
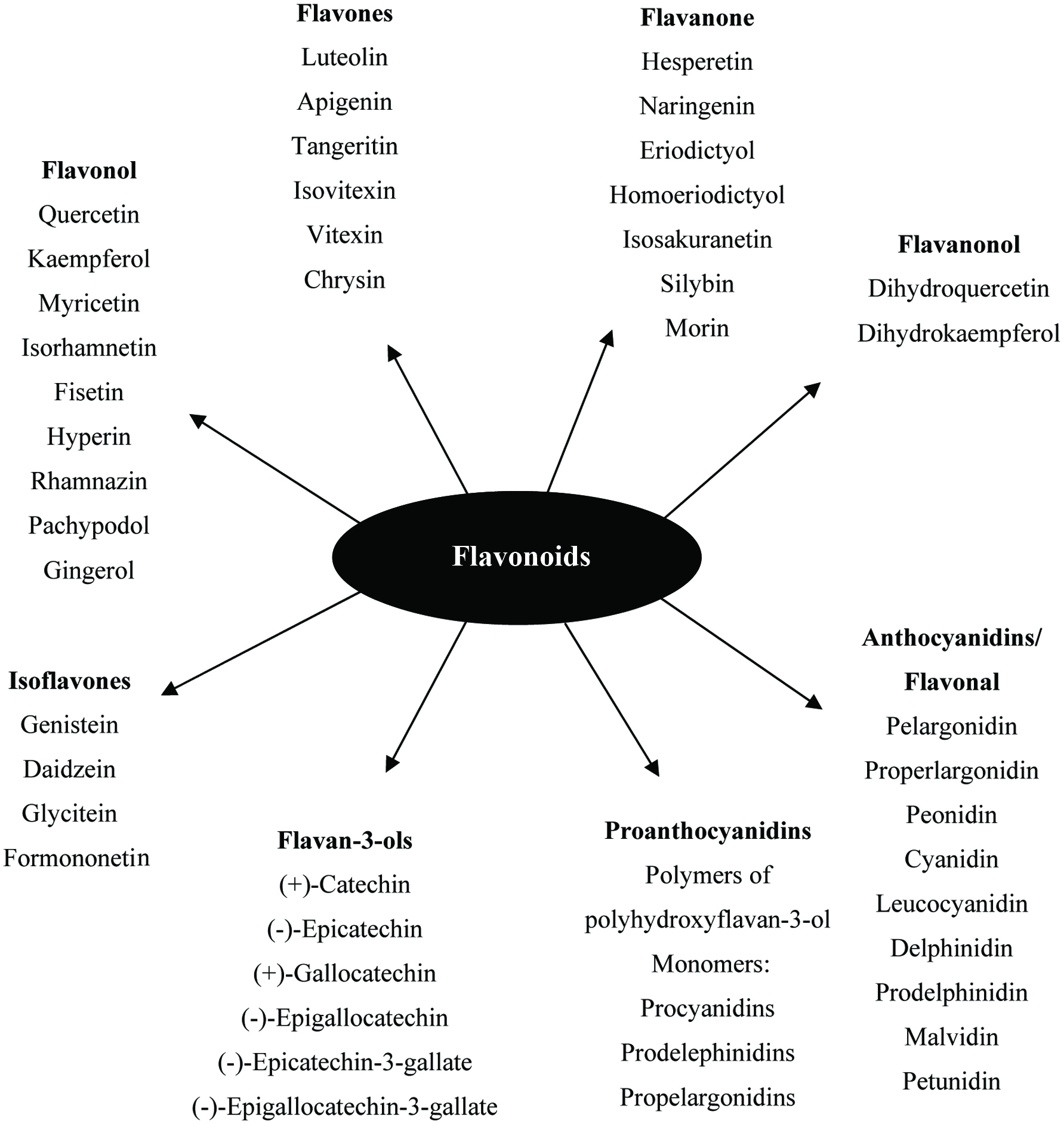
Figure 1. General breakdown of non-nutritive plant-base natural products.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 5, March 2019, pages 57-119
