| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 2, June 2018, pages 98-103
5-Demethylnobiletin is more effective than nobiletin in preventing AOM/DSS-induced colorectal carcinogenesis in ICR mice
Jia-Ching Wua, b, #, Yen-Chen Tunga, #, Yu-Nu Zhengc, Mei-Ling Tsaic, Ching-Shu Laic, Chi-Tang Hod, Min-Hsiung Pana, e, f, *
aInstitute of Food Science and Technology, National Taiwan University, Taipei 10617, Taiwan
bDepartment of Environmental and Occupational Health, National Cheng Kung University Medical College, Tainan 704, Taiwan
cDepartment of Seafood Science, National Kaohsiung Marine University, Kaohsiung 811, Taiwan
dDepartment of Food Science, Rutgers University, New Brunswick, NJ 08901, USA
eDepartment of Medical Research, China Medical University Hospital, China Medical University, Taichung 40402, Taiwan
fDepartment of Health and Nutrition Biotechnology, Asia University, Taichung, Taiwan
#These authors contributed equally to this work
*Corresponding author: Min-Hsiung Pan, Institute of Food Science and Technology, National Taiwan University, No. 1, Section 4, Roosevelt Road, Taipei 10617, Taiwan
DOI: 10.31665/JFB.2018.2144
Received: January 15, 2018
Revised received & accepted: February 19, 2018
| Abstract | ▴Top |
Nobiletin and 5-demethylnobiletin as polymethoxyflavone and hydroxy polymethoxyflavone, especially, have been reported to exhibit various beneficial biological activities for human health. In this study, the effects of NOB and 5-demethylnobiletin (DMNB), on azoxymethane/dextran sodium sulfate (AOM/DSS)-induced colorectal carcinogenesis in Institute for Cancer Research (ICR) mice were compared. We found that NOB and DMNB significantly alleviated the weight loss, colon length and reduced colon tumor formation in AOM/DSS-induced colorectal carcinogenesis mice. At the molecular level, our results from western blot and polymerase chain reaction (PCR) analysis showed that 0.025% of NOB and DMNB presented an anti-inflammation property by reducing cyclooxygenase-2 (COX-2) and interleukin 6 (IL-6) expression. Taken together, these results demonstrate that DMNB had a better chemo-preventive efficacy than NOB in AOM/DSS-induced colorectal carcinogenesis in ICR mice model.
Keywords: Nobiletin; 5-Demethylnobiletin; Colorectal carcinogenesis; inflammation; Chemoprevention
| 1. Introduction | ▴Top |
Colorectal cancer (CRC) is one of the most common encountered cancers worldwide (Torre, Bray et al. 2015). Diet, lack of physical activity, family history of sporadic CRC and disease duration are risk factors of CRC development (Mandal 2017). Nowadays, chronic inflammation is recognized as a major risk factor for CRC development and progression and the progressive colorectal tumors are also associated with high levels of inflammation environment(Terzić, Grivennikov et al. 2010, Mandal 2017, Ray, Berggren et al. 2017). Moreover, chronic inflammation involves in several immune cell, cytokine, and proinflammatory mediators during the process of carcinogenesis (Mandal 2017). The proinflammatory cytokine interleukin 6 (IL-6) has been identified as a major regulator of inflammatory colon diseases and is correlated with CRC development, tumor metastasis, and reduced survival rate (Waldner, Foersch et al. 2012, Kakourou, Koutsioumpa et al. 2015, Wang, Wu et al. 2015). Moreover, inflammation-associated enzymes, such as cyclooxygenase-2 (COX-2), contribute to colitis-related colon tumorigenesis by production of prostaglandin E2 (PGE2) and can be upregulated by azoxymethane/dextran sodium sulfate (AOM/DSS)-induced CRC in animal model that is a commonly used platform for colitis-related carcinogenesis intervention study (Snider, Bialkowska et al. 2016). Therefore, suppress severe inflammation responses in CRC development could be retarded the progressive of CRC.
