
Figure 1. Total number of papers published in each year from 2010 to 2020 related to the exploration of marine-based novel natural components (Carroll et al., 2022, 2021, 2019; Blunt et al., 2018, 2017, 2016, 2015, 2014, 2013, 2012).
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 19, September 2022, pages 4-96
Novel marine bioactives: application in functional foods, nutraceuticals, and pharmaceuticals
Figures


























Tables
| Compound | Classification of compounds | Species of sponge | Biological activity | References |
|---|---|---|---|---|
| SW1990 - Human pancreatic adenocarcinoma cell line, PANC-1 - Human pancreatic cancer cell line isolated from a pancreatic carcinoma of ductal cell origin, DU145 - Human prostate cancer cell line, Huh7 - hepatoma tissue cell lines, HepG2 - human liver cancer cell line. | ||||
| Zampanolides B, C and D | Macrolide | Cacospongia mycofijiensis | Microtubule stabilizing action with anti-mitotic and anti-proliferative properties | Taufa et al., 2018 |
| Gracilosulfates A, B, C, D, E, F and G | Steroid | Haliclona gracilis | Anti-tumor activity on the human prostate cancer cell lines | Shubina et al., 2020 |
| Monacolin X | Polyketide | Monascus sp. | Anti-migratory and anti-proliferative effects on human breast cancer cell lines | Nagabhishek and Madankumar, 2019 |
| Botryorhodines I and J | Depsidones | Setosphaeria sp. | Act as an anti-fungal agent against the phytopathogenic fungi Colletotrichum acutatum and Colletotrichum asianum | Pang et al., 2018b |
| Osirisynes G, H, and I | Long-chain highly oxygenated polyacetylenes | Haliclona sp. | Enzyme inhibition against proteasome kinase | Campos et al., 2020 |
| Mycalolide A, Mycalolide B, 38-hydroxymycalolide B | Macrolide | Mycale aff. nularosette | Incomplete cytokinesis due to actin depolymerization | Hayashi-Takanaka et al., 2019 |
| 19-methoxydictyoceratin-A | Sesquiterpene quinones | Dactylspongia elegans T3 | Anti-cancer effect on the human cancer cell lines SW1990, PANC-1, DU145 and Huh7 | Yu et al., 2019 |
| 5,6-dibromo-8,1′-dihydro-isoplysin A; 6-bromo-8,1′-dihydro-isoplysin A | Tryptophan derived alkaloids | Fascaplysinopsis reticulata | Antibacterial activity against Vibrio sp. | Campos et al., 2019 |
| Lectin | Glycoprotein | Axinella donnani | Anti-biofilm activity against biofilm-producing S. aureus and antibacterial activity against S. aureus bacterial cells | Sadanandan and Rauf, 2018 |
| 3,5-dibromo-2-(2,4-dibromophenoxy)-phenol 3,4,5-tribromo-2-(2,4-dibromophenoxy)-phenol | Polybrominated diphenyl ethers | Dysidea sp. | Inhibit the production of Hepatitis B viral cells in the cell line HepG2.2.15.7 | Yamashita et al., 2015 |
| Meroterpenes Alisiaquinone A Alisiaquinone B Alisiaquinone C Alisiaquinol | Verongula rigida (New Caledonian deep-sea sponge) | Anti-malarial activity against chloroquinone-resistant Plasmodium falciparum strain and enzymes protein farnesyltransferase and plasmodium kinase Pfnek-1 | Desoubzdanne et al., 2008 | |
| Alkaloid group | Sponge | Name of the compound | Biological activity | References |
|---|---|---|---|---|
| MCF-7 - human breast cancer cell lines, HCT-116 - human colorectal carcinoma cell lines, A549 - lung carcinoma cell lines, HT-29 - human colorectal adenocarcinoma cells, MDA-MB-231 – human breast cancer cell lines, L1210 - mouse lymphocytic leukemia cell line, KB - human epithelial carcinoma cells, THP-1 - human leukemia monocytic cell line, A2780 - human ovarian cancer cells, K-562 - myelogenous leukemia cell lines, P388 - lymphocytic leukemia cells, JB6C141 - mouse epidermal cell line, HL-60 - human leukemia cells, HepG2 - human liver cancer cell line, OVCAR - ovary carcinoma cells, HOP-92 - pulmonary carcinoma cells, PC3 - prostatic carcinoma cells, SK-MEL-28 - melanoma cells, UO-31 - kidney carcinoma cells, A431 - human epidermoid carcinoma cells, NSCLC-N6 - human bronchial epidermoid carcinoma cells, MDA-435 - metastatic human breast cancer cells, MRC-5 - human fetal lung fibroblast cells, PC9 - lung adenocarcinoma cell line, HM02 - well-differentiated gastric carcinoma cells, Huh7 - hepatoma tissue cell lines, C-38 - murine adenocarcinoma cell line, OVCAR-3 - ovarian adenocarcinoma cell line, MALME-3M - malignant human melanoma cell line, MM.1S - B lymphoblast cell, Sw620 - human colorectal adenocarcinoma cell lines, HCC-2998 - human colon carcinoma cell line, MOLT-4 - T lymphoblast cell line, mES - mouse embryonic stem, SKBR3 - human breast cancer cell line, B16 - murine tumor cell line, L929- fibroblast cell line, U138 - Colon cancer cells, SF-295 - human glioblastoma cell line, MDA-MB435 - Melanoma cell lines, HCT-8 - Colorectal cancer cell lines, A498 - renal cancer cell lines, PSN1 - human pancreatic adenocarcinoma cell line, LN-caP - prostate adenocarcinoma cell line, PANC-1 - human pancreatic cancer cell line, DU-145 - human prostate cancer cells, SKOV-3, IGROV, IGROV-ET - human ovarian cancer cell line, SK-BR-3 - human breast cancer cell line, MEL-28 - Melanoma cell lines, H-MEC-1 - Human microvascular endothelial cells, LoVo - lymph node metathesis cells, L5178Y - mouse lymphoma cell, SW480 - human colorectal carcinoma cell line, H460 and H292- human non-small-cell lung cancer cells, Du145 - human prostate cancer cells, BE2-C - neuroblastoma cell line, SJ-G2 – glioblastoma cancer cells, MIA - pancreatic cancer cells, SMA and U87 - human glioblastoma cell line, p53+/+, p53−/−, p21+/+, and p21−/− - isogenic HCT-116 human colon tumor cell lines, AsPC-1, BxPC3, and MIA PaCa2 - human pancreatic tumor cell line, P388D1 - mouse lymphoma cells, LU-1 - cancer cell line, SK-Mel-2 - human melanoma cell lines, 3Y1 - rat embryo fibroblast cell line, SK-MEL - Human malignant melanoma cells, BT-549 – Breast cancer cell lines, LLC-PK11, CLL - Chronic lymphocytic leukemia, LNCaP - human prostate adenocarcinoma cells, H4IIE - rat hepatoma cell lines. | ||||
| Acridine alkaloid | Dercitus sp. | Dercitin | Potent cytotoxic activity in nanomolar concentration | Burres et al., 1989 |
| Xestospongia sp. | Neoamphimedine; 5-methoxyneoamphimedine; Alpkinidine | Selective activity for solid tumors | Thale et al., 2002 | |
| β-carbolines | Acanthostrongylophora ingens | Acanthomine A; 1,2,3,4-tetrahydronorharman-1-one; Ingenine E; Annomontine | Potent cytotoxicity against human breast cancer (MCF7), colorectal carcinoma (HCT116), and lung carcinoma (A549) cell lines | Ibrahim and Mohamed, 2017 |
| Bromotyrosine | Jaspis sp.; Bubaris sp. | Aplyzanzine B | Strong cytotoxicity against human lung carcinoma epithelial cells (A549), colorectal adenocarcinoma(HT-29), and breast cancer (MDA-MB-231) cell lines | Tarazona et al., 2017 |
| Hexadella sp. | Anomoian B | |||
| Suberea sp. | Ma’edamines C; Ma’edamines D | Selective cytotoxicity on murine leukemia L1210 cell line | Kurimoto et al., 2019 | |
| Suberedamine A; Suberedamine B | Potent cytotoxic activity against mouse lymphocytic leukemia (L1210) and human epithelial carcinoma (KB) cell lines | Tsuda et al., 2001 | ||
| Psammoclemma sp. | Psammaplysene C; Psammaplysene D | Potent cytotoxic activities against human leukemia monocytic (THP-1) cell line | Buchanan et al., 2007 | |
