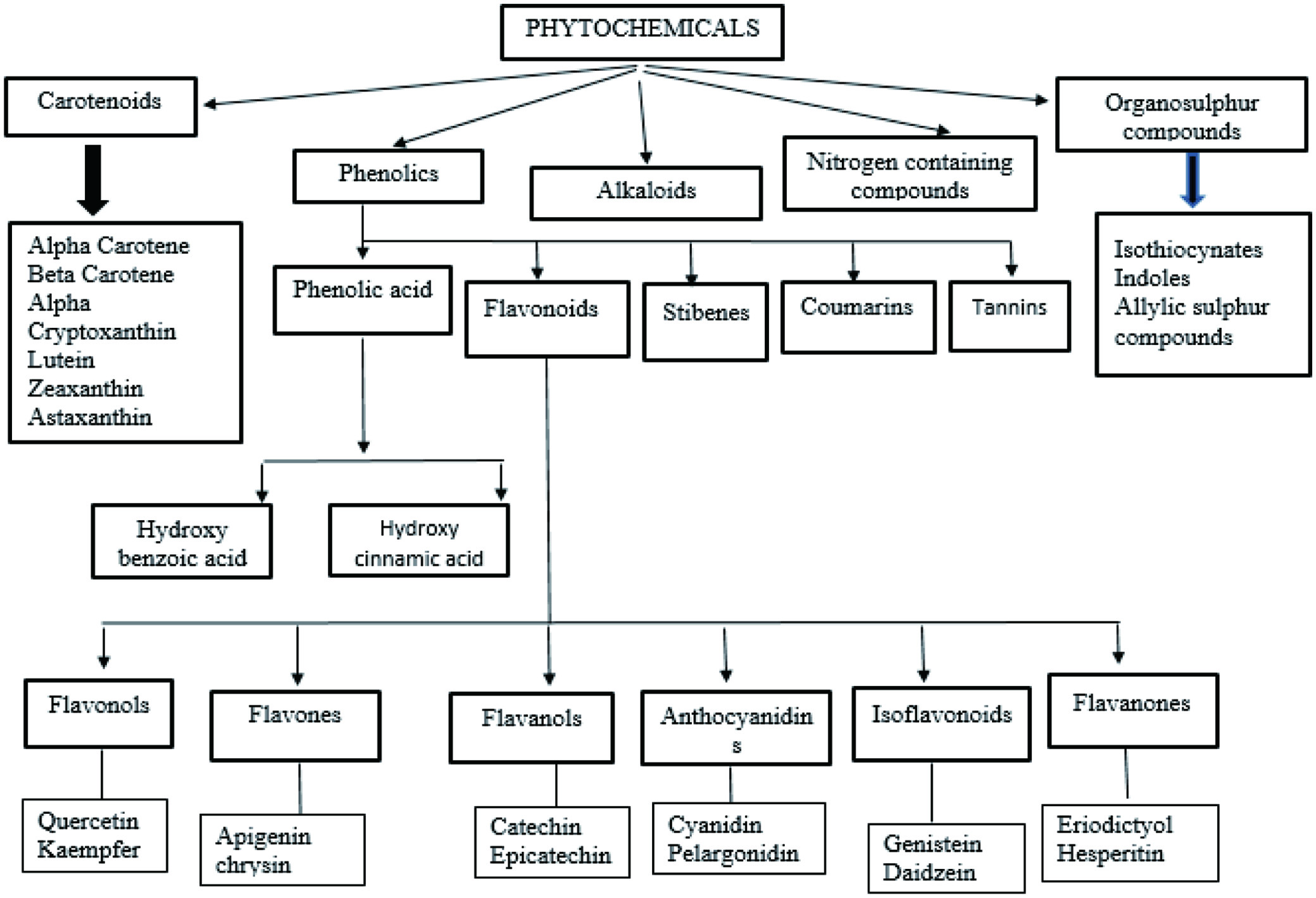
Figure 1. Classification of dietary phytochemicals.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 19, September 2022, pages 124-135
A review on fruits bioactive potential: an insight into phytochemical traits and their extraction methods
Figures

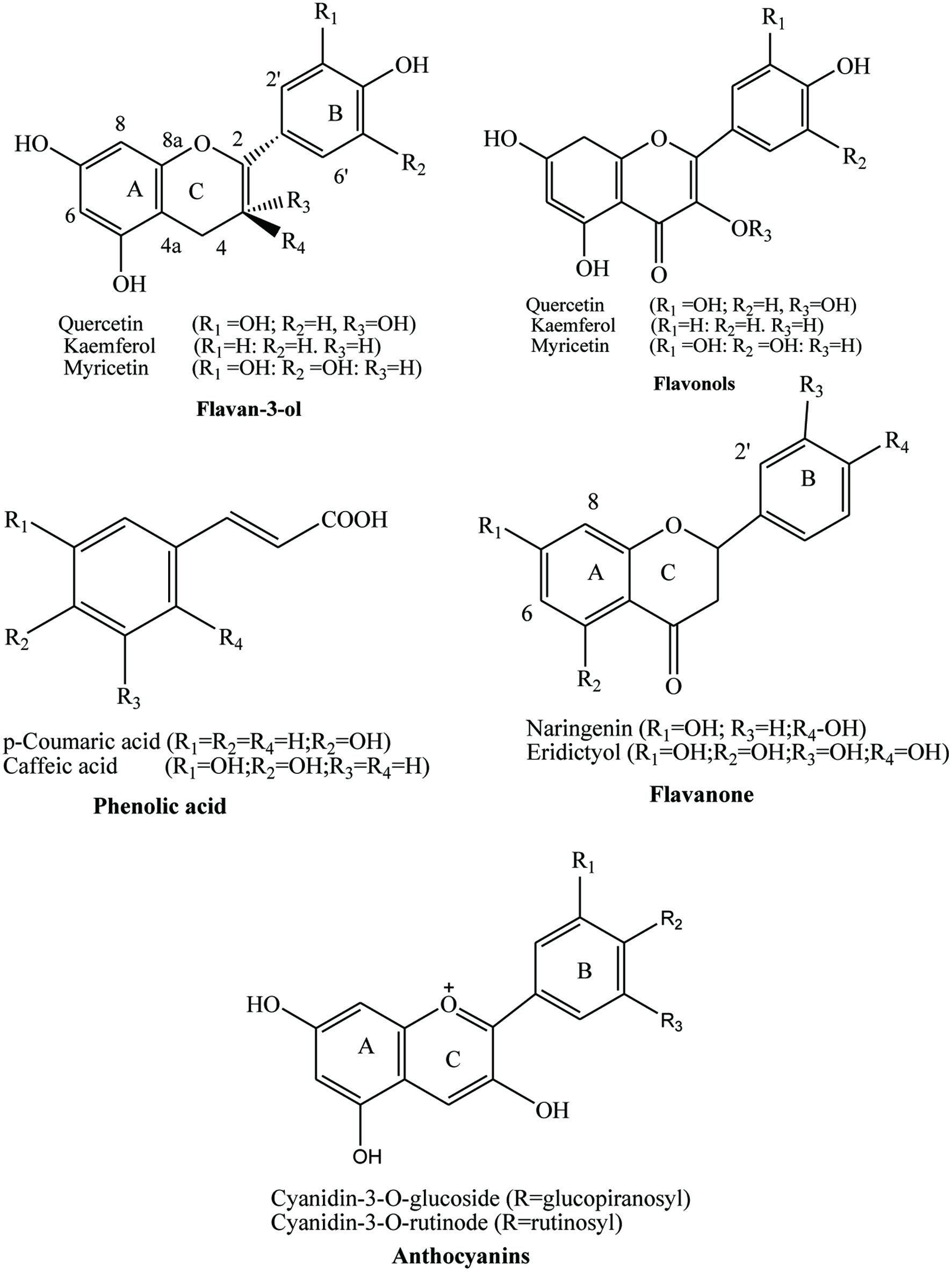
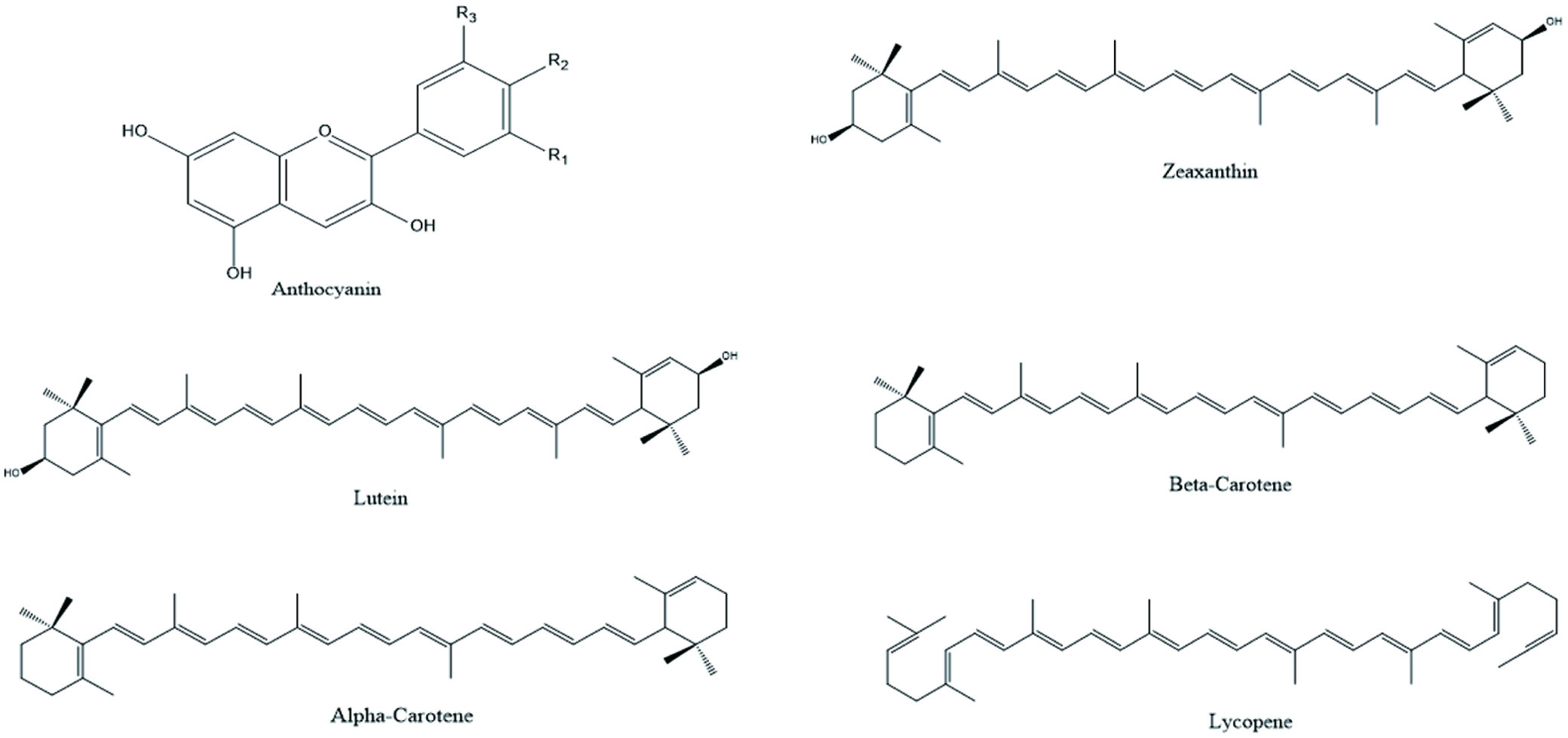
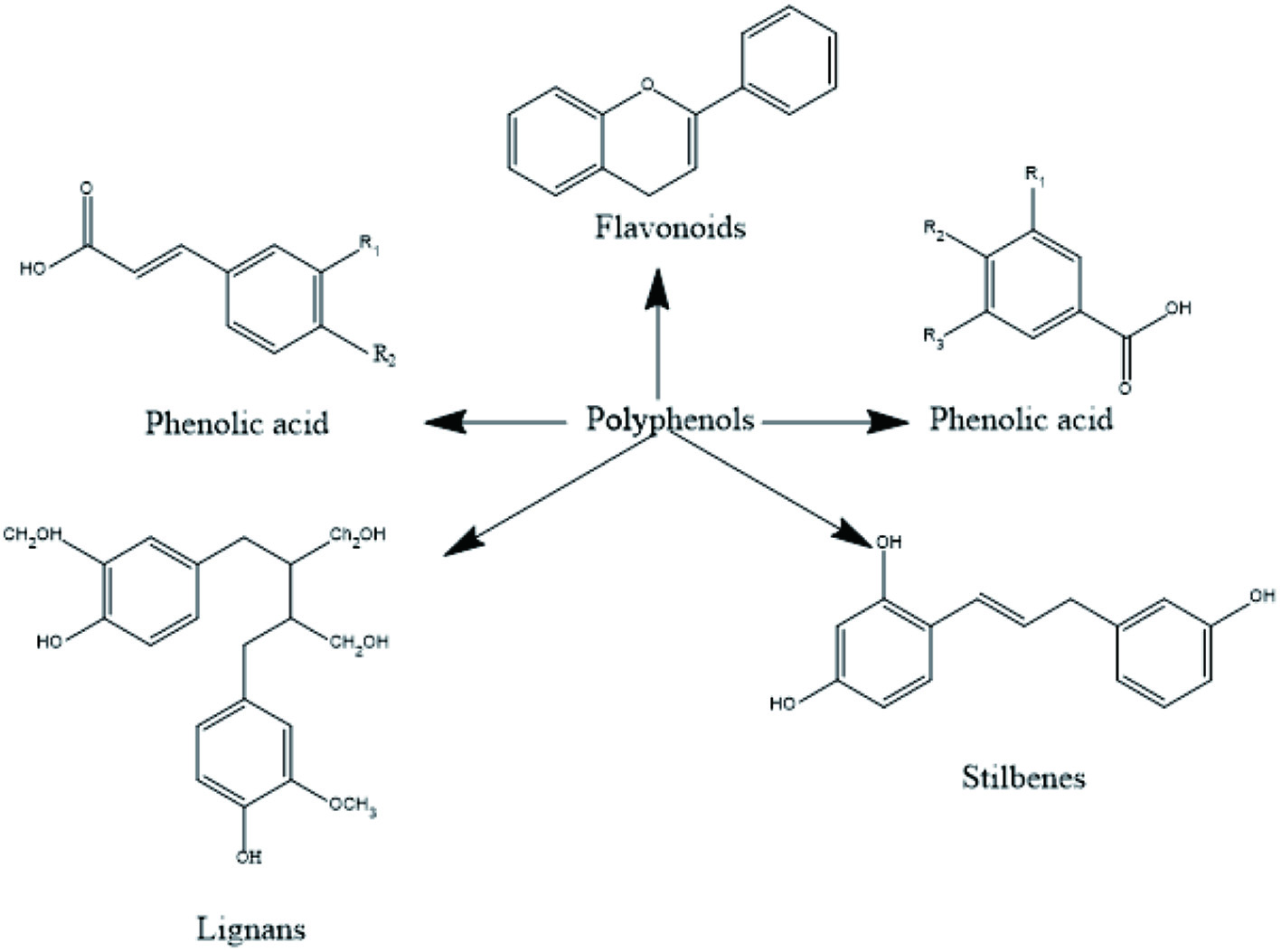
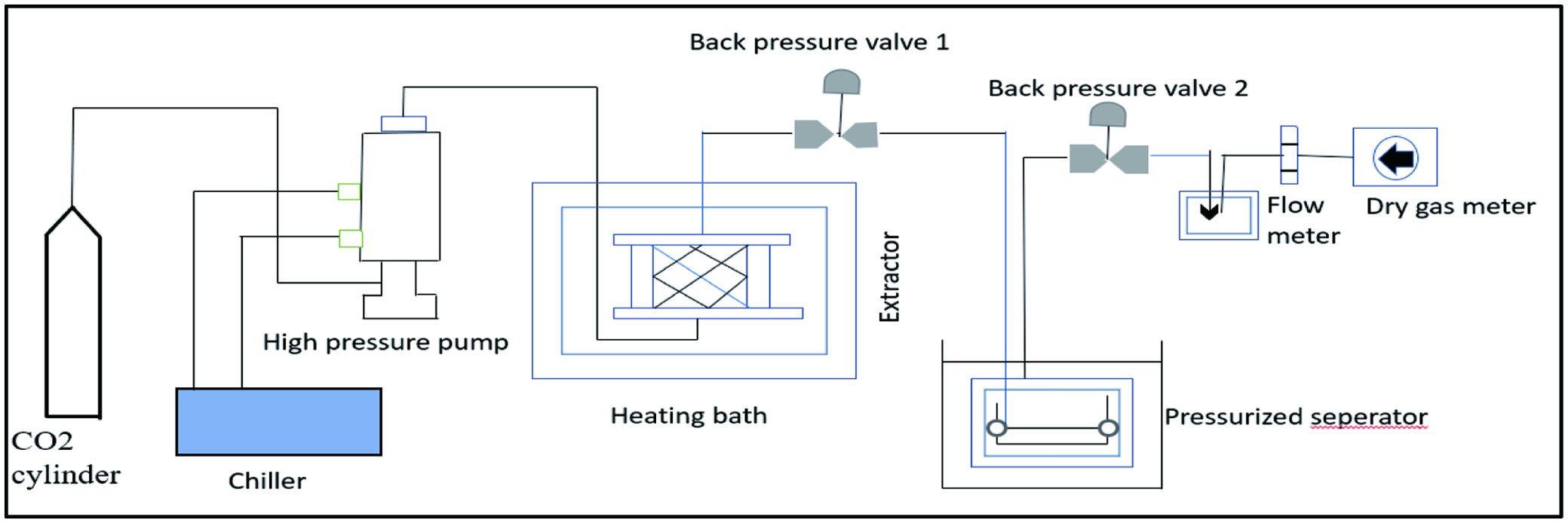


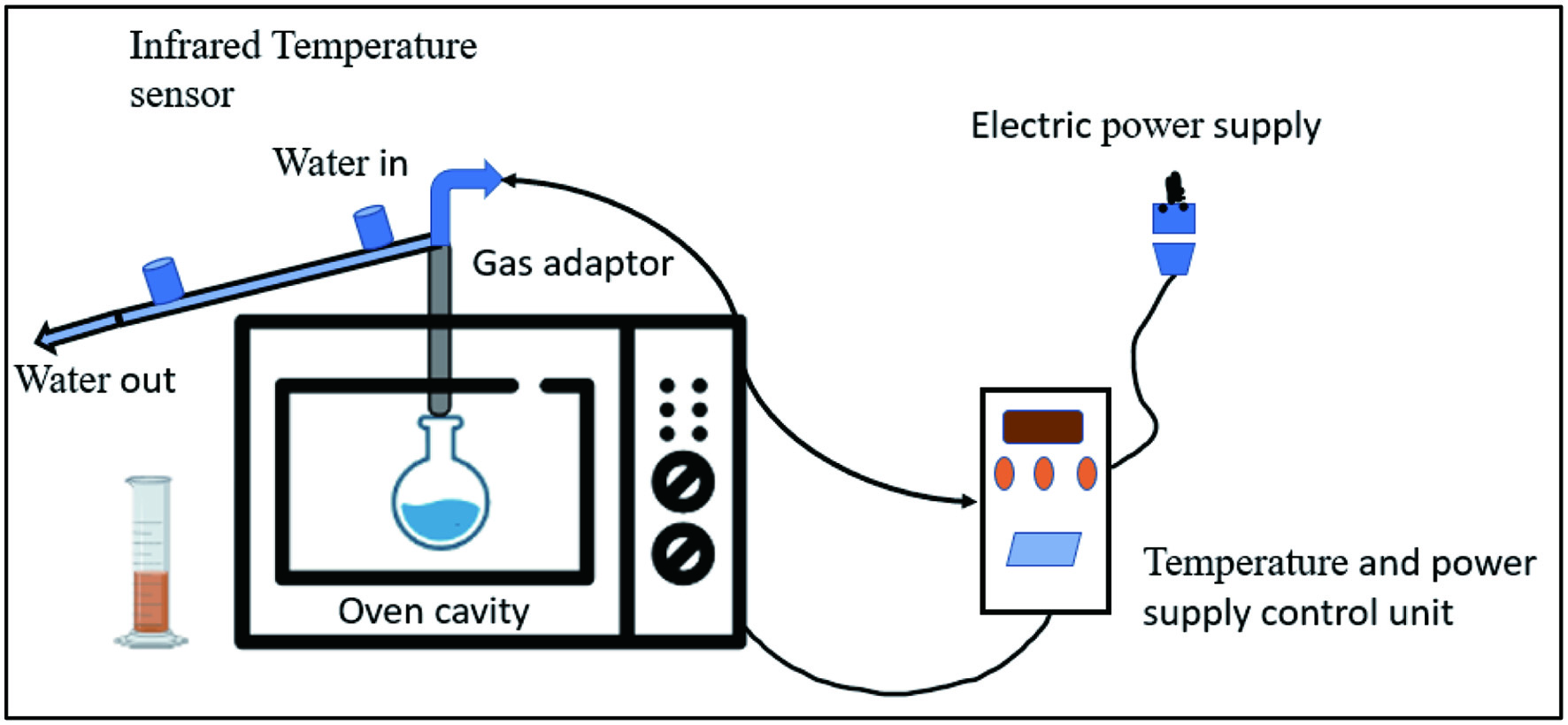
Tables
| Sources | Phytochemicals | Functions | References |
|---|---|---|---|
| Pomegranates, grapes, bilberry, choke-berry, elderberry, and all tart cherries | Anthocyanin | The antioxidants and free radical-fighting compounds may have anti-inflammatory, antiviral, and anti-cancer properties. | Yousuf et al., 2016 |
| Apples, berries, cherries, and citrus fruits | Flavonoids | The flavonoids aid in the regulation of cellular activities. They fight against free radicals that cause oxidative stress, are effective in the proper functioning of the body, and also shield it from everyday contaminants and stressors. | Waheed Janabi et al., 2020 |