Nobiletin (5,6,7,8,3′,4′-hexamethoxyflavone, NOB) (Figure 1A) is one of the major polymethoxyflavones in citrus peel and has gained increasing interest due to its beneficial properties including anti-inflammation, anti-cancer, and human health-promoting effects (Wu, Song et al. 2015, Li, Wang et al. 2017, Zheng, Bu et al. 2017). The compound 5-demethylnobiletin (5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone, DMNB) (Figure 1B) is a hydroxy polymethoxyflavone, which is also found in citrus peel and/or in aged tangerein peels. Both NOB and DMNB have shown anti-cancer effects, and regulate lipid metabolism (Chen, Wang et al. 2015, Tung, Li et al. 2016). NOB has shown its effect on CRC, however, the role of DMNB on CRC has not yet been investigated (Wu, Song et al. 2015). In this study, for the first time, chemo-preventive effect of NOB and DMNB, in AOM/DSS-induced colitis-associated carcinogenesis in ICR mice were investigated and offer possible molecular mechanism of their action suggested.
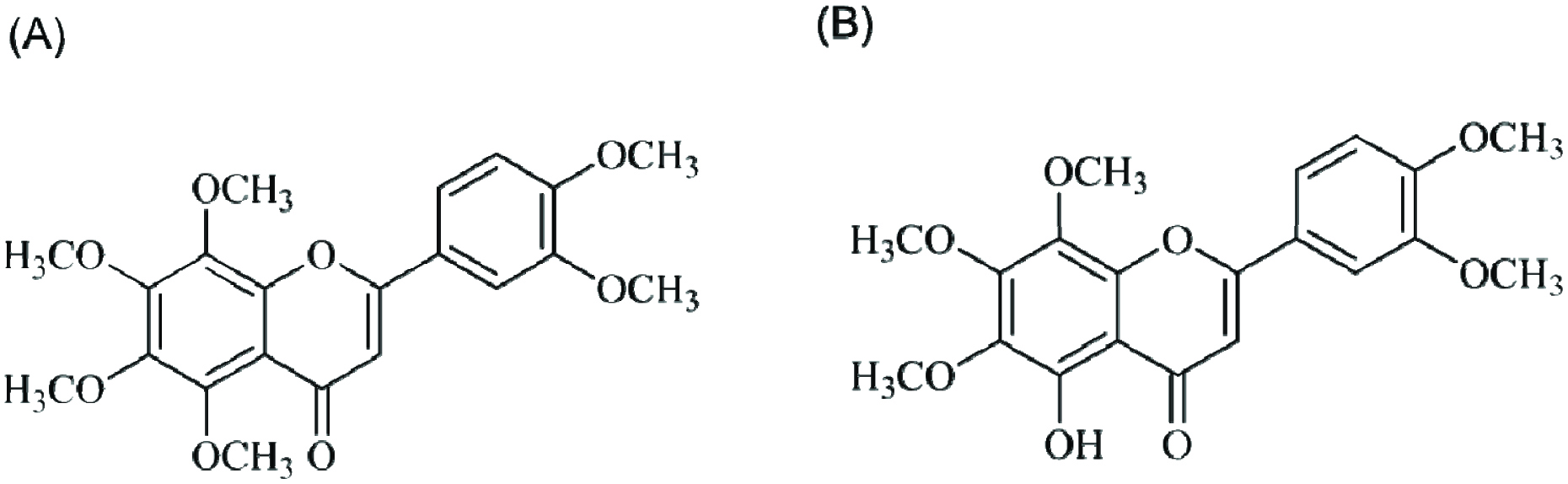 Click for large image | Figure 1. Structures of (A) nobiletin and (B) 5-demethylnobiletin. |
| 2. Materials and methods | ▴Top |
2.1. Reagents
The NOB and DMNB were obtained from Florida Flavors Company (Lakeland, FL, USA). The purity of NOB and DMNB was determined to be higher than 95% by high-performance liquid chromatography (HPLC).
2.2. Animals and experimental procedure
Male Institute for Cancer Research (ICR) mice of 4-6 weeks of age were purchased from the BioLASCO experimental animal center (Taiwan Co., Taipei, Taiwan). All animals were housed under a controlled atmosphere (25 ± 1 °C, 50% relative humidity, 12 h light/12 h dark cycle) and fed a standard AIN-76 diet and tap water ad libitum at all times. All experimental protocols used in these animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC 0101-AAAP-09) of National Kaohsiung Marine University.