| Druinella sp. | Purealidin S; Purpuramine J PurealidinQ; Aplysamine 2 Purpureamine I Aerophobin 2; Aerophobin1; Purealidin J; Araplysillin1; Araplysillin 2 | Potent to moderate cytotoxic activities against human ovarian cancer (A2780) and myelogenous leukemia (K562) cell lines | Tabudravu and Jaspars, 2002 | |
| Dibrominated and Brominated | Agelas dendromorpha | Agelastatin E | Strong cytotoxicity against human epithelial carcinoma (KB) cell lines | Tilvi et al., 2010 |
| Suberea sp. | Aerothionin | Potent cytotoxicity against HeLa cells | Shaala et al., 2015 | |
| Psammoclemma sp. | Echinosulfonic acid D; Echinosulfonic acid B | Potent cytotoxicity against human epithelial carcinoma (KB) cell lines | Rubnov et al., 2005 | |
| Aaptamine | Aaptos suberitoides | Aaptamine; Isoaaptamine; Demethylaaptamine | Proteasome inhibitory action and potent cytotoxicity against HeLa cell lines | Tsukamoto et al., 2010 |
| Suberitine A; Suberitine B; Suberitine C; Suberitine D | Selective and potent cytotoxicity against lymphocytic leukemia (P388), HeLa and myelogenous leukemia (K562) cell lines | Liu et al., 2012a | ||
| Guanidine | Monanchora pulchra | Monanchocidin A | Potent cytotoxicity against monocytic anemia cell lines THP-1, Hela, and JB6C141 cell lines | Guzii et al., 2010 |
| Monanchocidin B–E | Strong inhibitory activity against human leukemia (HL-60) cells | Makarieva et al., 2011 | ||
| Crame crame | Crambescidin-816 | Reduce cell viability of human liver cancer (HepG2) cell line; Affect the human tumor cell lines such as ovary carcinoma (OVCAR), pulmonary carcinoma (HOP-92), breast cancer cells (MCF-7), prostatic carcinoma (PC3), melanoma (SK-MEL-28), kidney carcinoma (UO-31), and colorectal adenocarcinoma (HT-29) | Rubiolo et al., 2014 | |
| Clathria bulbotoxa | Crambescidins 345; Crambescidins 361; Crambescidins 373; Crambescidins 359; Crambescidins 657 | Cytotoxic activity against human epidermoid carcinoma (A431) cell line | Kasmiati et al., 2018 | |
| Monanchora pulchra | Normonanchocidin A; Normonanchocidin B; Normonanchocidin D | Cytotoxicity against human leukemia monocytic (THP-1) and HeLa cells | Tabakmakher et al., 2015 | |
| Monanchomycalin C; Ptilomycalin A | Effect the human breast cancer (MDA-MB-231) cells | Tabakmakher et al., 2013 | ||
| Monanchoxymycalin C | Inhibit the colony formation of HeLa cells | Shubina et al., 2019 | ||
| Biemna laboutei | Netamine M; Netamine O; Netamine Q | Potent cytotoxicity against human epithelial carcinoma (KB) cell line | Gros et al., 2014, 2015 | |
| Anchinoe pauperta | Zanissine | Cytotoxic effects on murine pre- B cell lymphoma (P-388), KB, and human bronchial epidermoid carcinoma (NSCLC-N6) cell lines | Bouaicha et al., 1994 | |
| Monanchora sp. | Unguiculin A; Unguiculin B; Unguiculin C | Potent effect against KB cancer cell lines | El-Demerdash et al., 2018 | |
| Monanchoradin A; Dehydrocrambescin A2 418; Crambescidin 786; (−)-crambescidin 814; Monalidine A; (–)-crambescin 406; Crambescidin 800; Crambescidin 826; 20-norcrambescidic acid | Cytotoxic activity against human colorectal carcinoma cell (HCT-116), metastatic human breast cancer (MDA-435), human leukemia cells (HL-60), KB, and MRC-5 cell lines | El-Demerdash et al., 2016 | ||
| Imidazole | Leucetta chagosensis | (−)-calcaridine; (2E, 9E)-pyronaamidine-9-(N-methylimine) | Selective cytotoxicity against MCF-7 cell lines | Tang et al., 2019 |
| Pericharax heteroraphis | Naamidine J | Inhibited the growth of human breast cancer (K562) cell line | Wei et al., 2020 | |
| Naamidine H | Inhibitory effect on K562, HeLa, and adenocarcinomic human alveolar basal epithelial cells (A549) cell lines | |||
| Leucandra sp. | Naamine J | Inhibitory effect on MCF-7, A549, HeLa, and PC9 cell lines | Tang et al., 2016 | |
| Leucetta chagosensis | Naamidine I; Naamidine H | Cytotoxic effect on the HeLa cell line | Tsukamoto et al., 2007 | |
| Isonaamine C; Isonaamidine E | Growth inhibitory effect HM02, hepatocellular carcinoma (HepG2), and hepatoma tissue (Huh7) cell lines | Gross et al., 2002 | ||
| Chagosendine B; Chagosendine C | Potent inhibition against K562, HepG2, and HeLa cell lines | An et al., 2018 | ||
| Pyronaamidine | Inhibited K562 and HeLa cell lines | |||
| Leucosolenia sp. | Leucosolenamine B | Potent cytotoxic effect on the C-38 cell line | Ralifo et al., 2007 | |
| Indole, Bisindole, and Trisindole | Thorectandra sp. | Demethoxyfascaplysin | Cytotoxic effects on breast cancer cell line | Charan et al., 2004 |
| 1-deoxysecofascaplysin A | Inhibited the growth of MCF-7, OVCAR-3, and A549 | |||
| Fascaplysin | Cytotoxicity against MCF-7, OVCAR-3, MALME-3M and A549 | |||
| Lipastrotethya sp. | Dragmacidin G; Dragmacidin H; Topsentin B2 (bromotopsentin) Topsentin; B1 (topsentin) | Cytotoxic effect on HeLa cells | Hitora et al., 2016 | |
| Hyrtios sp. | Hyrtinadine A | Potent cytotoxicity on L1210 and KB cell lines | Endo et al., 2007 | |
| Hyrtios erectus | Hyrtioerectine A; Hyrtioerectine B; Hyrtioerectine C | Cytotoxic effects on HeLa cell line | Youssef, 2005 | |
| Callyspongia siphonella | 5-bromotrisindoline; 6-bromotrisindoline | Inhibitory effect on HT-29, OVCAR-3, and MM.1S cell line | El-Hawary et al., 2019 | |
| Smenospongia sp. | 5-bromo-l-tryptophan; 5-bromoabrine; 5,6-dibromoabrine; 5-bromoindole-3-acetic acid | Least cytotoxicity in a set of isogenic HCT116 cell lines | Tasdemir et al., 2002 | |
| Damiria sp. | Damirine A | Cytotoxic effect on MALME-3M, Sw620, HCC-2998, MOLT-4, and k562 cell lines | Tran et al., 2019 | |
| Spongosorites sp. | 6″-Debromohamacanthin A (DBHA) | Cytotoxic effect in mES cell line over 20 µM | Kim et al., 2013 | |
| Peptide alkaloid | Scleritoderma nodosum | Scleritodermin A | Cytotoxicity on HCT116, A2780, and SKBR3 cell lines | Schmidt et al., 2004 |
| Piperidine alkaloid | Arenosclera brasiliensis | Arenosclerin B; Arenosclerin C; Haliclonacyclamine E | Cytotoxic effects on HL-60, B16, L929, and U138 cancer cell lines | Torres et al., 2002 |
| Pachychalina alcaloidifera | Madangamine F; Haliclonacyclamine F; Arenosclerins D; Arenosclerins E | Cytotoxicity on on SF295, MDA-MB435, HCT8, and HL60 cell lines | de Oliveira et al., 2007 | |
| Neopetrosia proxima | Neopetrosiamine A | Inhibitory effect on MALME-3M, CCRF-CEM, and MCF7 | Wei et al., 2010a | |
| Mycale sp. | 1,5-diazacyclohenicosane | cytotoxic activity against A549, HT29, and MDA-MB-231 | Coello et al., 2009 | |
| Pachychalina sp. | Ingenamine G | Potent cytotoxicity against HCT-8, B16, and MCF-7 | de Oliveira et al., 2004 | |
| Neopetrosia cf exigua | Papuamine; Haliclonadiamine | Effect on human glioblastoma cell line (SF-295), and human renal cancer cell lines (UO-31 and A498) | Liang et al., 2015 | |
| Haliclona sp. | Papuamine | Cytotoxicity on MCF-7 cell | Kanno et al., 2013 | |
| Pyrimidine alkaloid | Theonella swinhoei | Lanesoic acid | Selective cytotoxicity against PSN1cells | Rodríguez et al., 2016 |
| Kirkpatrickia variolosa | Variolin B | Potent activity against several cell lines including prostate adenocarcinoma cell line (LN-caP), K-562, PANC-1, HT-29, DU-145, SKOV-3, IGROV, IGROV-ET, SK-BR-3, MEL-28, H-MEC-1 and A-549, and LoVo, cell lines | Fresneda et al., 2006 | |