Phenolic compounds in agri-food by-products, their bioavailability and health effects
Figures


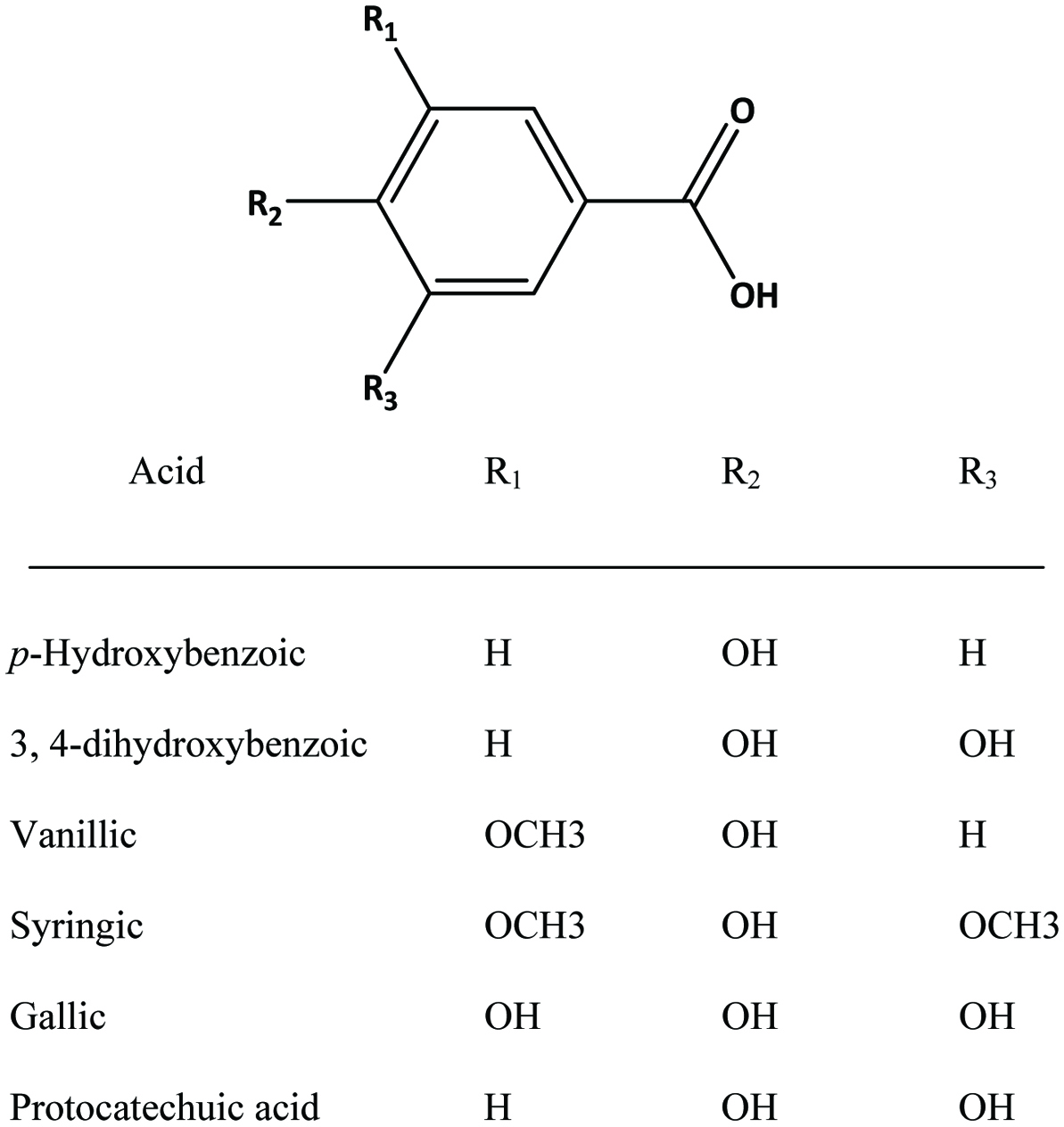


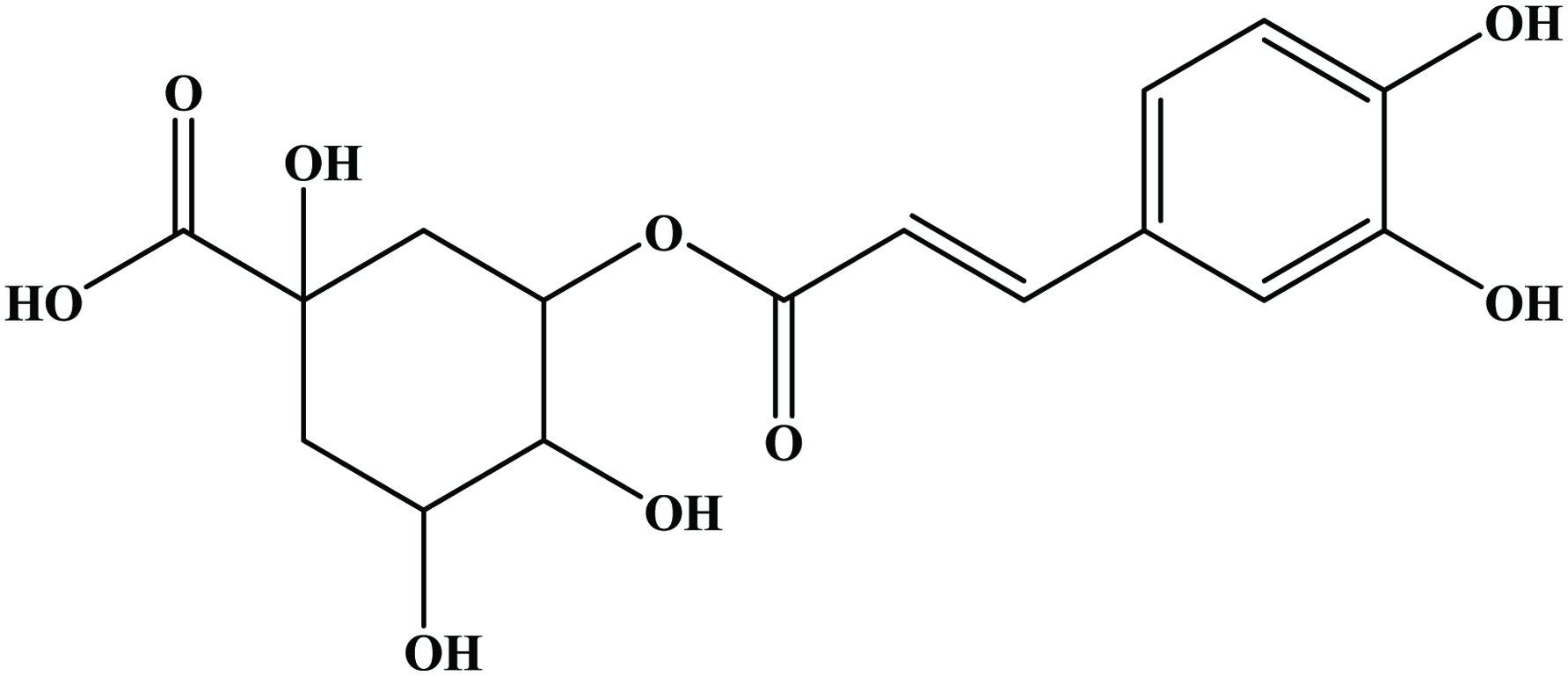
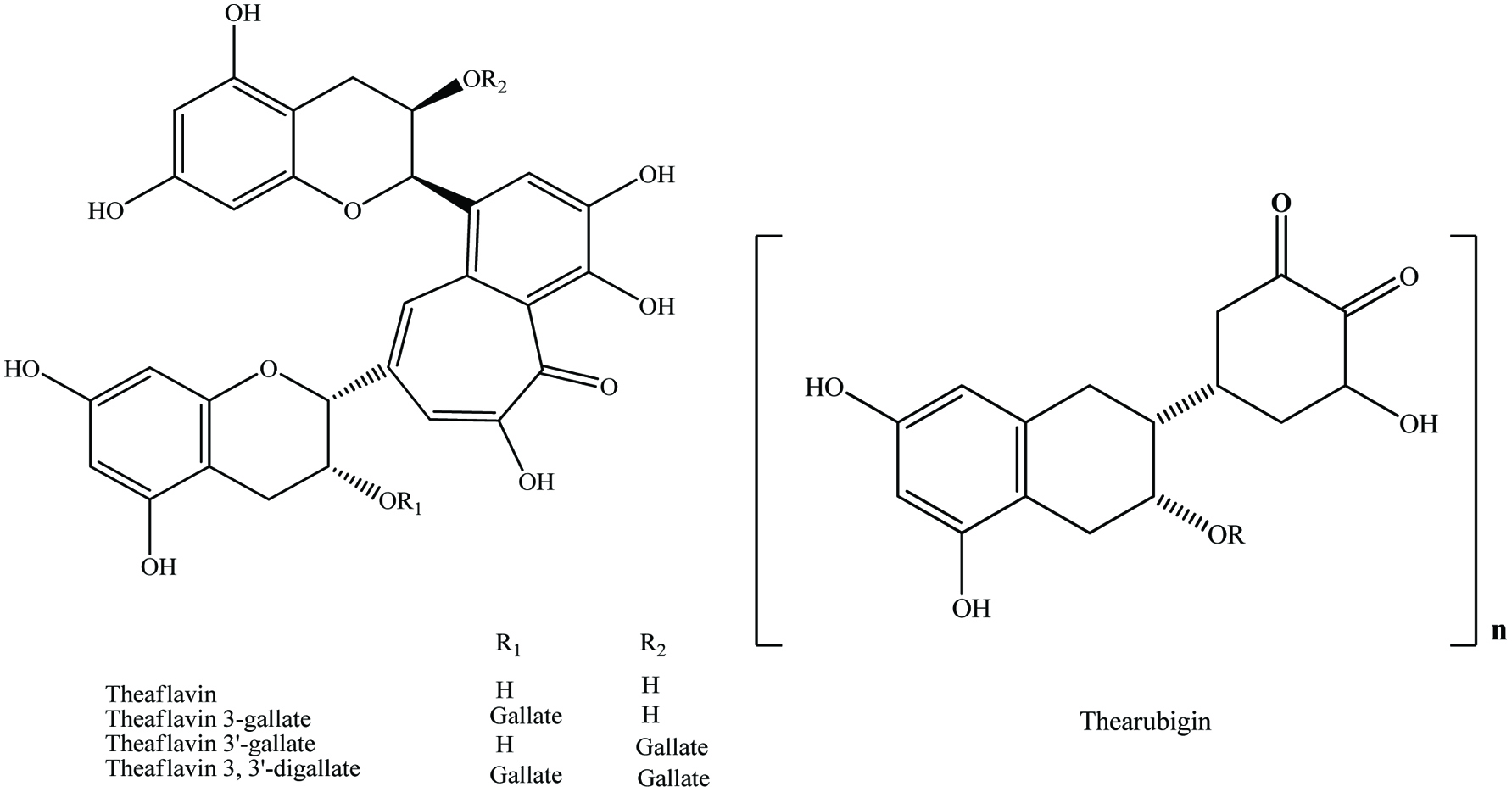