The animal experimental design in this study followed our previous study (Figure 2) (Lai, Yang et al., 2017). Briefly, all mice were randomly divided into six groups. Mice fed with normal diet and water as the control group. Mice in AOM/DSS treatment groups were administered AOM at a dose of 10 mg/kg via an intraperitoneal injection twice, followed by 2% DSS in drinking water as the AOM/DSS group. The NOB and DMNB treatment groups were fed diets containing 0.01 or 0.025% NOB and DMNB, respectively. At 15 weeks, all mice were euthanized by CO2 asphyxiation for further examination of molecular and histopathological changes in the colon tissues. The liver, spleen, and kidneys were also immediately collected and weighed.
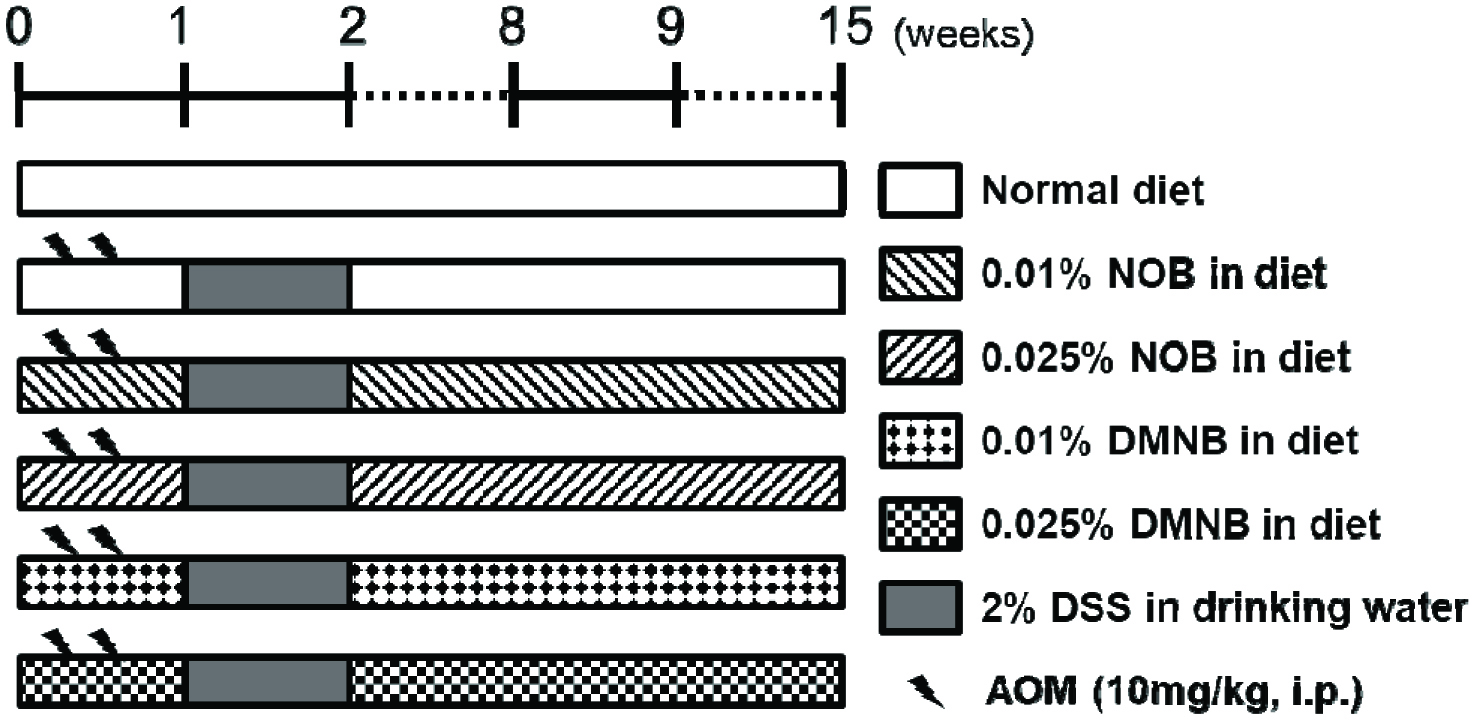 Click for large image | Figure 2. Experimental design of AOM/DSS-induced colorectal tumorigenesis in male ICR mice for 15 weeks. |
2.3. Reverse transcription polymerase chain reaction
Total RNA was extracted from the colonic mucosa using the TRIzol reagent (Invitrogen) according to the supplier’s protocol. The complementary DNA (cDNA) was synthesized with 1 mg total RNA, using SuperScript II RNA reverse transcriptase (RT) (Invitrogen). In the RT-PCR analysis, specific primers were designed to target IL-6 and COX-2. The RT-PCR primers used in this experiment are as follows:
IL-6, 5’-TGGAGTACCATAGCTACCTGGAG-3’, and 5’-TCCTTAGCCACTCCTTCTGTG-3’; COX-2, 5’-GGAGAGACTATCAAGATAGTGATC-3’ and 5’-ATGGTCAGTAGACTTTTACAGCTC-3’; and GAPDH, 5’-TCAACGGCACAGTCAAGG-3’ and 5’-ACTCCACGACATACTCAGC-3’. The PCR products were separated by electrophoresis on a 2% agarose gel and visualized using ethidium bromide (EtBr) nucleic acid staining (Yeastern Biotech, Taipei, Taiwan).
2.4. Immunohistochemical analysis
Colon tissues were fixed overnight in 10% buffered formalin and embedded in paraffin. Sections of 3-μm thickness were incubated with 0.3% hydrogen peroxide to quench the endogenous peroxidase activity and were then heated in 10 mM citrate buffer (pH 6.0) in a microwave oven for 10 min twice. Sections were then incubated with IL-6 primary antibody for 1 h at room temperature. Sections were incubated with a biotin-conjugated horseradish peroxidase secondary antibody, and 3,3’-diaminobenzidine tetrahydrochloride was used as the substrate.
2.5. Western blot analysis