| Pyridine alkaloids | Haliclona sp. | Cyano-3-dodecyl pyridine | Moderate cytotoxicity against A549, MCF-7 and HeLa cell lines | Zhang et al., 2016a |
| Amphimedon sp. | Amphimedoside A; Amphimedoside B; Amphimedoside C; Amphimedoside D; Amphimedoside E | Cytotoxicity against P388 cell lines | Takekawa et al., 2006 | |
| Xestospongia sp. | N-methylniphatyne A | Potent cytotoxicity against PANC-1 cells | Tsukamoto et al., 2000 | |
| Niphates sp. | Niphatyne A | Cytotoxic effect on P388 cells | Arai et al., 2016 | |
| Amphimedon sp. | Pyrinodemin B–D | Strong cytotoxicity on L1210 and KB cell lines | Hirano et al., 2000 | |
| Cribrochalina sp. | Pyrinadines B–G | Potent cytotoxic effects on L1210 cell line | Kariya et al., 2006 | |
| Pyrrole and Bromopyrrole | Stylissa carteri | (+)-dibromophakelline; (Z)-3-bromohymenialdisine | Cytotoxic effects with inhibition of growth of L5178Y cell line | Hamed et al., 2018 |
| Agelas oroides | Oroidin | Cytotoxic activity on MCF-7, A2780, HT-29, SW480, H460, A431, Du145, BE2-C, SJ-G2, MIA, SMA, and U87 cell lines | Dyson et al., 2014 | |
| Pyrroloimino-quinone | Latrunculia brevis | Discorhabdin L; Discorhabdin I | Potent cytotoxic effects on the HT-29 cell line | Reyes et al., 2004 |
| Smenospongia sp. | Makaluvamine O | Activity against p53+/+, p53−/−, p21+/+, and p21−/− cell lines | Tasdemir et al., 2002 | |
| Zyzzya cf. fuliginosa | Makaluvamine P | Inhibitory effect on the KB cell line | Casapullo et al., 2001 | |
| Batzella sp. | Isobatzelline A; Isobatzelline C; Isobatzelline D; Isobatzelline E | Cytotoxicity in all pancreatic cell lines (AsPC-1, BxPC3, and MIA PaCa2) | Guzmán et al., 2009 | |
| Quinoline and Quinolizinde | Xestospongia sponge | Renierol | Potent cytotoxic activity against L1210 cell line | McKee and Ireland, 1987 |
| Suberea sponge | Lihouidine | Moderate cytotoxicity against P388D cell line | Bowden et al., 2004 | |
| Reniera sarai | Saraine A–C; Saraine 1-3 | Preliminary cytotoxicity in the brine shrimp cytotoxic bioassay | Caprioli et al., 1992 | |
| Xestospongia muta | Meso-araguspongine C; Araguspongines A, C, E, L, N−P | Cytotoxic activities against human cancer cell lines HepG-2, HL-60, LU-1, MCF-7, and SK-Mel-2. | Dung et al., 2019 | |
| Tetrahydro- isoqouinoline | Jorunnaf unebris Xestospongia sp. | Jorunnamycin A | Potent cytotoxic activity versus H292 and H460 cell line | Sirimangkalakitti et al., 2016 |
| Genera Reniera, Haliclona, Xestospongia, Neopetrosia, and Cribrochalina Xestospongia sp. | Renieramycin M | |||
| Neopetrosia sp. | Renieramycin J | Powerful cytotoxicity on 3Y1, HeLa, and P388 cells | Oku et al., 2003 | |
| Steroidal alkaloid | Corticium niger | Plakinamine I–K; Dihydroplakinamine K | Potent cytotoxicity against HCT-116 cell lines | Ridley and Faulkner, 2003 |
| Plakinamine N; Plakinamine O; Plakinamine J | Enhanced inhibitory effects against all of the colon cell lines | Sunassee et al., 2014 | ||
| Manzamine | Acanthostrongylophora ingens | (+)-8-hydroxymanzamine A; (+)-manzamine A | Cytotoxic effects against SK-MEL, KB, BT-549, HepG2, and LLC-PK11 cell lines | Samoylenko et al., 2009 |
| Diterpene | Agelas citrina | Agelasine E | Potent cytotoxicity against CLL cell line | Stout et al., 2012 |
| Fasciospongia sp. | 19-oxofasciospongine A | Cytotoxic effect on LNCaP, LU-1, and MCF-7 cell lines | Yao et al., 2009 | |
| Agelas nakamurai | Isoagelasine C | Effect on HCT-116, K562, and HL-60 cell lines | Chu et al., 2017 | |
| Sesquiterpene Quinones/ Hydroquinones | Dysidea avara | (−)-4′-methylaminoavarone | Weak toxicity against HCT116 and H4IIE cell lines | Imperatore et al., 2020; Hamed et al., 2013 |
| (−)-N-methylmelemeleone-A | Weak cytotoxic activity on L5178Y, HCT116, and H4IIE cell lines | |||
| (−)-3′-methylaminoavarone | High cytotoxic activity L5178Y and weak toxicity against HCT116 and H4IIE cell lines | |||
| Group of compounds | Species | Compound | Bioactivity | References |
|---|---|---|---|---|
| CAKI-1 - human clear cell renal cell carcinoma, EKVX - human lung adenocarcinoma cell line, Daoy - human medulloblastoma, HEp-2 - human laryngeal carcinoma, WiDr - human colon adenocarcinoma, MCF-7 - human breast adenocarcinoma, P-388 - lymphocytic leukemia cells, A-549 - lung carcinoma epithelial cells, HT-29 - human colon cancer cell line, AGS - human gastric adenocarcinoma hyperdiploid cell line, DLD-1 - human colorectal adenocarcinoma, IMR-90 - human diploid lung fibroblast, MOLT-4 - human T lymphocyte leukemia cells, P388D1 - murine macrophage cells, CCRF-CEM- human T-cell acute lymphoblastic leukemia, HL-60- human premyelocytic leukemia, NBT-T2 - Nara Bladder Tumor II, HCMV - Human cytomegalovirus, Hep3B and HepG2 - human hepatocellular carcinoma, iNOS - Inducible nitric oxide synthase, HSV-1 - herpes simplex virus type 1, RKO - human colon tumor cells, HCT116 - human colon cancer cell line, COX-2 - cyclooxygenase-2. | ||||
| Diterpenoid | Clavularia sp. | Stolonidiol | Effect on nervous system (Potent choline acetyltransferase inducible activity at 0.01−10 μg/mL levels) | Yabe et al., 2000 |
| Clavularia koellikeri | Cembrane-type diterpenoid | Cytotoxic effect on human colorectal adenocarcinoma cells (IC50 4.2 μg/mL) and potent growth inhibitory activity against human T lymphocytic leukemia cells (IC50 0.9 μg/mL) | Iwashima et al., 2000 | |
| Junceella juncea | Juncin ZII | Antifoulant effect towards the settlement of barnacle Balanus amphitrite larva at nontoxic concentrations with EC50 values of 0.004 | Qi et al., 2009 | |
| Antillogorgia bipinnata | Caucanolide A and D | In vitro antiplasmodial effect towards the malaria parasite, Plasmodium falciparum | Ospina et al., 2005 | |
| Antillogorgia elisabethae | Pseudopterosin P and Q | Antimicrobial activity against the Gram-positive bacteria Enterococcus faecalis, Staphylococcus aureus, and Streptococcus pyogenes at a dose of 25 μg/ml. | Ata et al., 2004 | |
| Ileabethoxazole | Antituberculosis (92% inhibition of Mycobacterium tuberculosis H37Rv at the levels of 128–64 μg/mL) | Rodríguez et al., 2006 | ||
| Homopseudopteroxazole | Antituberculosis (80% growth inhibitory effect towards M. tuberculosis H37Rv at the concentration of 12.5 μg/mL) | Rodríguez and Rodríguez, 2003 | ||
| Elisapterosin B | In vitro anti-tuberculosis activity (79% inhibitory effect towards M. tuberculosis H37Rv at a concentration of 12.5 μg/mL) | Rodriguez et al., 2000 | ||
| Aberrarone | In vitro antimalarial effect on a chloroquine-resistant strain of the protozoan parasite Plasmodium falciparum (IC50 = 10 μg/mL) | Rodríguez et al., 2009 | ||
| Antillogorgia kallos | Bielschowskysin | Antimalarial activity against Plasmodium falciparum (IC50 = 10 μg/mL) Antitumor effect towards the CAKI-1 (GI50 = 0.51 μM) and EKVX (GI50 < 0.01 μM) cell lines | Marrero et al., 2004 | |
| Leptogorgia virgulata | Pukalide; Epoxypukalide | Antifoulant activity (inhibit barnacle settlement with EC50 values ranging from 19 to 55 ng/ml) | Gerhart et al., 1988 | |
| Eunicea sp. | Dolabellanes | Antimalarial effect towards the protozoan parasite Plasmodium falciparum with IC50 values ranging from 9.4–59.6 μM | Wei et al., 2010b | |