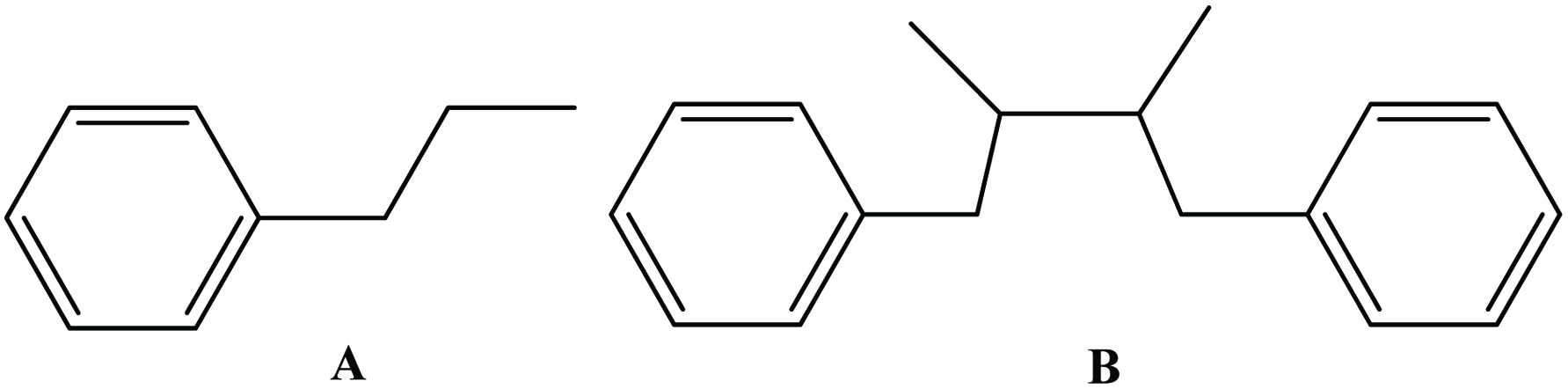
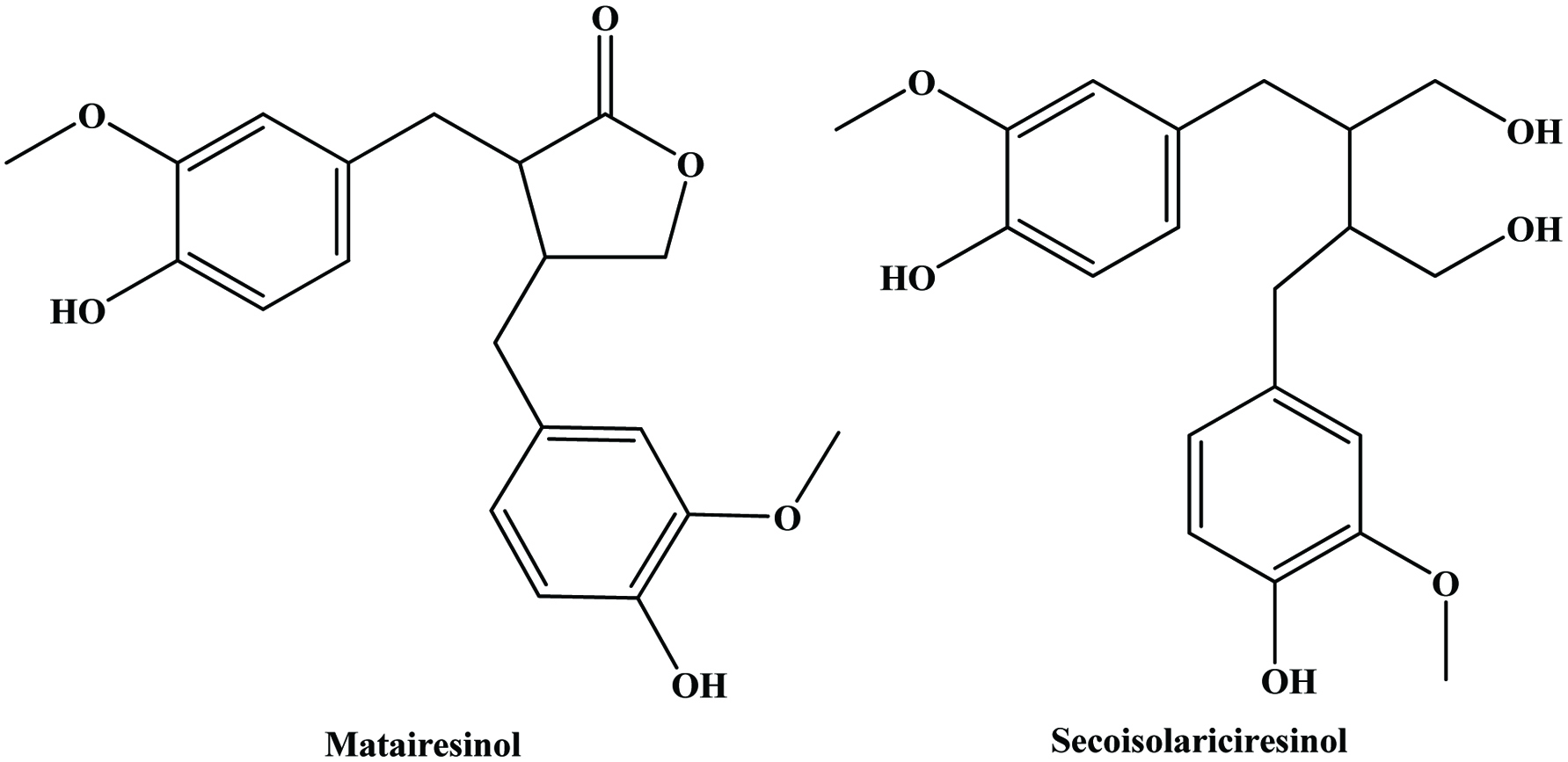
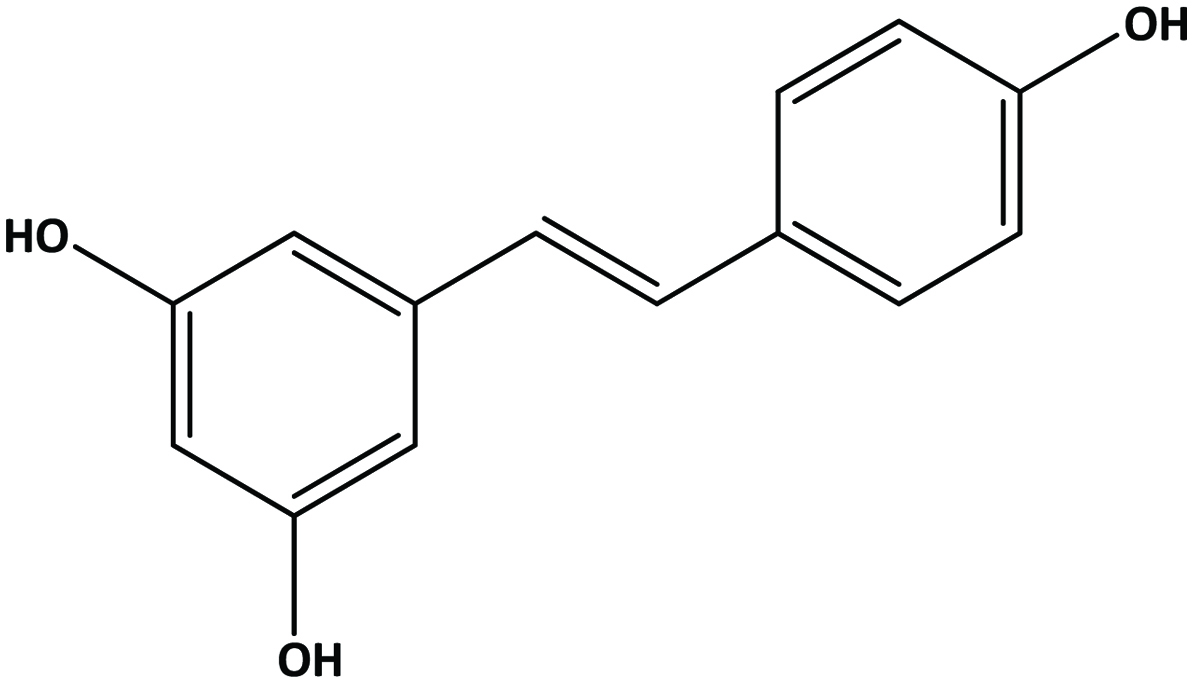


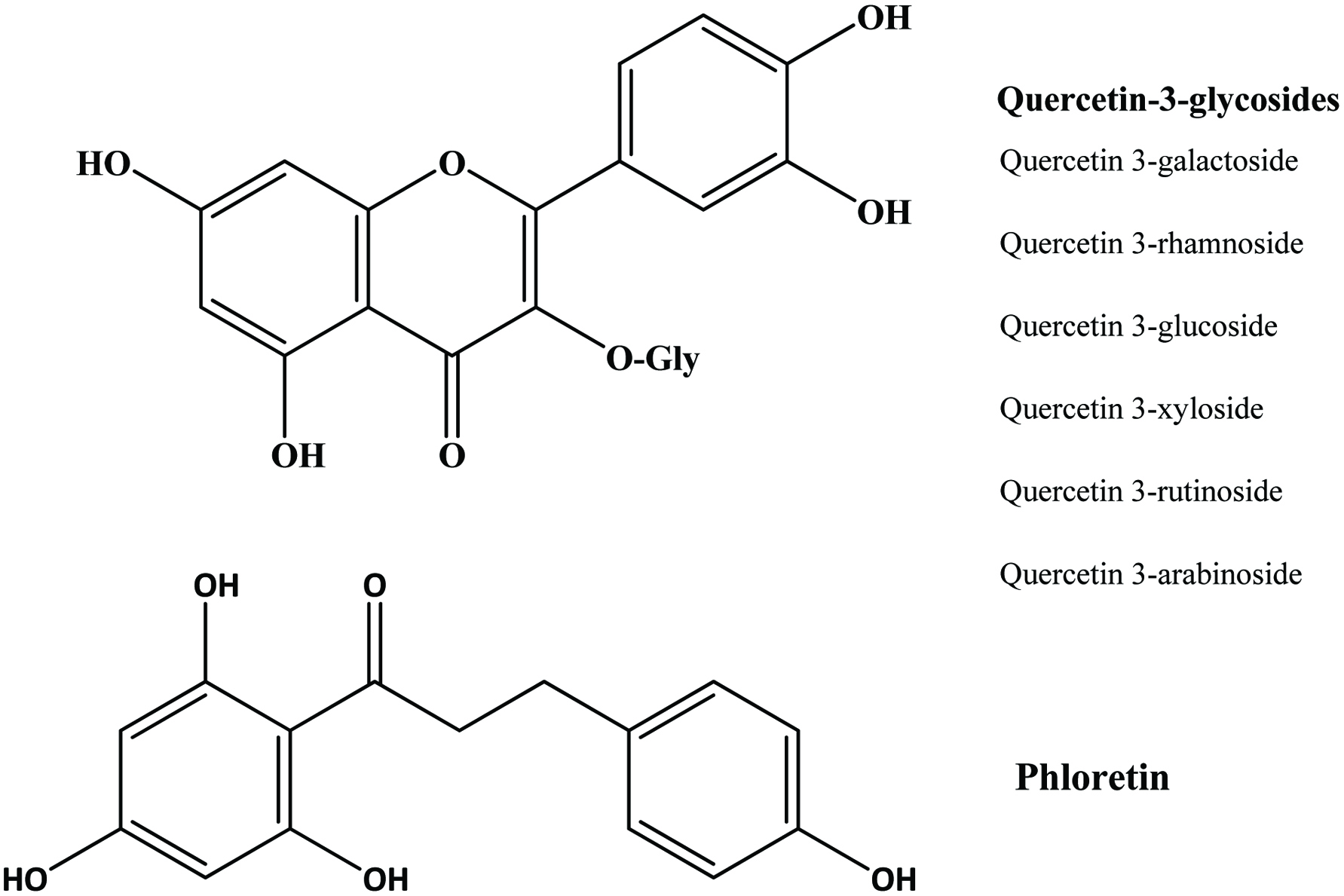




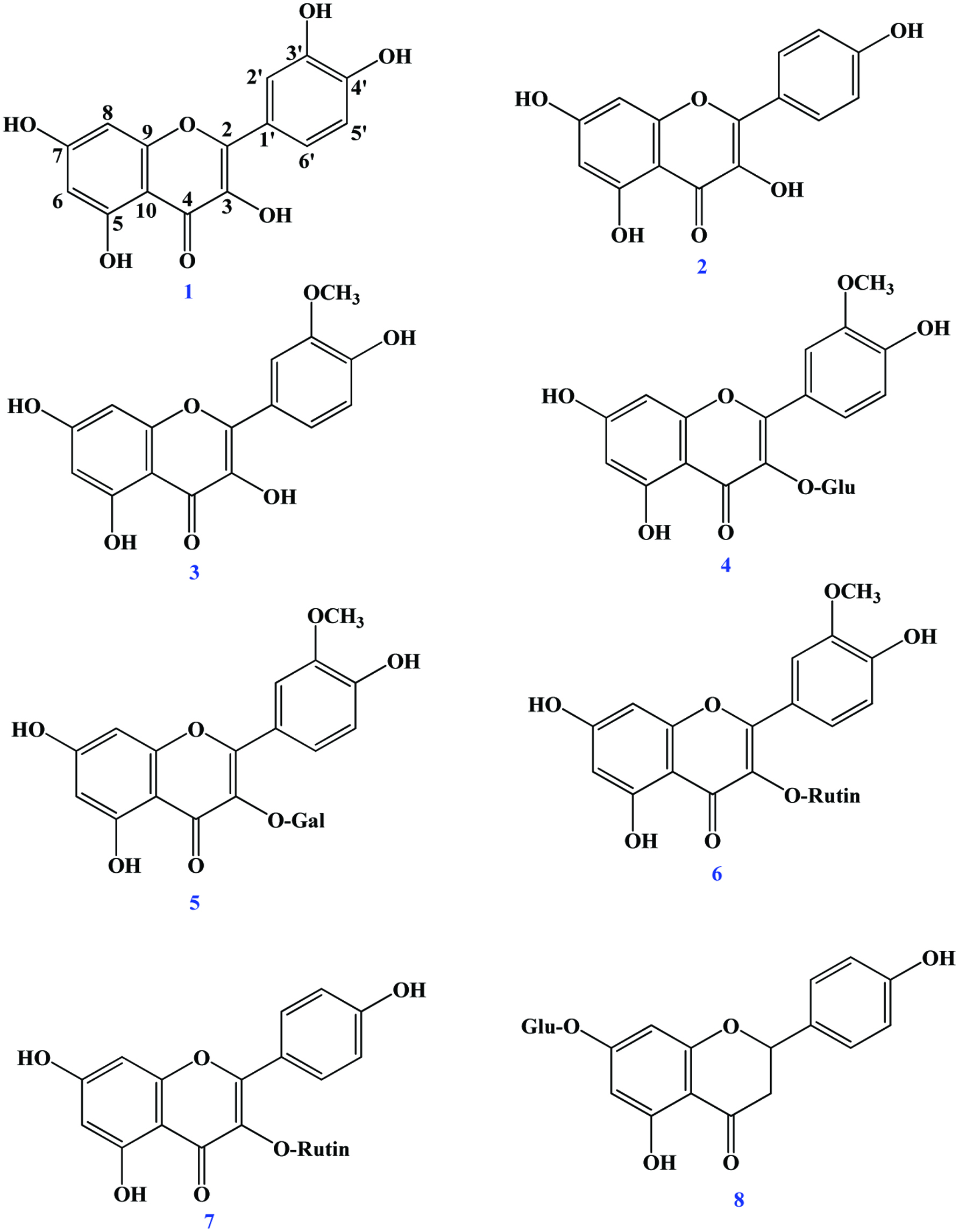