| Blueberries, raspberries, strawberries, and other berries | Phenolic acids | Phenolic acids are potentially effective antioxidants which prevent cell damage caused by free-radical oxidation processes, which can cause cancer and other diseases. They are easily absorbed through intestinal tract walls. Humans’ anti-inflammation capacity is also enhanced by Phenolic acids when consumed regularly. | Di Domenico et al., 2015 |
| Pumpkin and watermelon | Alpha-carotene and Beta-carotene | The two forms of -carotene are primarily considered vitamin A precursors. In addition to their therapeutic role as vitamin A precursors, these carotenoids are also associated with the prevention of various cancers, including skin cancer and breast cancer. | Glowka et al., 2019 |
| Kiwi fruit, grapes, and orange | Lutein and zeaxanthin | Zeaxanthin and lutein are powerful antioxidants responsible for protecting body against free radicals. Free radicals, in excess, can harm your cells, speed up the aging process, and accelerate the progression of diseases including heart disease, cancer, type 2 diabetes, and Alzheimer’s disease. | Becerra et al., 2020 |
| Watermelon, grapefruit, guava, papaya, etc. | Lycopene | Carotenoids in human blood, including lycopene, combat oxidative damage to lipids, proteins, and DNA. Lycopene is an important detoxifier of singlet oxygen (a reactive form of oxygen). | Suwanaruang, 2016 |
| Blueberries | Polyphenols, flavonoids, anthocyanins | It reduces the risk of cancer, cardiovascular activity, and diabetes. | Bhise and Morya, 2021 |
| Gooseberries | Gallic acid, Ascorbic acid | It has anti-ulcerous, anti-carcinogenic, and anti-diabetic properties. | Bhise and Morya, 2021 |
| Fruits | Bioactive principles | Health benefits | Reference |
|---|---|---|---|
| Pineapple | Bromelain | Anti-inflammatory, antioxidant activity, nervous system monitoring, and bowel movement easing | Ali et al., 2020 |
| Mango, blueberry, blackberry, strawberry | Gallic acid | Antioxidant, anti-diabetic, anti-bacterial, gastrointestinal health, anti-cancer, and anti-inflammatory properties | Hussain et al., 2020 |