| Eunicea fusca | Fuscoside A and B | Anti-inflammatory activity with potency similar to manoalide and indomethacin; Fuscoside B selectively prevent the synthesis of leukotrienes LTB4 and LTC4 | Shin and Fenical, 1991 | |
| Fuscoside E | Strong anti-inflammatory activity and antifouling activity against bacterial strains | Reina et al., 2011 | ||
| Asterospicularia laurae | Asterolaurin L | Moderate antitumor activity against Daoy, HEp-2, WiDr, and MCF-7 with ED50 values of 6.23, 4.12, 6.08, and 4.09 μg/ml, respectively | Lin et al., 2011 | |
| Cespitularia hypotentaculata | Cespitularin A–E | Cespitularin C showed potent cytotoxicity against P-388 and A549 cells; Cespitularin E exhibited strong cytotoxic effect against A549 cells; Cespitularins A, B, and D exhibited moderate cytotoxicity towards P-388 cells (With an ED50 of ≤4.0 μg/mL) | Duh et al., 2002 | |
| Xenia novaebritanniae | Xeniolide I | Antibacterial effect at a concentration of 1.25 μg/ml | Bishara et al., 2006 | |
| Novaxenicin B | Induce apoptosis in transformed mammalian cells at the levels of 1.25 μg/ml | |||
| Xenia plicata | Blumiolide C | Strong cytotoxicity against P-388 and HT-29 cells with an ED50 of ≤4.0 μg/mL | El-Gamal et al., 2005 | |
| Blumiolide A and B | Moderate cytotoxicity against P-388 cells with an ED50 of ≤4.0 μg/mL | |||
| Terpenoid | Junceella fragilis | Frajunolides C; Junceellolide E; 11α,20α-Epoxy-4-deacetoxyjunceellolide D | Mild anti-inflammatory effect at a concentration of 10 μg/ml | Shen et al., 2007 |
| Antillogorgia bipinnata | Bipinnapterolide B | Antituberculosis (66% inhibition against the growth of Mycobacterium tuberculosis H37Rv at 128 μg/mL) | Ospina et al., 2007 | |
| Antillogorgia elisabethae | Caribenols A and B | Strong inhibitory effect against the growth of Mycobacterium tuberculosis H37Rv at a concentration range of 128−64 μg/mL | Wei et al., 2007 | |
| Antillogorgia rigida | Curcuphenol | Antibacterial activity | McEnroe and Fenical, 1978 | |
| Isis hippuris | Suberosenol A | Potent cytotoxicity toward the above three cancer cells with an ED50 value of ≤ 4.0 μg/mL | Sheu et al., 2000 | |
| Suberosenol C | potent activity against the growth of P-388 and HT-29 cells with an ED50 value of ≤ 4.0 μg/mL | |||
| Prostanoid | Clavularia viridis | Claviridic acid A–D; Clavulones I–III | Cytotoxic activity against AGS tumor cells | Lin et al., 2008 |
| Halogenated prostanoids | Cytotoxicity against DLD-1, IMR-90, and MOLT-4 cells at IC50 values of 0.6, 4.5, and 0.52 μg/mL, respectively | Watanabe et al., 2001 | ||
| Claviridin A–D | Potent cytotoxic activity against four human cancer cells of Doay, Hep2, epitheloid carcinoma (HeLa), and WiDr | Shen et al., 2010 | ||
| Steroid | Clavularia viridis | Yonarasterols A, B, C, and E | Antitumor activity against human colorectal adenocarcinoma cells at IC50 3, 3, 50, and 0.02 μg/ml, respectively | Iwashima et al., 2001 |
| Carijoa sp. | Carijoside A | Anti-inflammatory activity (effects on elastase release (IC50 = 6.8 μg/mL) and superoxide anion generation (IC50 = 1.8 μg/mL) by human neutrophils; Moderate cytotoxicity against P388D1, DLD-1, CCRF-CEM, and HL-60 cell lines with ED50 of 10.4, 9.7, 13.1, and 12.0 μg/mL, respectively | Liu et al., 2010 | |
| Pseudopterogorgia sp. | Secosterols | Moderate inhibitory activity towards protein kinase C and potential anti-inflammatory and antiproliferative activity | He et al., 1995 | |
| Isis hippuris | Polyoxygenated gorgosterol (steroids) | Moderate cytotoxic effect against cultured NBT-T2 cells | Uddin et al., 2011 | |
| Polyoxygenated steroid | Inhibitory activity against HCMV, with an EC50 values of 2.0 μg/mL; Cytotoxicity against P-388 and A-549 cell lines with ED50 values of 3.2 and 3.86 μg/mL, respectively | Chen et al., 2011 | ||
| Isishippuric acid B | Potent cytotoxicity againstA549, P-388, and HT-29 tumor cells with ED50 values < 0.1 μg/mL | Sheu et al., 2004 | ||
| A-nor-22-epi-hippurin-2α-carboxylic acid | Antitumor activity against Hep3B and HepG2 cells with ED50 values at 6.9 and 3.6 μg/mL, respectively | |||
| Dendronephthya sp. | Isogosterones A–D | Antifoulant activity (Prevent the settlement of the barnacle Balanus amphitrite larva with an EC50 value of 2.2 μg/mL) | Tomono et al., 1999 | |
| Sesquiterpenoid | Lemnalia flava | Flavalin A and B | Neuroprotective activity | Lu et al., 2011a |
| Flavalin A | Anti-inflammatory activity (Dose-dependent inhibition of iNOS protein expression) | |||
| Paralemnalia thyrsoides | Paralemnolin Q and S | Neuroprotective activity | Huang et al., 2011 | |
| Echinogorgia pseudosassapo | 3β-methoxyguaian-10(14)-en-2β-ol | Antifoulant activity (antilarval effect against Balanus amphitrite larvae with EC50 value of 17.2 μg/mL and 50% inhibition towards the larval settlement of B. neritina at levels of 25 μg/mL); Mild anti-HSV-1 activity | Gao et al., 2011 | |
| Pyridine | Leptogorgia setacea; L. virgulata | Homarine (N-methyl-2-carboxypyridine) | Potential antifoulant activity (inhibited the growth of diatoms) | Targett et al., 1983 |
| Prostaglandin | Telesto riisei | Punaglandins | Antiproliferative activity (Induce apoptosis in RKO cells); Inhibit the activity of isopeptidase | Verbitski et al., 2004 |
| Dialkylamine | Antillogorgia acerosa | Bis(pseudopterane) amine | Antitumor activity against the HCT116 and HeLa cancer cell lines with the IC50 values of 4.2 μM and 42 μM | Kate et al., 2009 |
| Ergostanoid | Nephthea erecta | Ergostanoids | Anti-inflammatory activity (Decrease the levels of the iNOS and COX-2 protein); Cytotoxicity towards P-388 cell line with an ED50 value of 3.7 μg/mL | Cheng et al., 2009 |
| Secosteroid | Astrogorgia sp. | Astrogorgols | Inhibitory effects against human tumor related protein kinases | Lai et al., 2011 |
| Class of Echinodermata | Organism | Bioactive compounds | Group of the compounds | Biological activity | Reference |
|---|---|---|---|---|---|
| Holothuroidea | Isostichopus badionotus | Fucosylated chondroitin sulfate | Sulfated polysaccharides | Anticoagulant; Antiparasitic | Marques et al., 2016 |
| Cucumaria frondose; Thelenota ananas | Fucosylated chondroitin sulfate | Sulfated polysaccharides | Antihyperglycemic; Anticoagulant; Antithrombin; Insulin-sensitizing; Antiviral | Hu et al., 2014b; Huang et al., 2013 | |
| Ludwigothurea grisea | Fucosylated chondroitin sulfate, Glycosaminoglycans | Sulfated; polysaccharides | Antiparasitic; Anticoagulant; Antithrombin | Marques et al., 2016; Borsig et al., 2007 | |
| A. japonicus | Glucosamine; Galactosamine | Polysaccharides | Antihyperlipidemic; Antioxidant | Liu et al., 2012b | |
| S. japonicus | Holotoxin | Triterpene glycosides | Antifungal | Yano et al., 2013 | |
| Acaudina molpadioides | ACE inhibitory peptide | Peptide | Antihypertension | Zhao et al., 2009 | |
| Holothuria scabra | T-antigen-binding lectin | Peptide | Antibacterial | Gowda et al., 2008 | |
| Acaudina molpadioide; Bohadschia argus | Cerebrosides, galactocerebrosides, AMC-2 | Lipid | Anticancer; Antihyperlipidemic | Ikeda et al., 2009; Zhang et al., 2012a; Du et al., 2015 | |
| Holothuria atra | Lysophosphatidylcholine; L-PAF | Lysophospholipid | Anti-inflammatory | Nishikawa et al., 2015 | |
