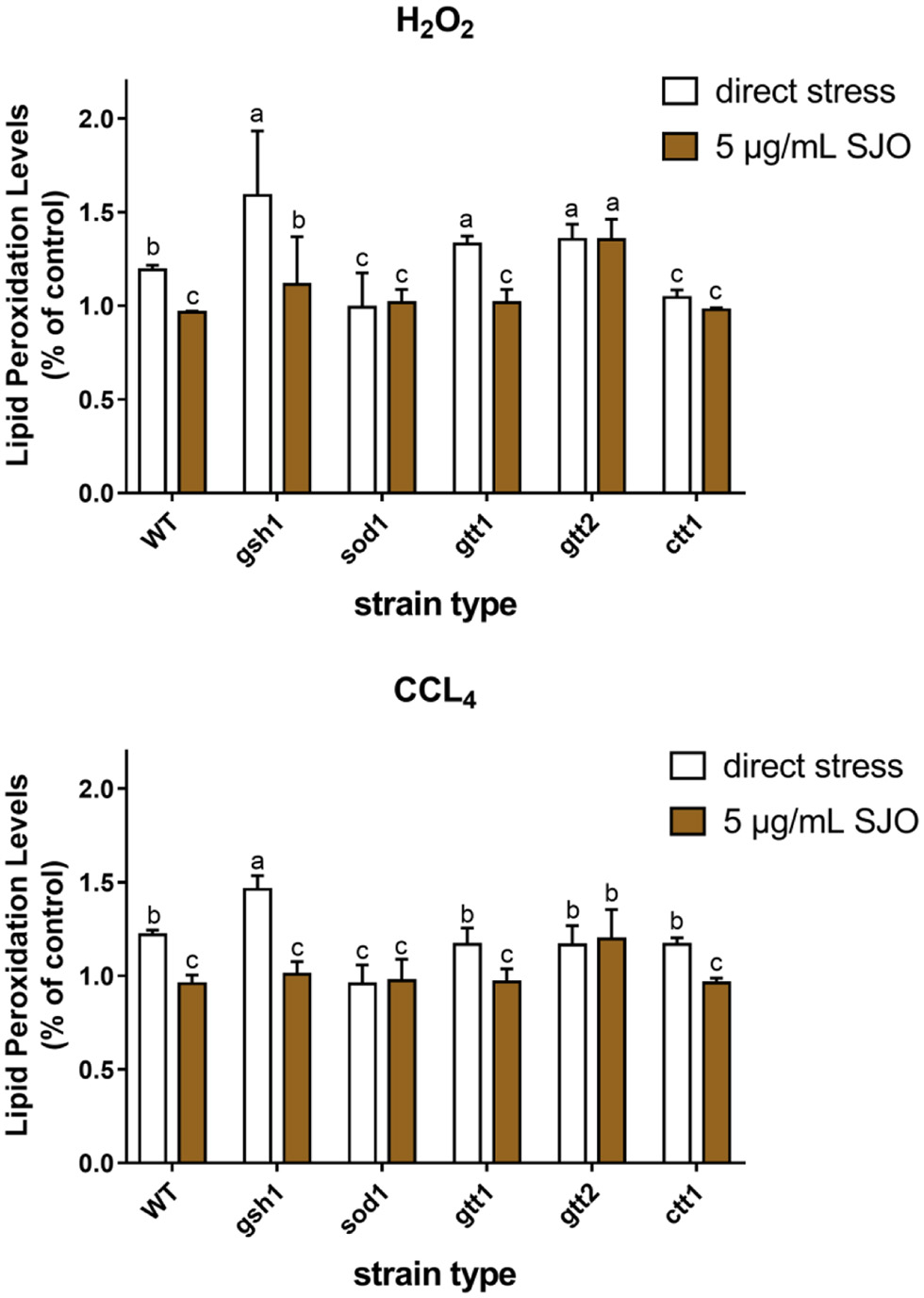
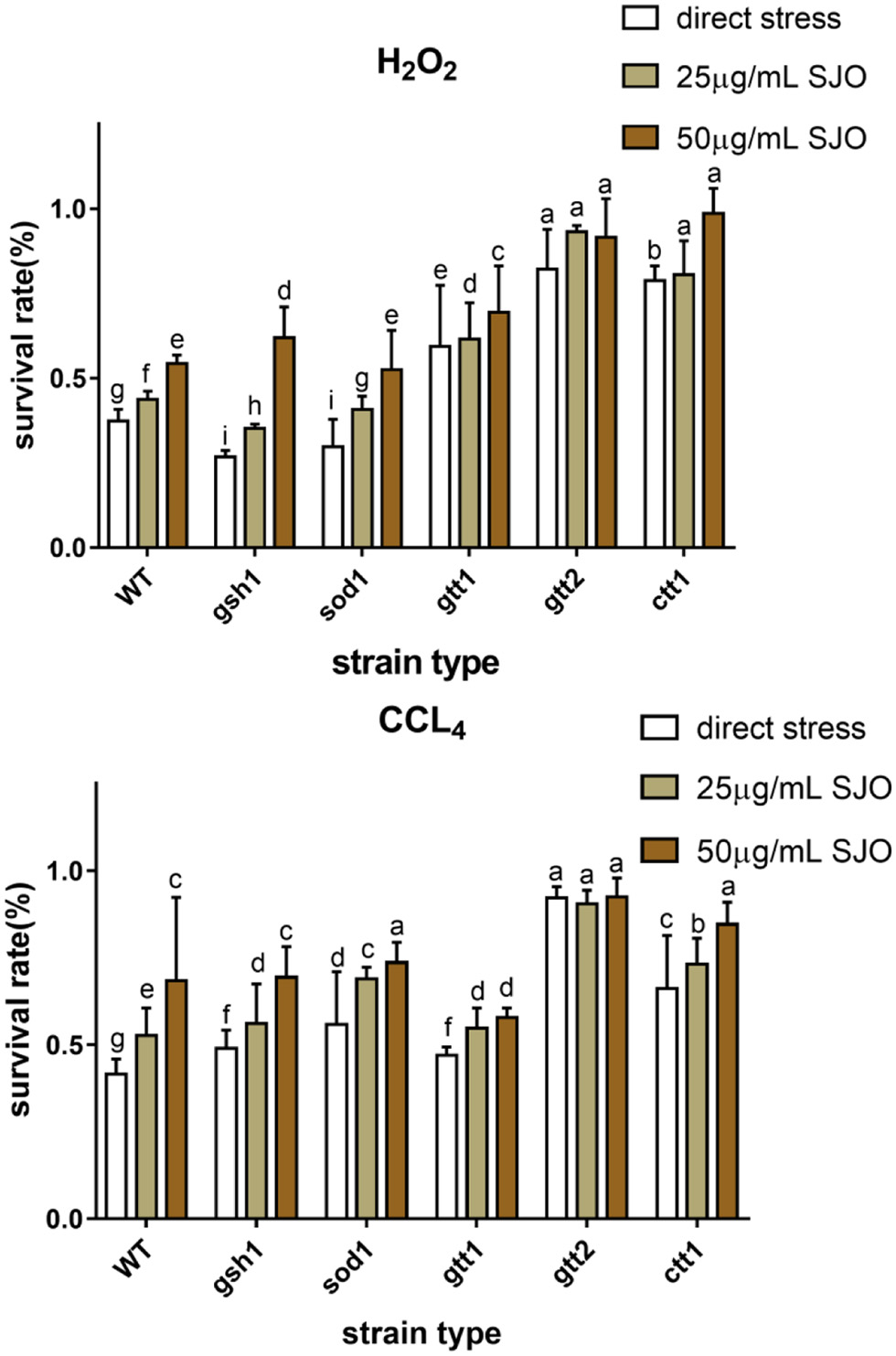
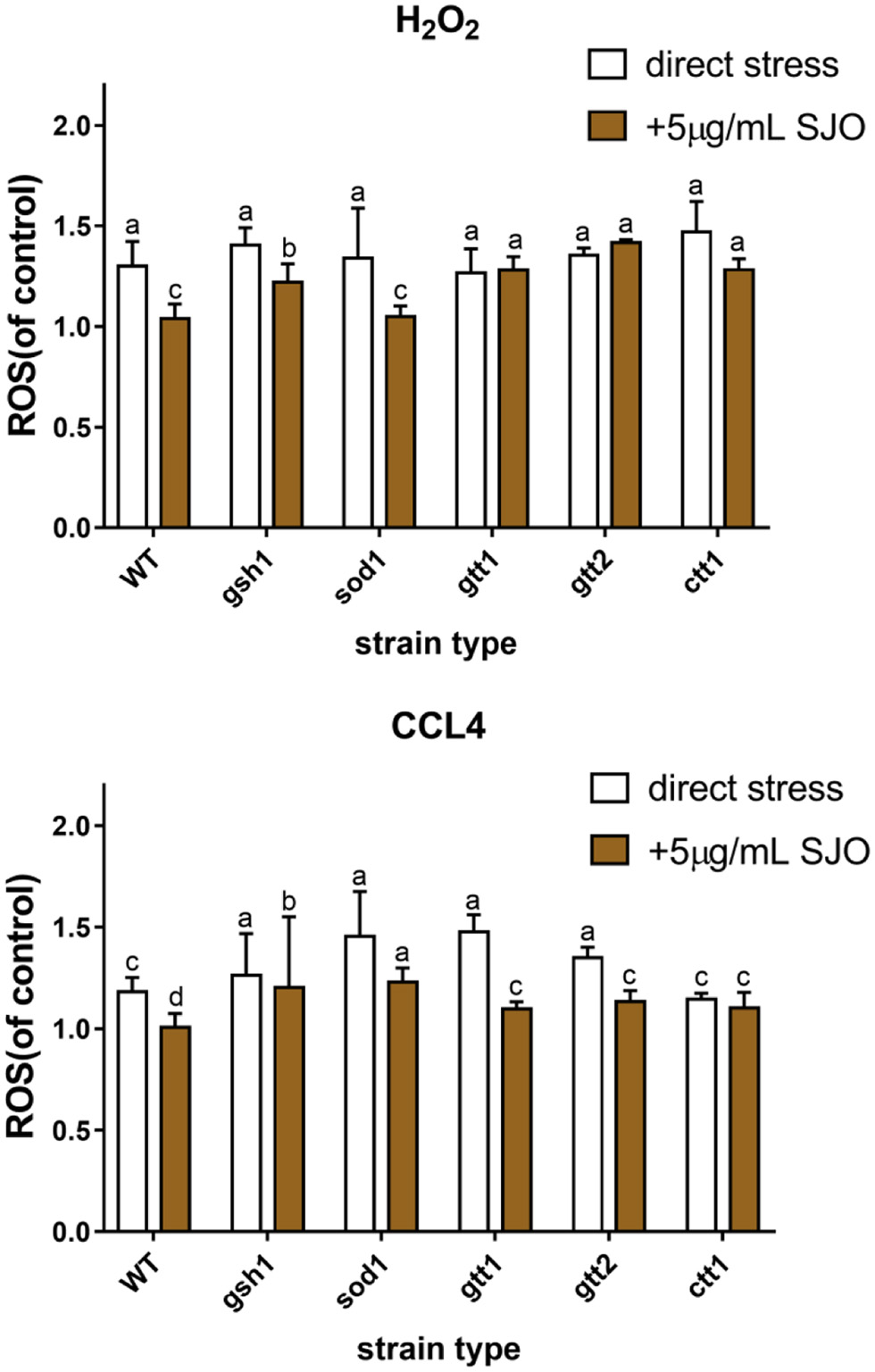
Table
| No. | Components | RT | Relative content | Molecular Formula |
|---|---|---|---|---|
| 1 | a-Pinene | 6.53 | 0.28 | C10H16 |
| 2 | y-Limonene | 6.58 | 0.33 | C10H16 |
| 3 | Didehydro pinane | 7.31 | 0.37 | C10H16 |
| 4 | D-Limonene | 8.5 | 17.87 | C10H16 |
| 5 | y -Terpinene | 9.37 | 4.23 | C10H16 |
| 6 | Terpinolene | 9.47 | 3.04 | C10H16 |
| 7 | 1,3,8-p-Menthatriene | 9.65 | 0.13 | C10H14 |
| 8 | trans-Alloocimene | 9.75 | 1.07 | C10H16 |
| 9 | cis-p-Mentha-2,8-dien-1-ol | 9.92 | 0.8 | C10H16O |
| 10 | d-Citronellal | 10.04 | 1.65 | C10H18O |