| Strawberries | Ellagic acid | Anti-inflammatory, anti-diabetic, cardioprotective, neuroprotective, and prebiotic properties | Nair and Agrawal, 2017 |
| Grapes | Resveratrol, chlorogenic acid, flavonoids. | Cardiovascular health, weight maintenance and eye vision | Kalt et al., 2019 |
| Papaya | Papain, carpaine and carposide | Anti-cancer properties. | Kumar and Devi, 2017 |
| Banana, Apple, Berries | Quercetin, catechin, gallic acid, phloretin | Improves lipid metabolism, antioxidant, gastrointestinal function, metabolic diseases (hyperglycemia, insulin resistance). | Skinner et al., 2018 |
| Test | Reagent | Endpoint | References |
|---|---|---|---|
| Alkaloids testing | |||
| Mayer Test | Mayer reagent | White creamy precipitate form | Banu and Cathrine, 2015 |
| Wagner test | Wagner reagent | Reddish-brown precipitate form | Shahzad et al., 2020 |
| Dragendroff’s test | Dragendroff’s reagent | Orange or orange-red precipitate form | Raal et al., 2020 |
| Hager test | Hager reagent (saturated solution of Picric acid) | Yellow precipitate form | Kancherla et al., 2019 |
| Carbohydrate testing | |||
| Molish’s test | Molisch’s reagent (a solution of ∝-naphthol in ethanol | purple or reddish-purple | Elzagheid, 2018 |
| Fehling’s test | Fehling reagent A: (CuSO4.5H20); Fehling reagent B (Sodium Potassium Tartrate) | Reddish-brown color appears | De-Silva et al., 2017 |
| Barfoed test | Barfoed reagent (solution of copper acetate added to acetic acid) | Red precipitate form | Rajasree et al., 2021 |
| Seliwanoffs test | Seliwanoffs reagent (resorcinol and concentrated HCL) | Cheery red color form | Sánchez-Viesca and Gómez, 2018 |
| Protein testing | |||
| Millon test | Millon’s reagent (mercuric nitrate and mercurous nitrate dissolve in concentrated Nitric acid) | Brick red colour form | Kamineni et al., 2016 |
| Ninhydrin test | Ninhydrin reagent | For primary/Secondary amines- deep purple color appears; For hydroxyproline and proline-yellow color appears; For asparagine-brown color appears | De-Silva et al., 2017 |