| S. japonicus, Acaudina; molpadioides | Cerebroside | Sphingolipid | Antioxidant | Duan et al., 2016; Xu et al., 2011a | |
| Holothuria parva | (Z)2,3-DPAN | Phenolic compounds | Anticancer | Amidi et al., 2017 | |
| Plesiocolochirus minaeus | β-carotene, echinenone, canthaxanthin, etc. | Pigments | Antioxidant | Maoka et al., 2015 | |
| C. frondosa | Frondanol A5 | Saponin | Anticancer | Jia et al., 2016; Janakiram et al., 2015 | |
| S. japonicus | SJAMP | Mucopolysaccharide | Antitumor; Immunomodulatory | Song et al., 2013 | |
| Asteroidea | Henricia leviuscula | Laeviusculosides | Polyhydroxysteroids | Hemolytic; Cytotoxic activity | Fedorov et al., 2008; Ivanchina et al., 2006 |
| Asteropsis carinifera | Thornasteroside A | Asterosaponins | Antitumor | Malyarenko et al., 2012 | |
| Linckia laevigata | Hexadecanoic acid | Lipids | Antifouling | Guenther et al., 2009 | |
| Ophiuroidea | Ophiomastix mixta | 2,3-Dimethyl butenolide | Terpene | Antitumor | Lee et al., 2007 |
| Astrotoma agassizii | Polyhydroxysterols | Steroidal compound | Antiviral | Comin et al., 1999 | |
| Ophiarachna incrassata | Steroidal glycosides | Steroidal glycosides | Antiviral | D’Auria et al., 1993 | |
| Ophioderma longicauda | Ophioxanthin | Carotenoid | Antioxidant | D’Auria et al.,1985 | |
| Echinoidea | Diadema setosum | DSG-A | Ganglioside | Neuritogenic | Yamada et al., 2008 |
| Lytechinus variegatus | Sulfated fucan | Polysaccharide | Anticoagulant | Pereira et al., 1999 | |
| Heliocidaris crassispina | Echinochrome A | Naphthoquinoid pigment | Anti-inflammatory; Antimicrobial; Antioxidant; Antitoxic agents | Jeong et al., 2014; Berdyshev et al., 2007 | |
| Strongylocentrotus droebachiensis | Strongylocins | Peptides | Antimicrobial | Li et al., 2008b | |
| Crinoidea | Comanthus parvicirrus | Naphthopyrones comaparvin | Naphthopyrones | Anti-inflammatory | Chen et al., 2014; Chovolou et al., 2011 |
| Neogymnocrinus richeri | Gymnochrome D | Anthraquinoid pigments | Antiviral | Laille et al., 1998 | |
| Anneissia bennetti | Rhodoptilometrin, crinemodin | Polyketides | Antipredatory | Rideout et al., 1979 |
| Species | Compound | Backbone Structure | References |
|---|---|---|---|
| D. auricularia | Dolabellol A (Bromo-chloro-diterpenoid) | 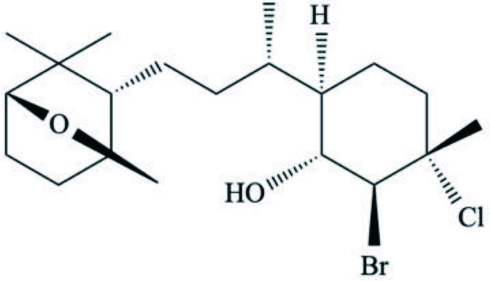 | Machida et al., 2017 |
| Phyllidiella pustulosa | Pustulosaisonitriles, 1–3 (Isonitriles) |  | White et al., 2017 |
| Spurilla neapolitana | Spurillin A (Cyclohexenyl terpenoid) |  | Ciavatta et al., 2017 |
| Spurilla sp. | Farnesol derivative | 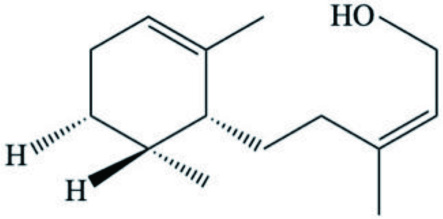 | |
| Spurillin B (Diterpene) | 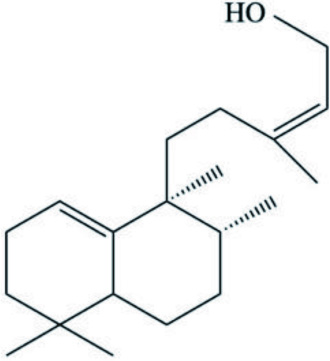 | ||
| Phyllodesmium longicirrum | Methylsarcoate analog |  | Bogdanov et al., 2017 |
| 2R secogorgosterol |  | ||
| Bisepoxide |  | ||
| Isosarcophine |  | ||
| Charcotia granulosa | Granuloside (Linear homosesterterpene) |  | Cutignano et al., 2015 |
| Onchidium sp. | Pyranone ester derivatives or analogs | 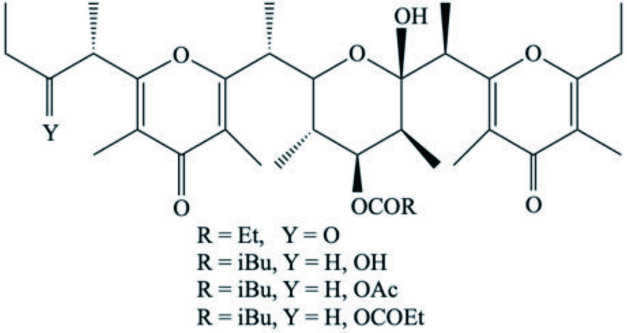 | Carbone et al., 2013 |
| Thuridilla splendens | Thuridillins (Diterpene metabolites) | 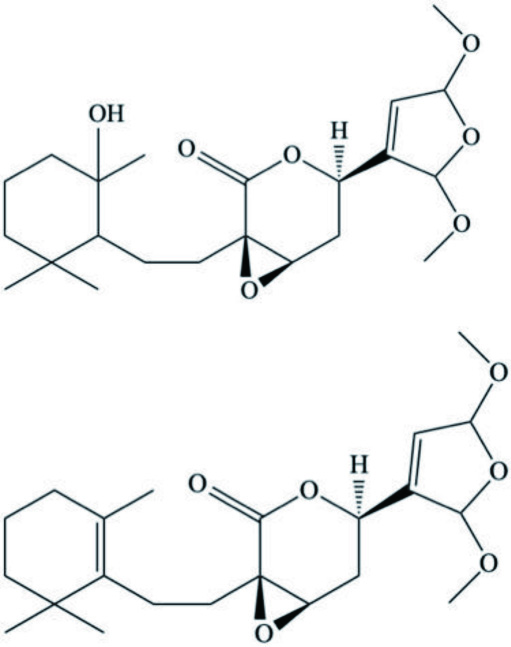 | Somerville et al., 2012 |
| Aplysia californica (Sea hare) – from ink | Mycosporine-type of amino acids |  | Kamio et al., 2011 |
| Philinopsis speciosa and Bulla occidentalis | Niuhinone A–C (Polypropionate derivatives) | 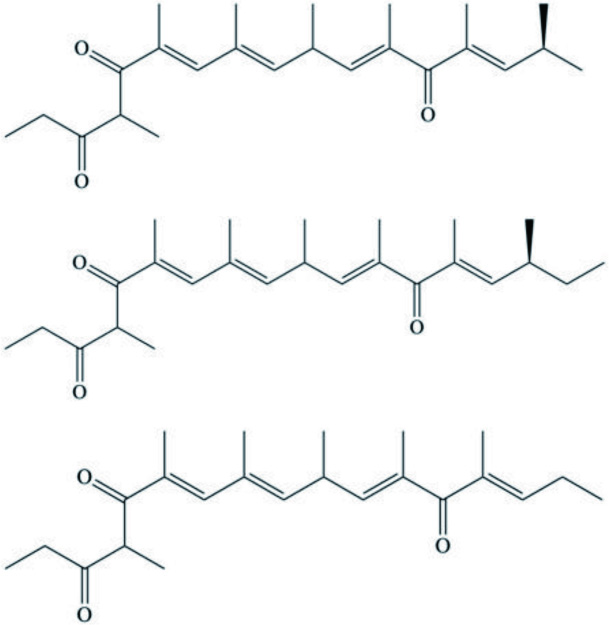 | Cutignano et al., 2011 |
| Doto pinnatida | Guanidine-bound terpene derivative |  | Putz et al., 2011 |
| Phyllodesmium magnum | Asteriscane sesquiterpenoid | 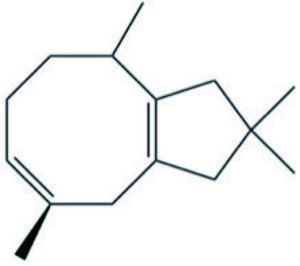 | Mao et al., 2011 |
| Tritoniopsis elegans | Tritoniopsin A–D (Pyran- enclosed cladiellane diterpene derivatives) |  | Ciavatta et al., 2011 |
| Tambja ceutae | Tambjamine K (Isopentyl containing alkaloid) | 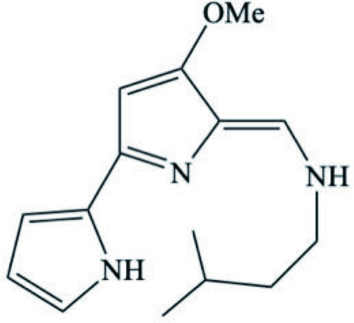 | Carbone et al., 2010 |
| Aplysia fasciata (sea hare) | Sesquiterpenoids |  | Ioannou et al., 2009 |
| Acetogenin |  | ||
| Diterpenoid | 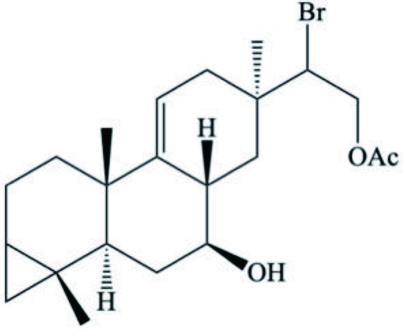 | ||
| Cadlinalutero marginata | Ansellone A (Sesterterpenoid) | 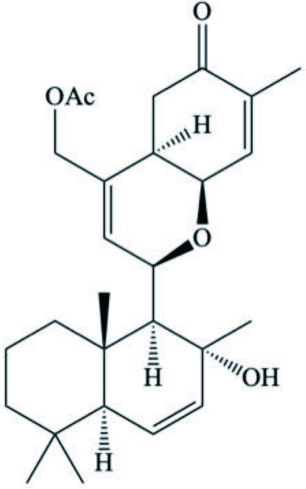 | Daoust et al., 2010 |
| Austrodoris kerguelenensis | Palmadorin A–C (Clerodane diterpenes) | 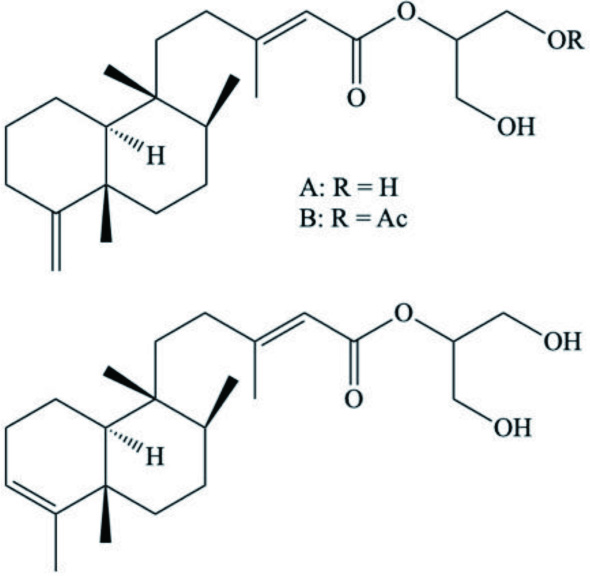 | Diyabalanage et al., 2010 |
| Aplysiopsis formosa (slug) | Aplysiopsene A–D (α-pyrone polyketides) |  | Ciavatta et al., 2009 |
| Onchidiopsis variegate | Ketosteroid derivative |  | Santalova et al., 2007 |
| Scaphander lignarius | Lignarenones (Aromatic benzene-enclosed compounds) |  | Sala et al., 2007 |
| Haminoea fusari | Fusaripyrones A and B (Polypropionate analogs) |  | Cutignano et al., 2007 |
| Bursatella leachii (sea hare) | 7R-configured malyngamide |  | Suntornchashwej et al., 2007 |
| H. sanguineus (Spanish dancer mollusc) | Sesquiterpenoid | 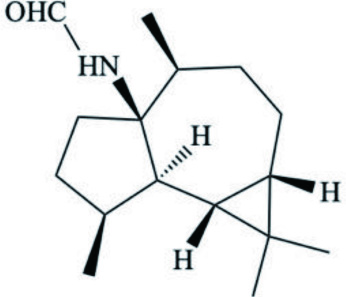 | Zhang et al., 2007b |
| Diterpenoid | 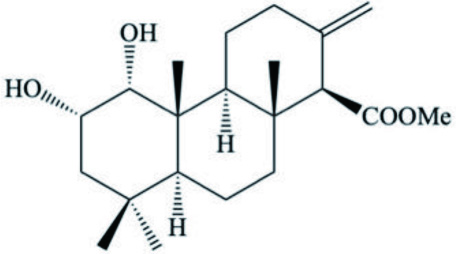 | ||
| Siphonaria lessoni | Siphonarienolone | 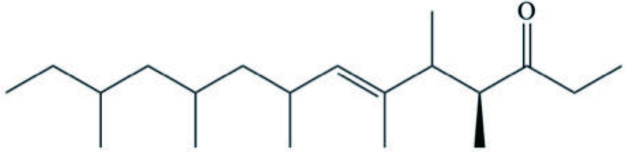 | Rovirosa and San-Martín, 2006 |
| Elysia cf. expansa | Dihydrocaulerpenyne | 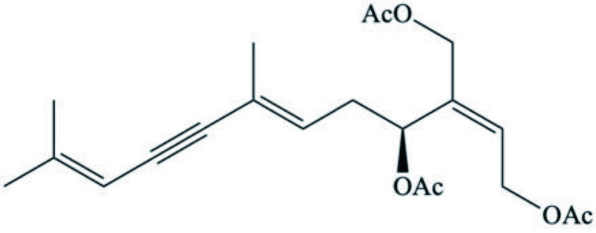 | Ciavatta et al., 2006 |
| Expansinol |  | ||
| Elysia diomedea | Elysiapyrone metabolites A–B |  | Cueto et al., 2005 |
| D. pelseneeri | 15- acetoxy-ent-pallescensin-A |  | Gaspar et al., 2005 |
| Dendocarbin-A |  | ||
| Euryfuran |  | ||
| Drimane ester mixture |  | ||
| A. parvula | (3Z)-bromofucin |  | McPhail and Davies-Coleman, 2005 |
| Syphonota geographica | Aplykurodinone 1–2 |  | Gavagnin et al., 2005 |
| Doriopsilla pelseneeri | Pelseneeriols-1 and 2 (Furanosesquiterpene alcohol derivatives) |  | Gaspar et al., 2005 |
| Phyllidiella pustulosa | Sesquiterpenes |  | Manzo et al., 2004 |
| A. kerguelenensis | Austrodoric acid | 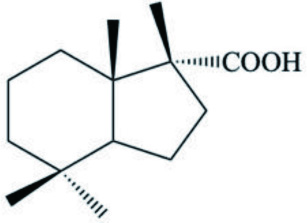 | Gavagnin et al., 2003 |
| Placida dendritica | Placidenes C–F (Asymmetrical polypropionate derivatives) | 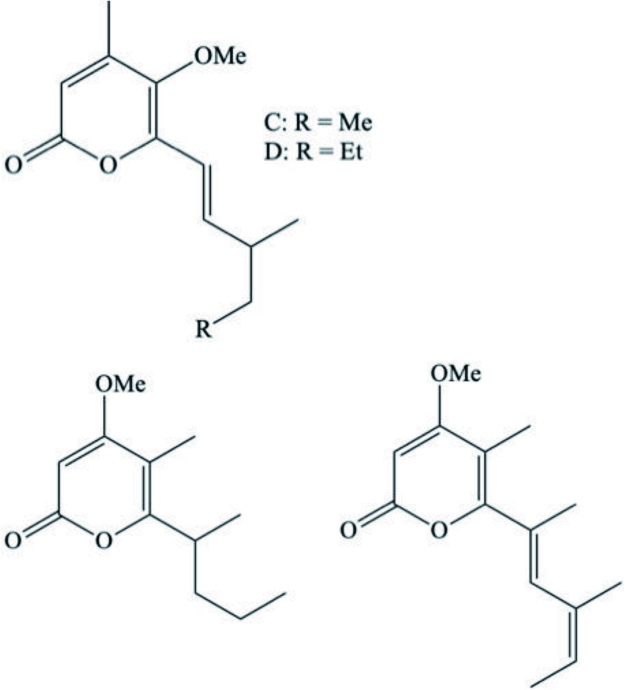 | Cutignano et al., 2003 |
| Aldisas maragdina | Progesterone analog | 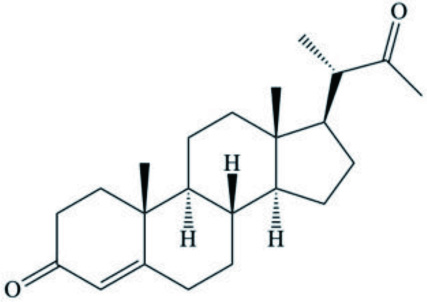 | Gavagnin et al., 2002 |
| A. dactylomela | Johnstonol | 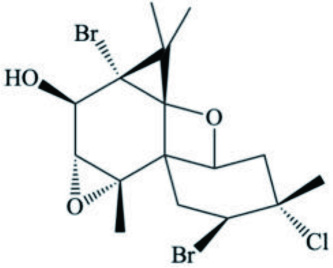 | Kaiser et al., 2001 |
| Pacifenediol |  | ||
| Pacifidiene | 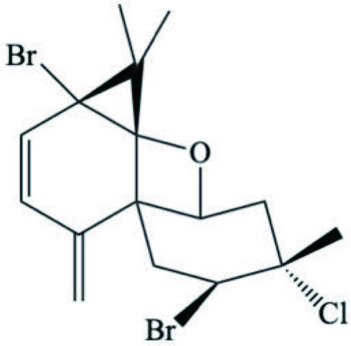 | ||
| Tryptophan-based dipeptides |  | Appleton et al., 2001 | |
| A. dactylomela | Puertitol-B acetate (Sesquiterpenes) |  | Wessels et al., 2000 |
| Caespitenone (Sesquiterpenes) | 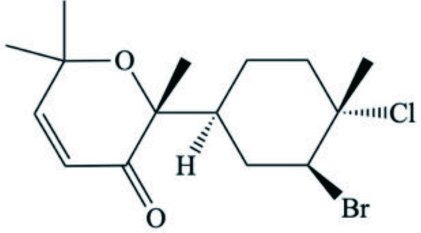 | ||
| 8-acetyl-caespitol (Sesquiterpenes) |  | ||
| Dactylopyranoid (Diterpenoids) | 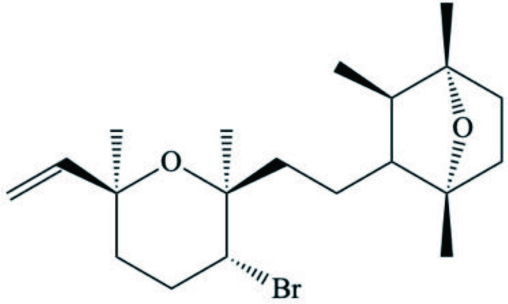 | ||
| Isopinnatol B (Diterpenoids) | 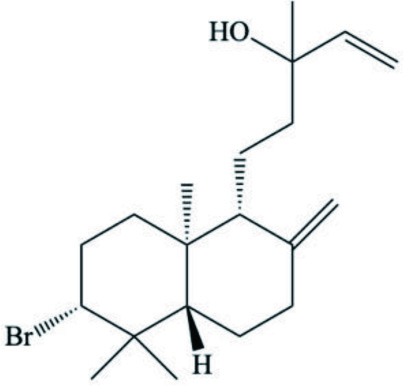 | ||
| P. ocellatus | Tridachiapyrones G–J (Polypropionate pyrones) |  | Fu et al., 2000 |
| Tridachiahydropyrons B–C (Polypropionate pyrones) |  | ||
| Asco bullaulla | Ascobullins A and B (Sesquiterpenes) |  | Gavagnin et al., 2000 |
| Species | Compound | Backbone structure | References |
|---|---|---|---|
| V. cyprinoides (Corbiculid clam) | 19-(10→5) abeo-20-methyl-pregnenyl-3-hexenoate (Abeo-pregnane-type sterol derivative) |  | Joy and Chakraborty, 2018a |