For protein analyses, scraped colon mucosa tissue was homogenized with lysis buffer (50 mM Tris-HCl, 1 mM NaF, 150 mM NaCl, 1 mM ethylene glycol tetraacetic acid (EGTA), 1 mM phenylmethanesulfonyl fluoride, 1% NP-40, and 10 μg/mL leupeptin) on ice for 30 min, followed by centrifugation at 12,000 × g for 30 min at 4 °C to extract total protein. The 50-μg protein samples were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and the gel was electroblotted onto 0.45 μm polyvinylidene difluoride membrane. The membranes were blocked with 1% bovine serum albumin (BSA) solution and immunoblotted overnight at 4 °C with a primary antibody followed by a secondary antibody conjugated with horseradish peroxidase and visualized using enhanced chemiluminescence (ECL; Amersham Bioscience, Piscataway, NJ, USA). The densities of the bands were quantified using a densitometer (AlphaImagerTM 2200 System Alpha densitometer, Innotech Corporation, San Leandro, CA, USA).
2.6. Statistical analysis
All data were expressed as mean ± SD for the indicated number of independently performed experiments. Statistical differences were determined using student’s t-tests, one-way analysis of variance (ANOVA), and Duncan’s multiple range tests using the SAS statistical software package (version 8.0). Differences of a p-value < 0.05 were considered statistically significant.
| 3. Results and discussion | ▴Top |
3.1. DMNB exhibited higher activity in preventing colonic tumor formation than NOB in an AOM/DSS-induced CRC model
The AOM/DSS-induced colitis-associated colorectal tumorigenesis model is a recognized experimental model for investigating CRC development, and it has been considered an effective platform for evaluating the chemopreventive and chemotherapeutic activity of drugs and phytochemicals (Neufert, Becker et al. 2007, Lai, Yang et al. 2017). To evaluate and compare the effects of NOB and DMNB on AOM/DSS-inducedCRC model, we design the experimental model of AOM/DSS and NOB or DMNB treatment as shown in Figure 2. A previous study showed that 0.05% of NOB could alleviate the weight loss and decrease colon tumor incidence in vivo (Wu, Song et al. 2015). In this study, we found that the body weight decrease was accompanied with tumor formation in the AOM/DSS group (43.3 ± 3.3 g) compared with the control group (50.7 ± 3.3 g) Mice were fed with NOB or DMNB to retard body weight loss caused by AOM/DSS. The relative organ weight in each group only the spleen weight of AOM/DSS group was heavier than other groups, due to the DSS cause inflammation in spleen (Perše and Cerar 2012) (Table 1).