| Xanthoproteic test | Xathoproteic reagent (test solution: tyrosine, phenylalanine, albumin, tryptophan; Nitric acid NaOH | Dark yellow or orange-colored solution form | Tiwari et al., 2011 |
| Test for Flavonoids | |||
| Lead acetate test | Lead acetate solution in water NaOH | Black precipitate at the bottom of the flask will form | Kamineni et al., 2016 |
| Test for Phytosterols | |||
| Salkwoski Test | Chloroform solution of steroid is mixed with concentrated sulphuric acid | Reddish-brown color will form | Laveena and Chandra, 2017 |
| Libermann-Burchard’s test | The concentrated solution of chloroform, acetic anhydride, sulphuric acid | Green or greenish-blue color will form | Noormazlinah et al., 2019. |
| Test for Phenols | |||
| Tannin Test | Ferric chloride | Greenish to black color will form | Hayat et al., 2020 |
| Advanced techniques | Process | References |
|---|---|---|
| Gas chromatography | The sample is distributed between the gas and liquid phase. Migration of sample depends upon the quantity of chemical which is distributed in the liquid phase. | Rasul, 2018 |
| HPLC (High-performance liquid chromatography) | In this technique, components are separated on the basis of interaction between solid particles of column and solvent of the mobile phase. This technique needs 400 bar pressure and components are not decomposed or eluted in this technique. | Marques et al., 2018 |
| HPLC–MS (High-performance liquid mass spectroscopy) | A mixture of components is separated first followed by ionization and separation of ions according to mass/ charge ratio. | Katanić Stanković et al., 2020 |
| HPTLC (High-performance thin layer chromatography) | Separation of components is done by using an advanced work station. The layers are coated with sorbent having particle size (5–7 microns) and thickness (150–200 microns). The reduction in layer and particle size results in increasing plate efficiency with the nature of separation. | Blebea and Negreș, 2018 |
| OPLC (Optimum performance laminar chromatography) | Combination of TLC and HPLC. It separates about 10–15 mg of sample. | Banjo et al., 2018 |