| 241, 242 dihomocholesta-5, 22, 241-trienol (Cholestenols) |  | ||
| 241- homocholesta-5, 22-dien-3, 241-diol (Cholestenols) |  | ||
| P. malabarica | C18 sesquiterpenoid with prenylated irregular farnesene framework (2H-pyranoids) | 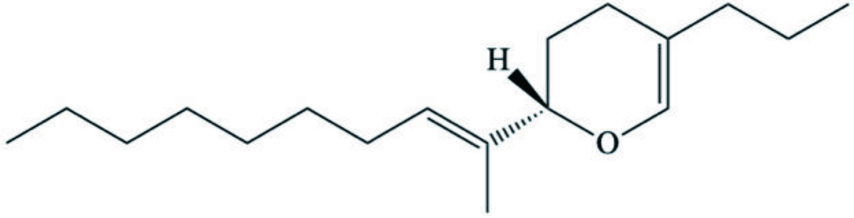 | Joy and Chakraborty, 2017a, 2017b |
| C21 prenylated bisabolene-type meroterpenoid (2H-pyranoids) | 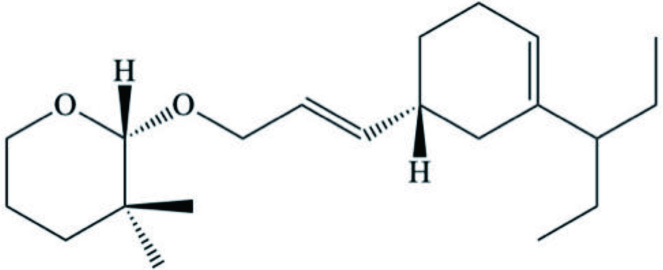 | ||
| 18 (4 →14), 19 (4 → 8)-bis-abeo C19 norditerpenoid (Isopimarane derivative) | 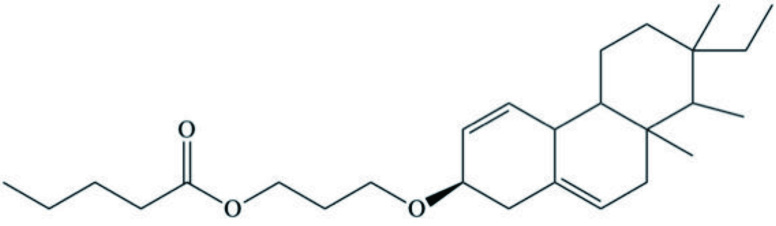 | ||
| M. galloprovincialis | Carotenoids (19′-hexanoyloxyfucoxanthin derivatives) |  | Maoka et al., 2011 |
| M. chinensis (Chinese surf clam); R. philippinarum M. petechialis | Fucoxanthin fatty acid esters | 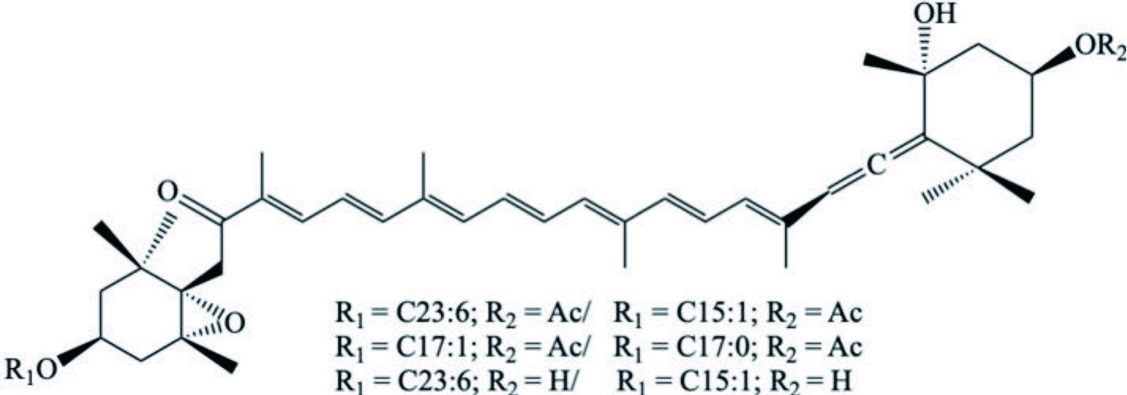 | Maoka et al., 2010, 2007 |
| Fucoxanthinol fatty acid esters |  | ||
| Paphia amabills P. amabillis | Amarouciaxanthin A and its ester derivatives |  | Maoka et al., 2008 |
| C37-skeletal carotenoids |  | ||
| Calyptogena phaseoliformis (deep-sea clam) | n-4 PUFAs |  | Saito, 2007 |
| Venus verrucose (Brackish water clam) | Thio-arsenosugars | 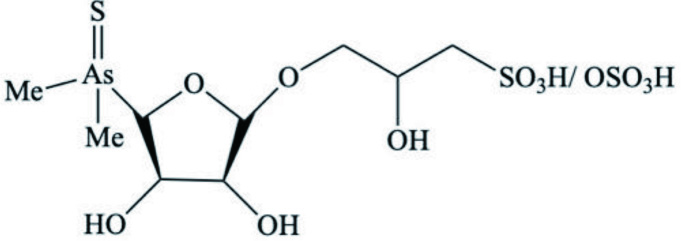 | Nischwitz et al., 2006 |
| O. rivularis | Ostrerine A |  | Ouyang, 2006 |
| M. edulis | 20-methyl spirolide G |  | Aasen et al., 2005 |
| C. gigas | Carotenoids | 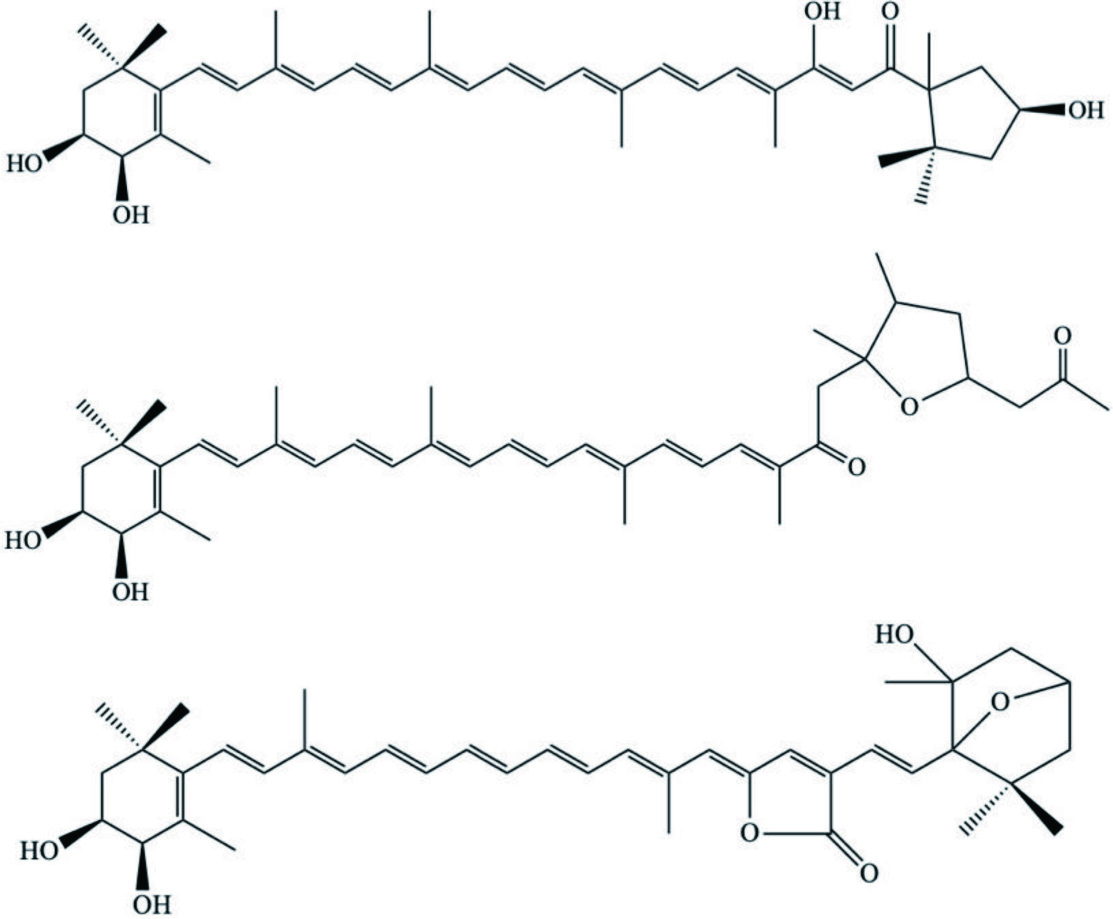 | Maoka et al., 2005b |
 | Maoka et al., 2001 | ||
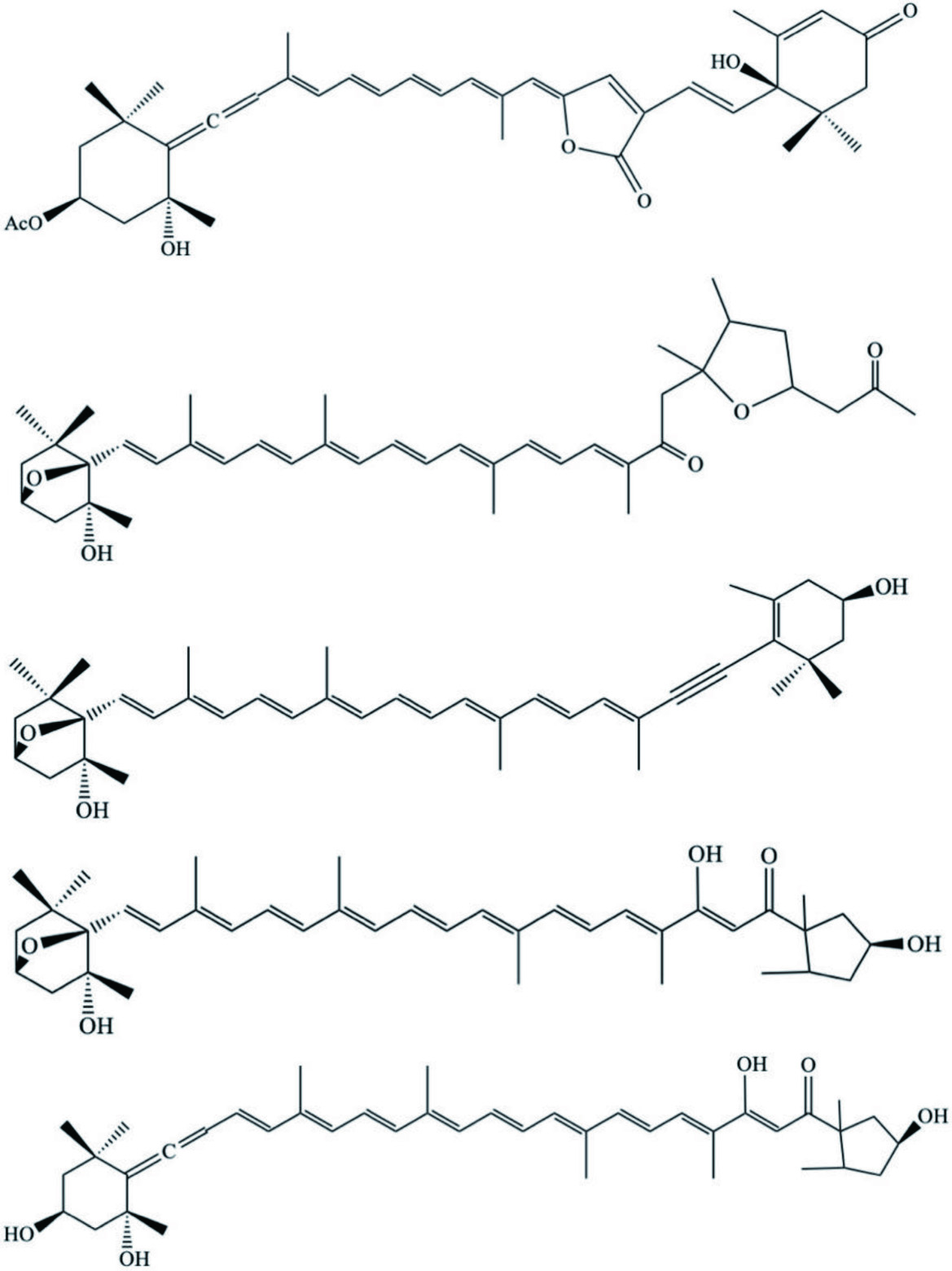
| M. galloprovincialis | Chlorosulfolipid | 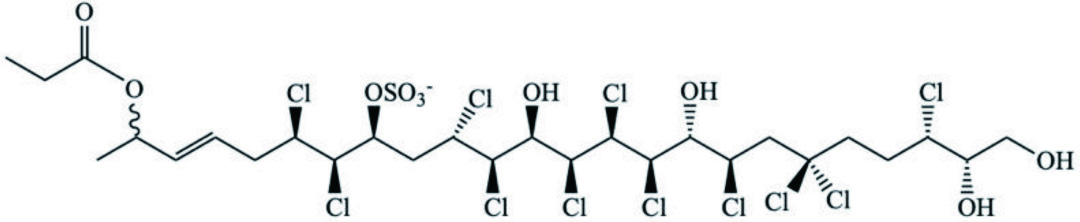 | Ciminiello et al., 2004 |
| Polychlorinated sulfolipid |  | Ciminiello et al., 2002 | |
| Oxazinins 1–3 (Bioactive alkaloids) |  | Ciminiello et al., 2001 |
| Class of compound | Name of the compound | Ascidian species | Biological activity | References |
|---|---|---|---|---|
| P388 - mouse lymphocytic leukemia cells, V-ATPase - Vacuolar-type ATPase, NCI-H460 - Lung cancer cell line. | ||||
| Alkaloids | Didemnidines A and B (indole spermidine) | Didemnum sp. | Inhibitors of farnesyltransferase and phospholipase A2 enzyme without cytotoxicity | Finlayson et al., 2011 |
| Meridianins (brominated 3-(2-aminopyrimidine)-indoles) | Aplidium meridianum | Potential antitumor activities; Prevent cell proliferation and induce cell apoptosis; Promising kinase-inhibitory | Núñez-Pons et al., 2015 | |
| Herdmanines A–D (nucleoside derivatives) | Herdmania momus | Inhibit the production and the expression of mRNA of pro-inflammatory cytokines; C and D show moderate suppressive effects on the production of Lipopolysaccharide induced nitricoxide | Li et al., 2012b; Li et al., 2011a | |
| Polypeptides | Vitilevuamide (bicyclic peptide) | Didemnum cuculiferum; Polysyncranton lithostrotum | In vivo cytotoxic effect against P388 cells | Edler et al., 2002 |
| Diazonamides (macrocyclic peptides) | Diazona angulata | Exhibits antitumor activities; Diazonamide A is a potent chemotherapeutic agent | Cruz-Monserrate et al., 2003 | |
| Chondromodulin-1 (glycoprotein) | Ciona savignyi | Potential antioxidant and antitumor agent; Protects H2O2 oxidative injury; Suppresses cell proliferation of human neuroblastoma and cervical cancer cells; Prevents angiogenesis of human umbilical vein endothelial cells | Dou et al., 2018 | |
| CS5931 | C. savignyi | Cytotoxic effects on several cancer cell; Induces apoptosis through a mitochondrial-mediated pathway | Zhao et al., 2013; Cheng et al., 2012 | |
| Polyketides | Palmerolide A (macrocyclic polyketide) | Synoicum adareanum | selective cytotoxicity toward melanoma by inhibiting V-ATPase | Noguez et al., 2011 |
| Mandelalides A and B (glycosylated polyketides) | Lissoclinum sp. | Potent cytotoxicity against Neuro-2a neuroblastoma cells in mouse and NCI-H460; Strong antifungicidal activity on Candida albicans | Nazari et al., 2017; Sikorska et al., 2012 | |
| Phosphoeleganin (phosphorylated polyketide) | Sidnyum elegans | Inhibitory effect against the protein tyrosine phosphate 1B | Imperatore et al., 2016 | |
| Group of compounds | Name of the compound | Seaweed source | Bioactivity | References |
|---|---|---|---|---|
| Polysaccharides | Fucoidans | Brown seaweeds | Anticoagulative, antioxidative, anti-inflammatory, antitumor, and antimicrobial activities | Song et al., 2012; Berteau and Mulloy, 2003 |
| Ascophyllum nodosum | Inhibits the invasion of breast cancer and colon adenocarcinoma cells | Haroun-Bouhedja et al., 2002 | ||
| Fucus vesiculosus | Immunomodulatory activity | Jintang et al., 2010 | ||
| Alginates | Brown seaweeds | Strong antibacterial and anti-inflammatory properties; Cholesterol-lowering and antihypertensive effects; Chelate metal ions; Inhibit the absorption of toxic substances; Play a crucial role as dietary fiber | Kim and Lee, 2008; Nishide and Uchida, 2003; Murata and Nakazoe, 2001 | |
| Laminarin | Brown seaweeds | Antibacterial, antiviral, antitumor, and anticoagulant, and antioxidant property | Li and Kim, 2011 | |
| Reduce the systolic blood pressure, boosts the immune system, and lower the serum cholesterol | Hoffmane et al., 1995; Holdt and Kraan, 2011 | |||
| Sulfated polysaccharides | Caulerpa prolifera Sargassum filipendula | Cell proliferation inhibition | Costa et al., 2011 | |
| Fucoxanthin | Phaeodactylum tricornutum | Anti-proliferative effect | Neumann et al., 2019 | |
| Undaria pinnatifida | Strong radical scavenging activity | Yan et al., 1999 | ||
| Saccharina japonica Sargassum horneri | Antihypertensive activity | Sivagnanam et al., 2015 | ||
| Ulvans | Ulva lactuca Ulva rigida | Anticoagulant activity | de Carvalho et al., 2018; Adrien et al., 2019 | |
| Codium fragile | Strong immunomodulatory activity | Lee et al., 2010 | ||
| Xyloarabinogalactans (Sulfated polysaccharides) | Codium fragile | Anticoagulant activity | Jurd et al., 1995 | |
| Fucans | Padina gymnospora | Anticoagulant activity | Silva et al., 2005 | |
| Protein | Lectin | Eucheuma spp. Gracilaria sp.; Ulva sp. | Anti-inflammation, cancer metastasis, induction of apoptosis, antibacterial, and antiviral activities | Holdt and Kraan, 2011; Chojnacka et al., 2012 |
| Protien hydrolyzates | Gracilariopsis lemaneiformis | Antioxidant | Zhang et al., 2019b | |
| Dipeptides | Undaria pinnatifida | ACE inhibitory activity | Kim and Wijesekara, 2010 | |
| Undecapeptide | Chlorella vulgaris | Antiproliferative and antioxidative activity | Sheih et al., 2010 | |
| Lipids | Phospholipids | Absorption of cholesterol, fatty acids, and other lipophilic components; Ease of digestion; Act as natural emulsifiers | Sørensen, 2009 | |
| Polyphenols | Pholorotannins (Eckol) | Ecklonia cava subsp. stolonifera | Antioxidant | Manandhar et al., 2019 |
| Pholorotannins | Bifurcaria bifurcata | Anti-proliferative activity | Gonçalves-Fernández et al., 2019 | |
| Pholorotannins (Eckmaxol) | Ecklonia maxima | Neuroprotective effect | Wang et al., 2018b | |
| Pholorotannins (Phloroglucinol) | Eisenia bicyclis | Anticancer activity | Shibata et al., 2002 | |
| Pholorotannins (Dioxinodehydroeckol, phlorofucofuroeckol A) | Eisenia stolonifera | Anti-allergenic effect | Shim et al., 2009 | |