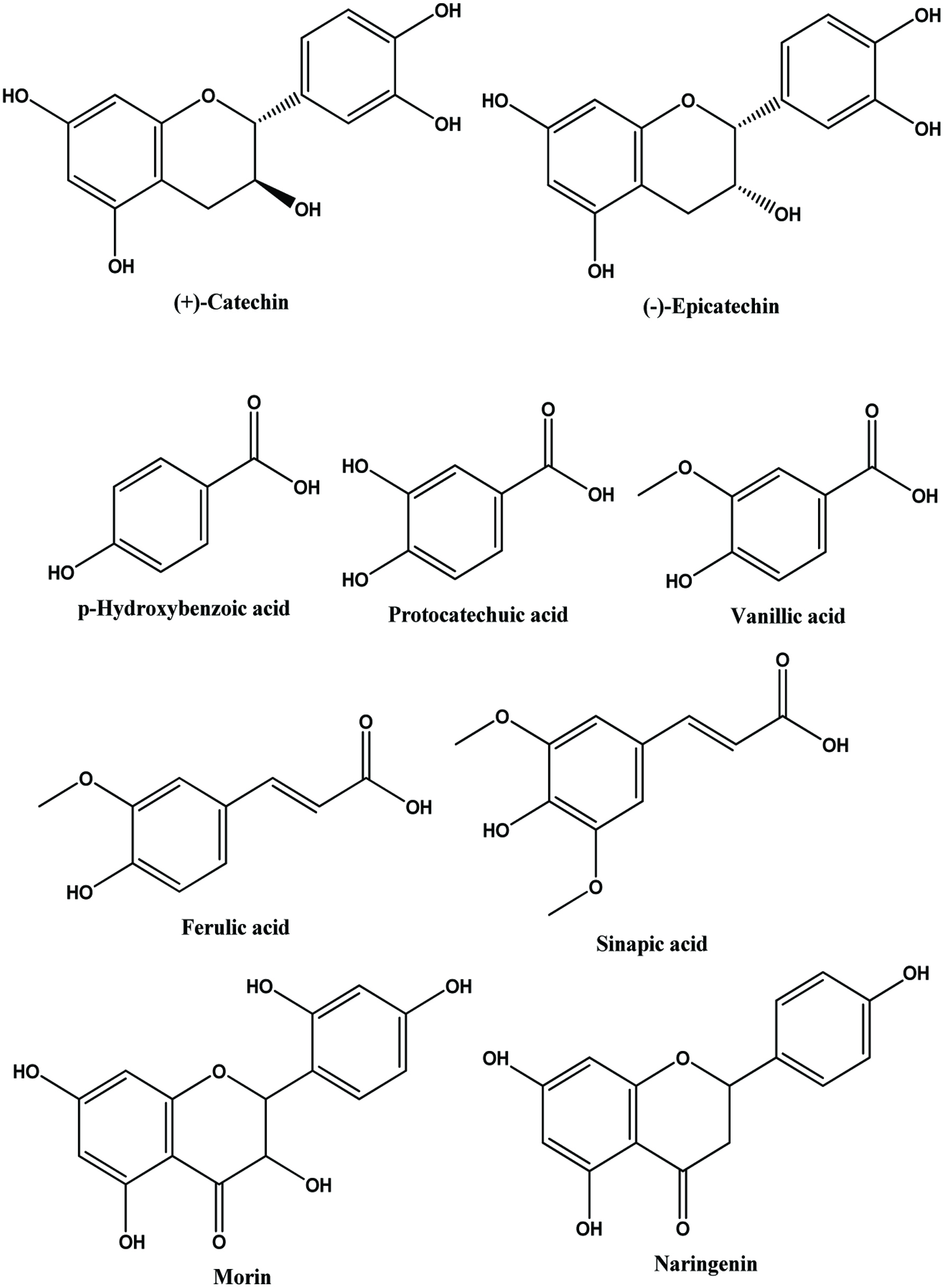

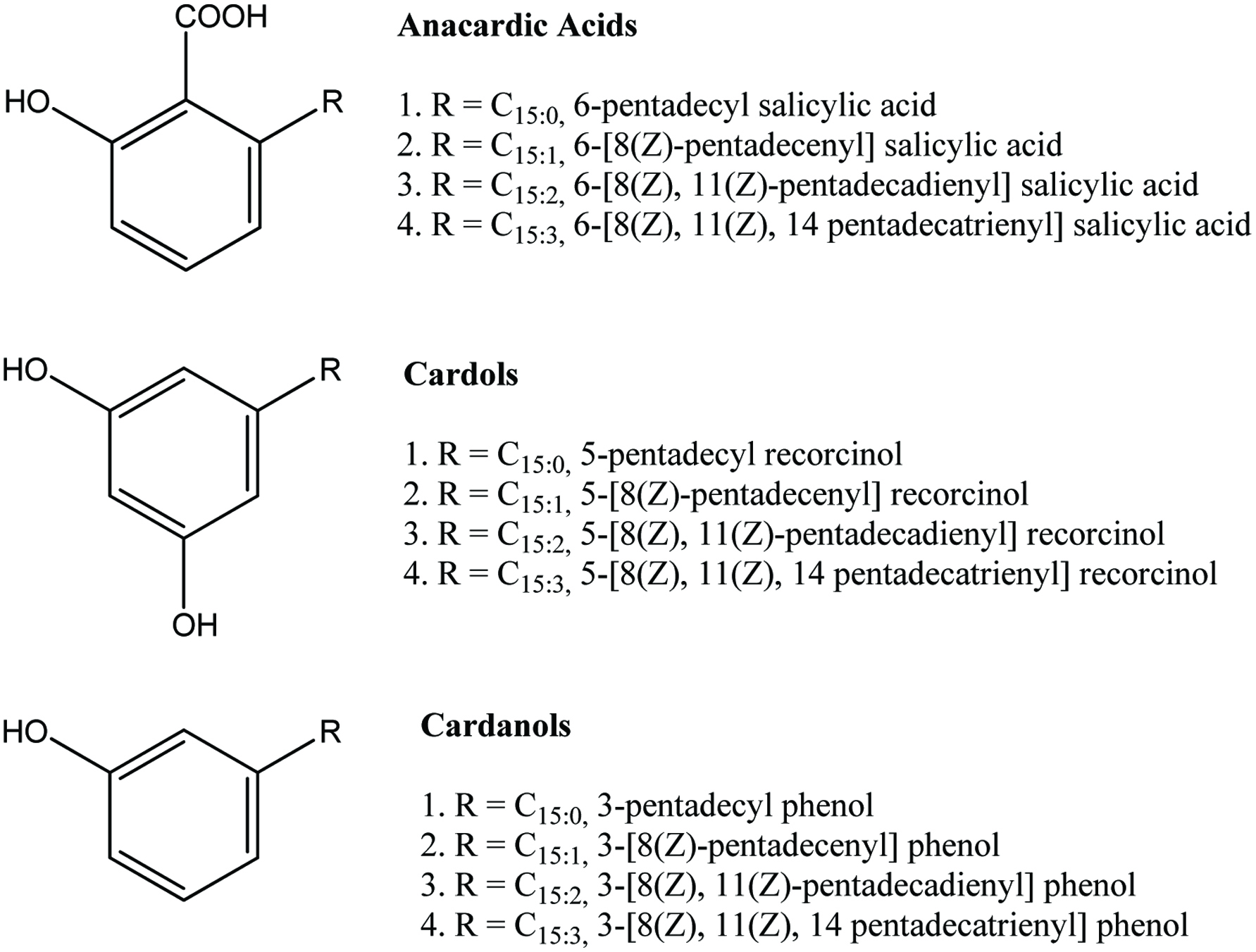

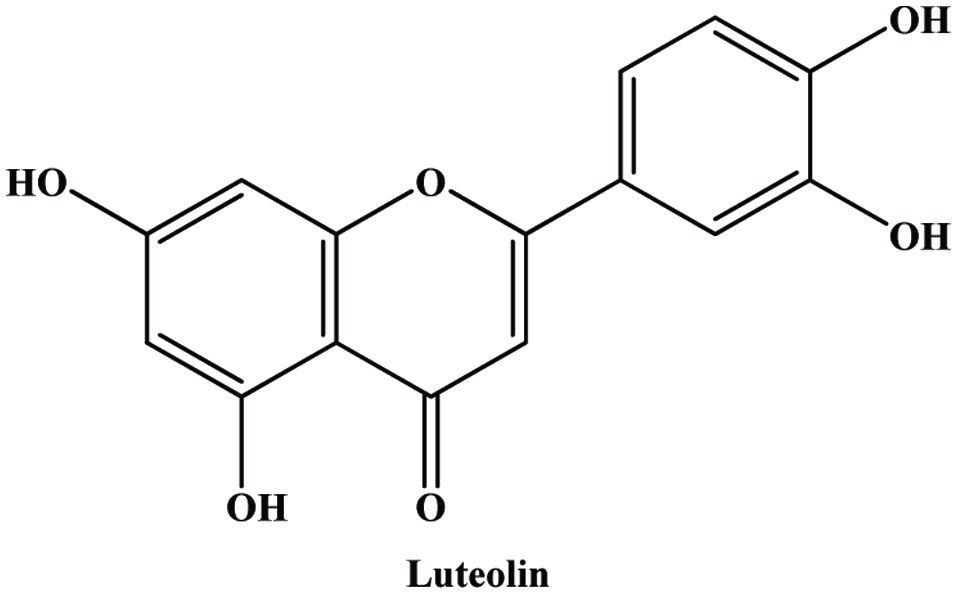




Table
| By-products | Major phenolic compounds | Total Phenolic contenta | Reference |