| 11 | Cyclohexene, 3-(3-methyl-1-butenyl)-, (E)- | 10.28 | 0.17 | C10H18 |
| 12 | Sabinene hydrate | 10.33 | 0.16 | C10H18O |
| 13 | Terpinen-4-ol | 10.51 | 2.93 | C10H18O |
| 14 | a-terpineol | 10.73 | 4.68 | C10H18O |
| 15 | Isopiperitenol | 10.84 | 0.11 | C10H16O |
| 16 | Citronellol | 11.08 | 2.18 | C10H20O |
| 17 | methyl thymol ether | 11.17 | 0.65 | C11H16O |
| 18 | Carvone | 11.39 | 0.6 | C10H14O |
| 19 | piperitone | 11.53 | 0.18 | C10H16O |
| 20 | perillyl aldehyde | 11.84 | 1.88 | C10H14O |
| 21 | Thymol | 11.96 | 3.79 | C10H14O |
| 22 | Carvacrol | 12.24 | 2.16 | C10H14O |
| 23 | y -Elemene | 12.64 | 0.15 | C15H24 |
| 24 | cis-carane | 12.77 | 0.55 | C10H18 |
| 25 | Geranyl acetate | 12.93 | 0.38 | C12H20O2 |
| 26 | a-Copaene | 13.25 | 1.73 | C15H24 |
| 27 | β-Copaene | 13.46 | 1.24 | C15H24 |
| 28 | Methyl N-methylanthranilate | 13.91 | 15.3 | C8H9NO2 |
| 29 | β-Caryophyllene | 14.05 | 3.05 | C15H24 |
| 30 | a-Caryophyllene | 14.53 | 0.78 | C15H24 |
| 31 | Germacrene D | 14.87 | 0.49 | C15H24 |
| 32 | a-Farnesene | 15.11 | 9.5 | C15H24 |
| 33 | Cadina-1(10),4-diene | 15.46 | 2.16 | C15H24 |
| 34 | a-Elemol | 15.94 | 0.44 | C15H26O |
| 35 | Dodecanoic acid | 16.16 | 0.5 | C12H24O2 |
| 36 | a-Patchoulene | 16.45 | 0.37 | C15H24 |
| 37 | Caryophyllene oxide | 16.51 | 0.38 | C15H24O |
| 38 | Tetradecanal | 16.64 | 0.2 | C14H28O |
| 39 | aromadendrene oxide | 17.17 | 0.11 | C15H24O |
| 40 | T-muurolol | 17.26 | 0.2 | C15H26O |
| 41 | β-eudesmol | 17.44 | 0.45 | C15H26O |
| 42 | β-santalol | 17.63 | 0.31 | C15H24O |
| 43 | a-sinensal | 18.46 | 6.34 | C15H22O |
| 44 | 2-Amylnonen-2-al | 19.72 | 0.18 | C14H26O |
| 45 | Hexadecanoic acid | 20.36 | 1.85 | C16H32O2 |
| 46 | p-Camphorene | 21.09 | 0.15 | C20H32 |
| 47 | Methyl Linoleate | 22.09 | 0.23 | C19H34O2 |
| 48 | Methyl Linolenate | 22.16 | 0.14 | C19H32O2 |
| 49 | Linoleic acid | 22.55 | 0.69 | C18H32O2 |
| 50 | Dodecenylsuccinic anhydride | 22.72 | 0.15 | C16H26O3 |