| Phlorotannins (Phlorofucofuroeckol A, dieckol, and eckol) | Ecklonia stolonifera | ACE inhibitory activity | Jung et al., 2006 | |
| Bromophenol | Leathesia nana Rhodomela confervoides | Anticancer activity | Dong et al., 2020 | |
| Symphyocladia latiuscula | Antidiabetic activity | Paudel et al., 2019b | ||
| Polysiphonia morrowii | Antiviral and anti-inflammatory activity | Kim et al., 2011; Choi et al., 2018 | ||
| Flavonoids | Sargassum cristaefolium | Anticoagulant | Manggau et al., 2019 | |
| Enteromorpha prolifera | Antidiabetic activity | Yan et al., 2019 | ||
| Caleurpa spp. | Antioxidant activity | Tanna et al., 2018 | ||
| Polyphenol extract | Ascophyllum nodosum Fucus vesiculosus | Antidiabetic activity | Zhang et al., 2007a; Lordan et al., 2013 | |
| Pigments | Pheophorbidea (Chlorophyll-a derivatives) | Enteromorpha prolifera | Strong antioxidant activity | Cho et al., 2011 |
| C-phycocyanin | Spirulina platensis | Anticancer activity against HeLa cells | Li et al., 2006 | |
| Others | Cyclic diterpene | Dictyota menstrualis | Antiviral activity | Cirne-Santos et al., 2019 |
| Meroditerpenoids (Epitaondiol and Stypodiol) | Stypopodium flabelliforme | Anti-bacterial and anticancer activities | Pereira et al., 2011 | |
| Sesquiterpene (Zonarol) | Dictyopteris undulata | Neuroprotective effect | Shimizu et al., 2015 |
| Class of phenolic compounds | Name of the compound | Seaweed source | Reference |
|---|---|---|---|
| Phlorotannins | Fuhalol; Phlorethols Fucophlorethols; Eckol | Sargassum fusiforme | Li et al., 2017b |
| Dieckol | Ecklonia stolonifera | Yoon et al., 2013 | |
| Octaphlorethol A | Ishige foliacea | Lee et al., 2016 | |
| Dieckol; Phlorofucofuroeckol A; Heptafuhalol A; 8,8′-bieckol; 6,6′-bieckol | Ecklonia cava | Oh et al., 2019; Cotas et al., 2020 | |
| Eckol; Dieckol; 8,8′-bieckol; 6,6′-bieckol; Phlorofucofuroeckol-A | Ecklonia bicyclis | Kim and Kwak, 2015 | |
| Bromophenols | 5′-hydroxyisoavrainvilleol | Avrainvillea nigricans | Colon et al., 1987 |
| Brominated monoterpenoid quinol | Cymopolia barbata | Estrada et al., 1987 | |
| 3-bromo-4,5-dihydroxy benzoic acid methyl ester; 3-bromo-4,5 dihydroxy-benzaldehyde-bromophenols; Bis(2,3-dibromo-4,5-dihydroxybenzyl) methane | Rhodomela confervoides | Popplewell and Northcote, 2009; Wu et al., 2015 | |
| 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether and its derivates | Scotinophara latiuscula | Paudel et al., 2019b | |
| 5-bromo-3,4-dihydroxybenzaldehyde | Polysiphonia morrowii | Ko et al., 2019 | |
| Flavonoids | Isoflavones (daidzin or genistein) | Red seaweeds (Chondrus crispus, Porphyra/Pyropia spp.); Brown seaweeds (Sargassum muticum, Sargassum vulgare) | Cotas et al., 2020 |
| Flavonoid glycosides | Durvillae antarctica; Lessonia spicata; Macrocystis pyrifera | Santos et al., 2019 | |
| Flavonoids C-glycosides | Nitell Hookeri (Green seaweed) | Markham and Porter, 1969 | |
| Catechin; Epicatechin; Epigallocatechin; Catechin gallate; Epicatechin gallate; Epigallocatechin gallate | Acetabularia ryukyuensis Ecklonia bicyclis; Padina arborescens; Padina minor; Neopyropia yezoensis; Gelidium elegans; Portieria hornemannii | Yoshie et al., 2000 | |
| Kaempferol; Quercetin | Caleurpa spp. | Tanna et al., 2018 | |
| Rutin hydrate | Caleurpa corynephora; Undaria pinnatifida | Tanna et al., 2019 | |
| Phenolic acids | Coumarins | Dasycladus vermicularis | Feng et al., 2007 |
| Vanilic acid derivatives | Cladophora socialis | Feng et al., 2007 | |
| Hydroxybenzoic acid; Rosmarinic acid; Quinic acid derivatives | Ascophyllum nodosum; Bifurcaria bifurcata; Fucus vesiculosus | Agregan et al., 2017 | |
| Benzoic acid; p-hydroxybenzoic acid; Salicylic acid; Gentisic acid; Protocatechuic acid; Vanillic acid; Gallic acid; Syringic acid | Genus Gracilaria | Farvin and Jacobsen, 2013 |
| Bacteria | Bioactive compound | Bioactivity | Reference |
|---|---|---|---|
| HCT116 - human colon cancer cell line, A549 - human lung adenocarcinoma cell line, HL-60 - human leukemia cell line, K-562 - myelogenous leukemia cell lines, HepG2 - human liver cancer cell line. | |||
| Verrucosispora sp. | Abyssomicin (Six known Abyssomicin and proximicin analogs) | Antiviral effects against the Influenza A virus | Zhang et al., 2020 |
| Streptomyces sp. | Four 2-alkyl-4-hydroxyquinoline compounds | Antifungal activity against Candida albicans | Kim et al., 2019 |
| Streptomyces sp. Al Dhabi-90 | 3-methylpyridazine n-hexadecanoic acid; Indazol-4-one; Octadecanoic acid; 3a-methyl-6-((4-methylphenyl) sul | Antibacterial activity against S. aureus, Klebsiella pneumoniae, E. coli, Pseudomonas aeruginosa, Proteus mirabilis, and Enterococcus faecium | Al-Dhabi et al., 2019 |
| Microbacterium aurantiacum FSW-25 | Exopolysaccharide | Antioxidant activities | Sran et al., 2019 |
| Streptomyces sp. ZZ745 | Bagremycins (five analogs including two new ones) | Antibacterial activity against E. coli | Zhang et al., 2018 |
| Streptomyces sp. S2A | Pyrrolo[1–a]pyrazine-1,4-dione,hexahydro-3-(2-methylpropyl) | Antimicrobial activity against pathogenic bacteria and fungi; Inhibitory activity against α-glucosidase and α-amylase enzymes; Cytotoxic activities against different cell lines; Antioxidant activities | Siddharth and Vittal, 2018 |
| Streptomyces sp. Bacillus sp. | Dentigerumycin E | Antiproliferative and antimetastatic activities against human carcinoma | Shin et al., 2018 |
| Aerococcus uriaeequi HZ | Exopolysaccharides | Antioxidant activities | Wang et al., 2018a |
| Streptosporangium sp. | Hexaricins (pradimicin-like polyketides) | Potent antioxidant activity | Gao et al., 2018 |
| Streptomyces sp. IMB094 | Neo-actinomycins A and B (chromopeptides) | Cytotoxic activities against human cancer HCT116 and A549 cell lines; Moderate inhibitory activities against vancomycin-resistant Enterococci strains and methicillin-resistant Staphylococcus aureus | Wang et al., 2017a |
| Streptomyces sp. HZP-2216E | Streptopertusacin A (Alkaloid) | Antibacterial activity against methicillin-resistant Staphylococcus aureus | Zhang et al., 2017 |
| Chromobacterium sp. HS-13-94 | Chromopeptide A (depsipeptide) | Cytotoxic activity against HL-60, K-562, and Ramos cells. | Zhou et al., 2015 |
| Saccharothrix sp. | Saccharothrixones A–D | Moderate cytotoxicity against the HepG2 cancer cell line in vitro (Saccharothrixone D) | Gan et al., 2015 |
| Shewanella piezotolerans | Shewanellines A–C | Antitumor activity against the HL-60 cell line | Wang et al., 2014b |
| Serinicoccus profundi sp. | 3-((6-methylpyrazin-2-yl) methyl)-1H-indole (Alkaloid) | Antibacterial activity against Staphylococcus aureus | Yang et al., 2013 |
| Fungi | Bioactive compound | Biological activity | Reference |
|---|---|---|---|
| HepG2 - human liver cancer cell line, A549 - human lung adenocarcinoma cell line, LNCaP - human prostate adenocarcinoma cells, B16F10 - murine melanoma cell line, A-375 - human melanoma cell line, CNE2 - human nasopharyngeal carcinoma cell line, LoVo - lymph node metathesis cells, 95-D – human lung cancer cells. | |||
| Aspergillus sp. LS116 | Aspergillsteroid A | Antibacterial effect on aquatic pathogen Vibrio harveyi | Xu et al., 2020 |