 Click to view | Table 1. Effects of NOB or DMNB on body weight and relative organ weight in AOM/DSS-induced CRC model |
Table 2 shows that AOM/DSS treatment significantly reduced the colon length, which was also observed in several other studies (Neufert, Becker et al. 2007, Chou, Suh et al. 2017, Zhang, Su et al. 2018). Dietary supplementation with NOB and DMNB restored the colon length (mm) to 78.3 ± 14.3 and 90.6 ± 14.4, and 81.2 ± 10.2 and 101.3 ± 11.4, at 0.01 and 0.025% NOB or DMNB, respectively, compared to 67.4 ± 6.0 in the AOM/DSS-treated group. We also observed that the AOM/DSS treatment group showed 100% tumor incidence; the total number of tumors in the AOM/DSS-treated group was 24.4 ± 8.5. The total number of colonic tumors was markedly and dose-dependently decreased by 0.01 and 0.025% NOB or DMNB consumption (10.2 ± 3.8, 8.9 ± 2.5 and 8.0 ± 3.6, 7.6 ± 3.1, respectively) (Figure 3 & Table 2). These results revealed that both compounds could restore more colon length and decrease the number of tumor in AOM/DSS-induced colorectal tumorigenesis animal model.
 Click to view | Table 2. Effects of NOB or DMNB on the colon length and number of tumors in AOM/DSS-induced CRC model |
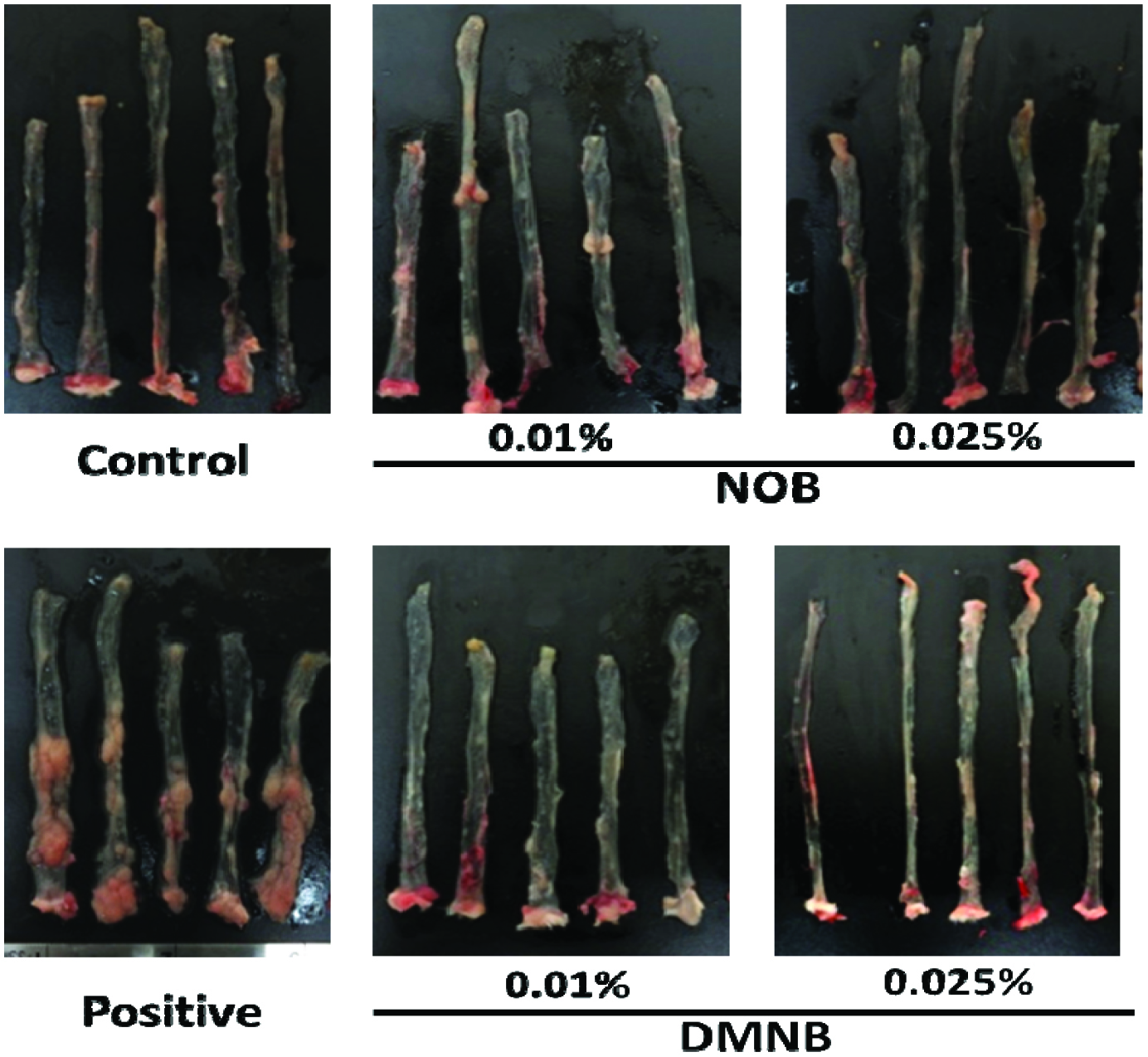 Click for large image | Figure 3. Morphology of colonic mucosal surface of AOM/DSS-treated mice. |
3.2. Orally administrated DMNB significantly prevented AOM/DSS-induced inflammation through decreased IL-6 and COX-2 expression levels in colonic mucosal tissue