| Penicillium minioluteum ZZ1657 | N-acetyl-L-valine conjugated drimarane sesquiterpenoids | Antimicrobial activity against methicillin-resistant S. aureus, E. coli, and C. albicans | Ma et al., 2020 |
| Drimane sesquiterpenoids | Antiproliferative activities against human glioma cells | ||
| Aspergillus sp. | Asperphenin A | Antitumoral activity against human colon cancer cells | Bae et al., 2020 |
| Penicillium chrysogenum | Tyrosol | Anti-quorum sensing activity against P. aeruginosa and Chromobacterium violaceum | Chang et al., 2019 |
| Penicillium sclerotiorin | Azaphilone derivative | Anti-inflammatory activity | Liu et al., 2019 |
| Penicillium sp. | 2-[(5-methyl-1,4-dioxan-2-yl) methoxy] ethanol; 2-[(2R Hydroxypropanoyl) amino] benzamide, 4- hydroxybenzandehyde | Antimicrobial activity | Le et al., 2019 |
| 20,30-Dihydrosorbicillin | Anti-α-glucosidase activity | ||
| Penicillium citrinum HDN-152–088 | Dicitrones | Antioxidant activity | Wang et al., 2019b |
| Penicillium sp. IMB17-046 | Trypilepyrazinol; 3b-Hydroxyergosta-8,14,24(28)-trien-7-one | Antiviral activity against HIV-1, hepatitis C virus and influenza A virus | Li et al., 2019 |
| (+)-Neocitreoviridin | Antibacterial against Helicobacter pylori; Antiviral activity against influenza A virus | ||
| Curvularia sp. IFB-Z10 | Spirocurvulaide | Cytotoxic effect on model cells of hepatomas | An et al., 2019 |
| Chaetomium sp. NA-S01-R1 | Chaephilone, chaetovirides | Antimicrobial activities; Cytotoxic effects on Hep G2 and HeLa cells | Wang et al., 2018d |
| Penicillium sp. ZZ901 | (+)-Scleroderolide | Inhibited the growth of glioma cells; Antibacterial activity against pathogenic S. aureus and E. coli | Li et al., 2018a |
| Trichoderma sp. SCSIO41004 | 5-acetyl-2-methoxy-1,4,6-trihydroxy-anthraquinone | Antiviral activity against human Enterovirus 71 | Pang et al., 2018a |
| Aspergillus niger AKV-MKBU | L-asparaginase | Anticancer activity | Vala et al., 2018 |
| Truncatella angustata | Isoprenylated cyclohexanols | Antiviral effect on HIV-1 and swine-origin influenza A virus | Zhao et al., 2018b |
| Fusarium equiseti | (11S)-1,3,6-trihydroxi-7-(1-hydroxyethyl)-anthracene-9,10-dione | Cytotoxic activity against HepG2, A-549, and cervical (HeLa) carcinoma human cell lines | Zhao et al., 2018a |
| 7-acetyl-1,3,6-trihydroxyanthracene-9,10-dione | Antifungal activity against Alternaria brassicicola and Pestallozzia theae | ||
| Alterperylenol | Antibacterial effect on Clavibacter michiganensis | ||
| Hansfordia sinuosae | Hansforesters A–M (Polyketides) | Strong inhibitory activity against numerous bacterial strains (Hansforester A) | Wu et al., 2018 |
| Cladosporium sp. OUCMDZ-1635 | Cladodionen | Cytotoxicity against several cancer cells lines | Zhu et al., 2018 |
| Penicillium sumatrense | Sumalactones A–D; Curvularin; Dehydrocurvularin (Polyketides) | Dehydrocurvularin showed anti-inflammatory activity via inhibiting NO production | Wu et al., 2017 |
| Penicillium chrysogenum V11 | Penochalasin K | Antimicrobial activities against Rhizoctonia solani and Colletotrichum gloeosporioides; Moderate activity against a panel of cancer cell lines | Zhu et al., 2017 |
| Penicillium sp. ZJ-SY2. | Peniphenone; Methyl peniphenone | Immunosuppressive activity | Liu et al., 2016 |
| Chaetomium globosum | Cytoglobosins H and I (cytochalasan alkaloids) along with seven analogs | Antiproliferative activity against LNCaP and B16F10 cell lines | Zhang et al., 2016c |
| Penicillium citrinum | Penicitrinine A | Inhibitory effects on different tumor types, particularly on human malignant melanoma cell A-375 | Liu et al., 2015a |
| Hansfordia sinuosae | Punctaporonins H–M (caryophyllene-type sesquiterpenoids) | Potent lipid-lowering activity by reducing the intracellular levels of triglycerides and total cholesterol (Punctaporonins K) | Wu et al., 2014 |
| Aspergillus ochraceus Jcma1F17 | 6b,9a-dihydroxy-14-p nitrobenzoyl cinnamolide (nitrobenzoyl sesquiterpenoid); Insulicolide A | Anticancer effects towards a panel of cancer cell lines | Fang et al., 2014 |
| Chondrostereum sp. | Chondrosterins A–E (triquinane-type sesquiterpenoids) | Cytotoxic activities against the cancer lines A549, CNE2, and LoVo (Chondrosterins A) | Li et al., 2012a |
| Penicillium sp. | Penicinoline (pyrrolyl 4-quinolinone alkaloid) | Inhibitory activity against 95-D and HepG2 cell lines | Shao et al., 2010 |
| Technique | Principle | References |
|---|---|---|
| Supercritical Fluid Extraction (Supercritical CO2 Extraction) | Increasing pressure and temperature above their critical points while maintaining liquid and gas characteristics. Carbon dioxide (CO2) is the frequently used solvent. Numerous combinations of temperature and pressure can be used. | Sánchez-Camargo et al., 2017 |
| Subcritical Water Extraction | Placing liquid water for a short time (5–10 min) at high pressure (10–60 bar) and temperatures above its boiling point (100–374°C) | Zakaria and Kamal, 2016 |
| Pulse Electric Field-Assisted Method | Disrupting cell membranes (by forming pores) using the electric field of 0.5–1.0 kV/cm field strength for 100–10,000 seconds, or 1–10 kV/cm field strength for 5–100 s. Commonly used treatment is –20 kJ/kg specific energy with 0.7–3 kV/cm electric field intensity. | Grosso et al., 2015 |
| Microwave-Assisted Method | Due to microwaves, the moisture in the cells evaporates as the temperature rises, resulting in high pressure in the cell. It causes the rupture of cell walls and increases of porosity of the matrix | Bruno et al., 2019 |
| Ultrasound-Assisted Method | These mechanical waves in the range of 20–1000 kHz travel through the cell matrices and make them porous. These waves create a pressure difference in the solvent and stimulate bubble formation. Then these bubbles burst, resulting in cavitation and triggering the rupture of particles. Thus, releasing bioactive chemicals into the matrix. | Ojha et al., 2020 |
| Pressurized Liquid Extraction | High pressures (10–15 MPa), temperatures in the range of 50 to 200°C, and short time duration are used along with small volumes of non-toxic solvents (hot water can also be used). When the temperature rises, the surface tension and viscosity of liquid reduce, whereas diffusivity rises, increasing the efficiency and speed of extraction. | Otero et al., 2019 |
| High Hydrostatic Pressure | Under high pressure (100–1000 MPa) and at a temperature of 5–35°C, cell permeability increases due to the deprotonation of charged particles, breakdown of salt bridges, and weak interactions (hydrogen bonds, electrostatic bonds, and hydrophobic bonds) | Ali et al., 2021 |
| Membrane Separation Technologies | Compounds are isolated depending on their molecular size by semi-permeable membranes and sometimes, depending on their chemical composition by microporous membranes | Nędzarek et al., 2017 |
| Enzymatic Extraction | Hydrolysis of compounds by using enzymes. It is a safe method, which saves time and energy. More widely used in nutraceutical applications | Ghosh et al., 2022 |
| Fermentative Extraction | Hydrolysis of compounds by microbial fermentation. It is a better approach for deproteination, proteolysis, and demineralization | Routray et al., 2019 |