Growing evidence indicates that chronic inflammation is a major and determining factor in initiation and promotion of CRC (Do, Hwang et al. 2016). For example, IL-6 is an important cytokine in the development of colitis and CRC by acting as a tumor-promoting factor and increasing the expression level of IL-6, which is correlated in AOM/DSS-treated rodents with colitis and patients with inflammatory bowel disease (Li, de Haar et al. 2010, Wang and Sun 2014, Chen, Ma et al. 2015). In this study, the results showed that NOB and DMNB significantly suppressed IL-6 expression in the colon tissue by immunohistochemical analysis. We also found that NOB and DMNB suppressed IL-6 protein and mRNA expression compared with AOM/DSS group (Figure 4). (Figures 5A & 5C). Furthermore, COX-2, which is an inflammation biomarker that contributes to colon tumorigenesis by the production of prostaglandin E2 and our previous study showed that AOM/DSS treated mice had higher protein level of COX-2 (Figure 5B & 5C)(Takahashi and Wakabayashi 2004, Lai, Yang et al. 2017). Wu et al. (2017) showed that a nobiletin mixture containing three metabolites (3′-demethylnobiletin, 4′-demethylnobiletin and 3′,4′didemethylnobiletin) suppressed colitis-associated colon carcinogenesis by down-regulating iNOS in AOM/DSS induced CRC model (Wu, Song et al. 2017). Another study showed that hydroxylated PMFs decreased the number of aberrant crypt foci in colonic tissues by significantly decreasing the levels of inducible nitric oxide synthase, COX-2 in AOM-induced colonic tumorigenesis model (Lai, Tsai et al. 2011). In this study, AOM/DSS significantly induced COX-2 expression, and it was suppressed by mice fed with NOB or DMNB. Interestingly, DMNB group reduced more COX-2 expression than NOB group. Consequently, our results demonstrated that the chemo-preventive effect of DMNB was more potent than that of NOB in this chemopreventive effect and was associated with a decreased inflammation and tumor formation in the AOM/DSS-induced CRC model.
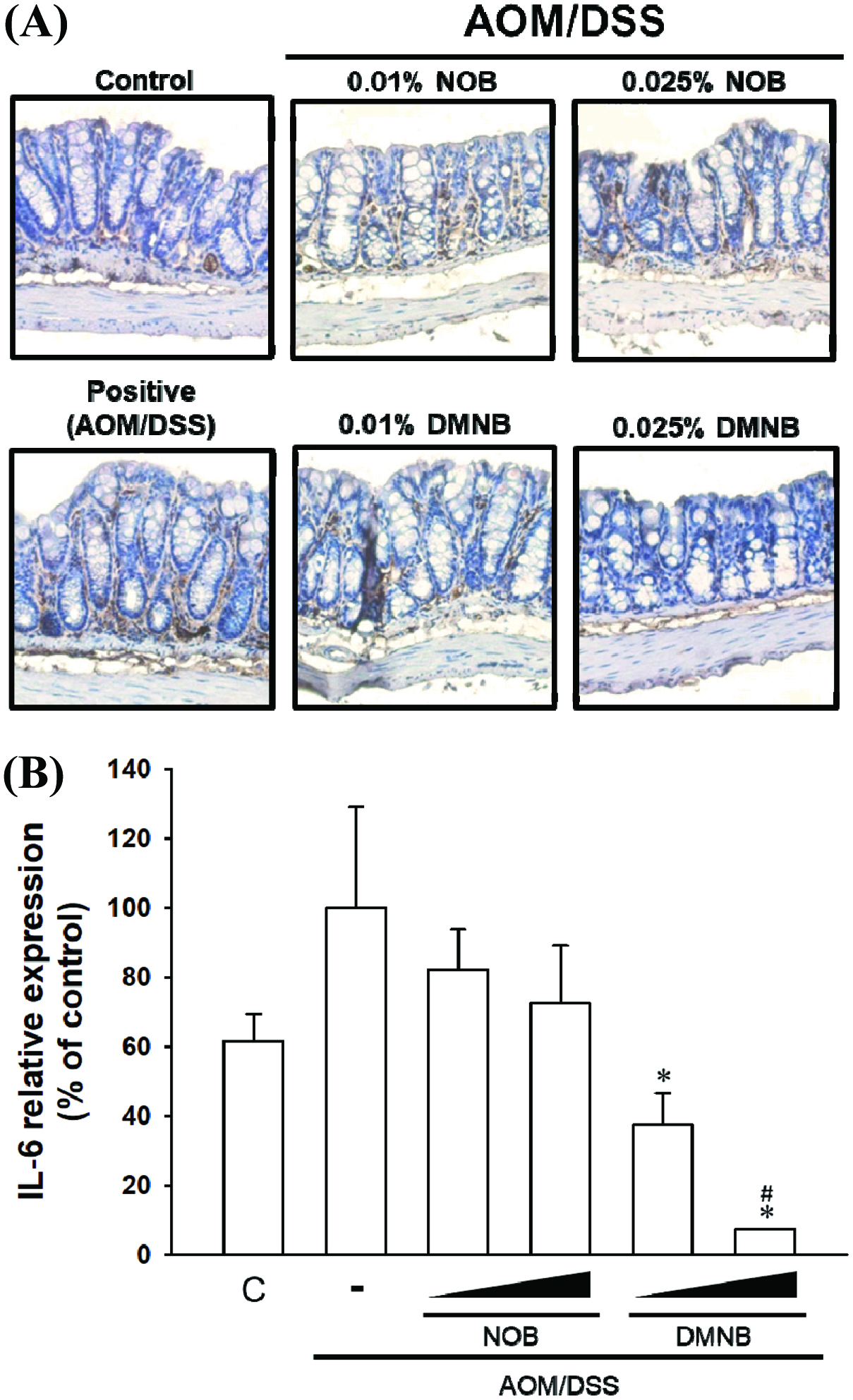 Click for large image | Figure 4. Inhibitoy effect of NOB and DMNB on AOM/DSS-induced IL-6 expression and distribution in colorectal tissue. |
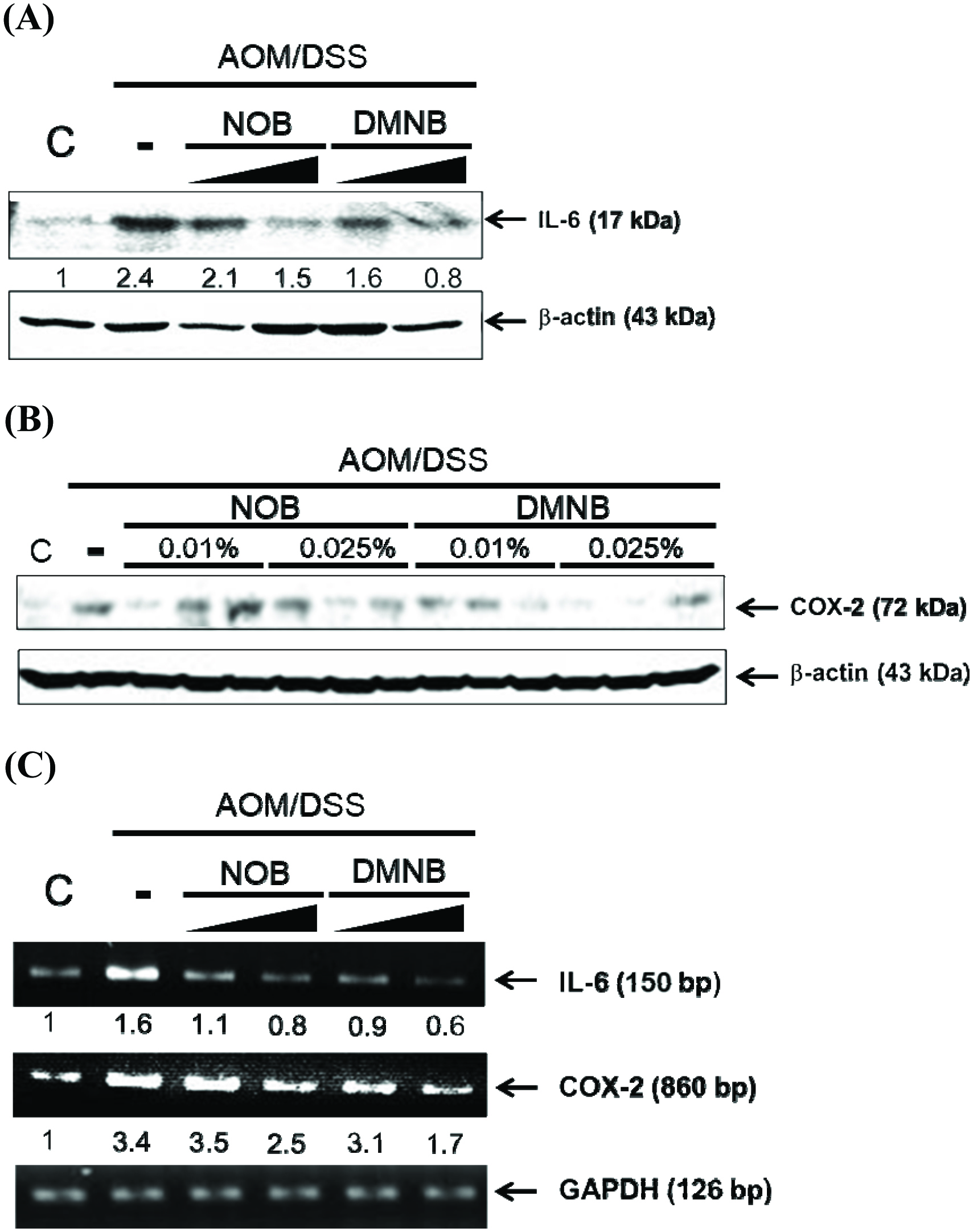 Click for large image | Figure 5. The NOB and DMNB significantly prevented AOM/DSS-induced inflammatory responses in colonic mucosa tissue. |
| 4. Conclusion | ▴Top |
The dietary administration of NOB and DMNB suppressed AOM/DSS-induced CRC by improving colon shortening, tumor formation, and inflammatory responses. More importantly, DMNB interfered with IL-6, and COX-2 expression and this could be a possible mechanism for better prevention against the CRC effect than NOB. These results show that DMNB has great potential as a novel chemopreventive agent that can be used in the prevention of colitis-associated CRC.
Acknowledgments
This study was supported by the Ministry of Science and Technology [105-2320-B-002-031-MY3, 105-2628-B-002-003-MY3].
Conflict of interest
The authors declare no conflict of interest.
| References | ▴Top |