|---|---|---|---|
| Abbbreviations: GAE: gallic acid equivalents; CE: catechin equivalents; TAE: tannic acid equivalents; SE: sinapic acid equivalents; CGE: chlorogenic acid equivalents. aExpressed as dry weight. | |||
| Apple peel | Quercetin glycosides, phloretin glycosides, catechins, procyanidins, phloridzin, caffeic acid, chlorogenic acid, cyanidin glycosides | 33.42 mg/g (GAE) | Wijngaard et al. (2009), Wolfe and Liu (2003) |
| Apple seed | Amygdalin, phloridzin | 2.17 mg/g | Schieber et al. (2003a), Lu and Foo (1998) |
| Apple pomace | Phloridzin, chlorogenic acid, quercetin glycosides | 4.22–8.67 (CGE) | Schieber et al. (2003a), Ćetković et al. (2008) |
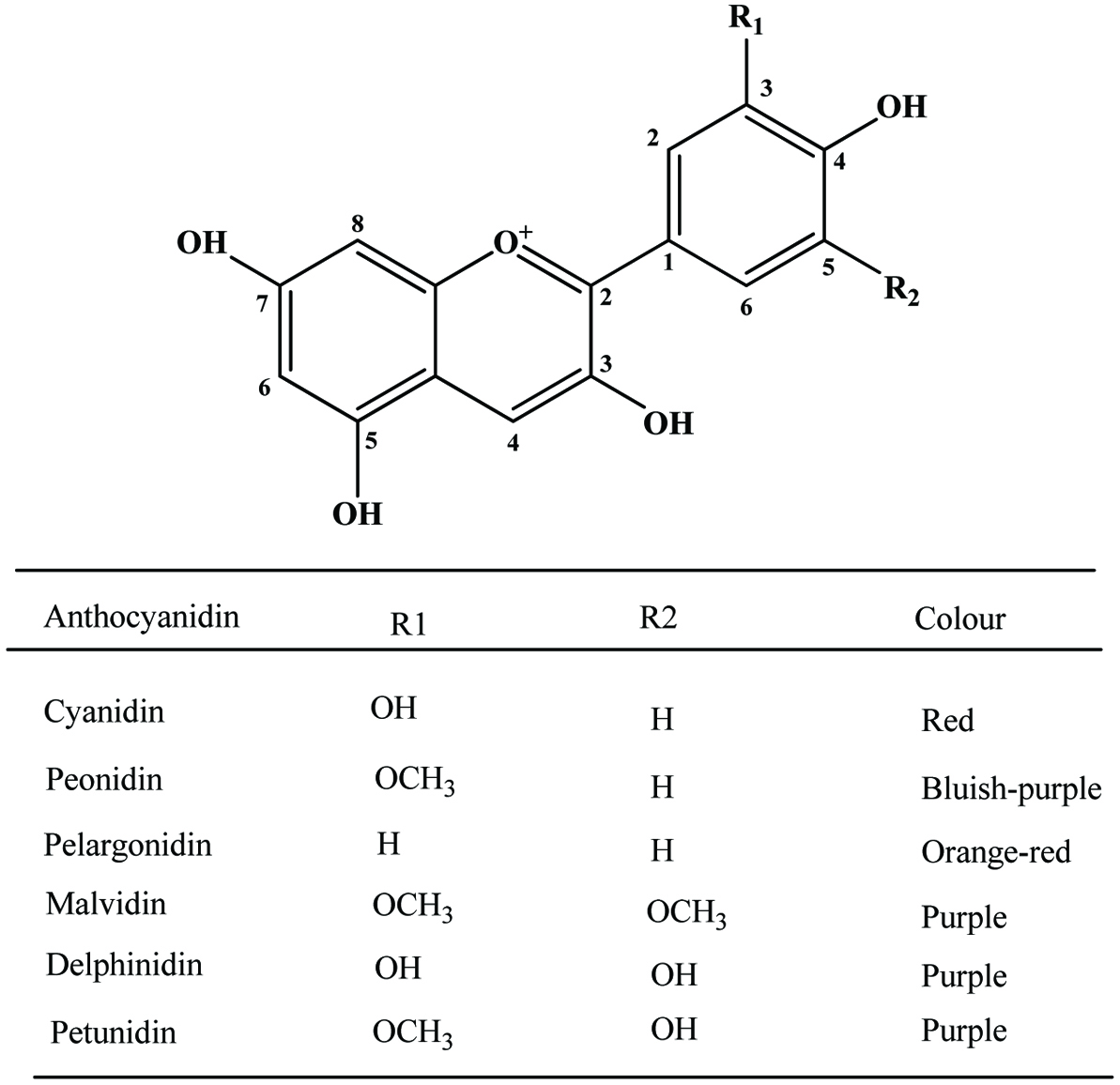
| Berry pomace | Cyanidin-3-O-glucoside, quercetin-3-O-glucoside, gallic acid, protocatechuic acid | 21.7–47.4 mg/g (GAE) | Zhou et al. (2009) |
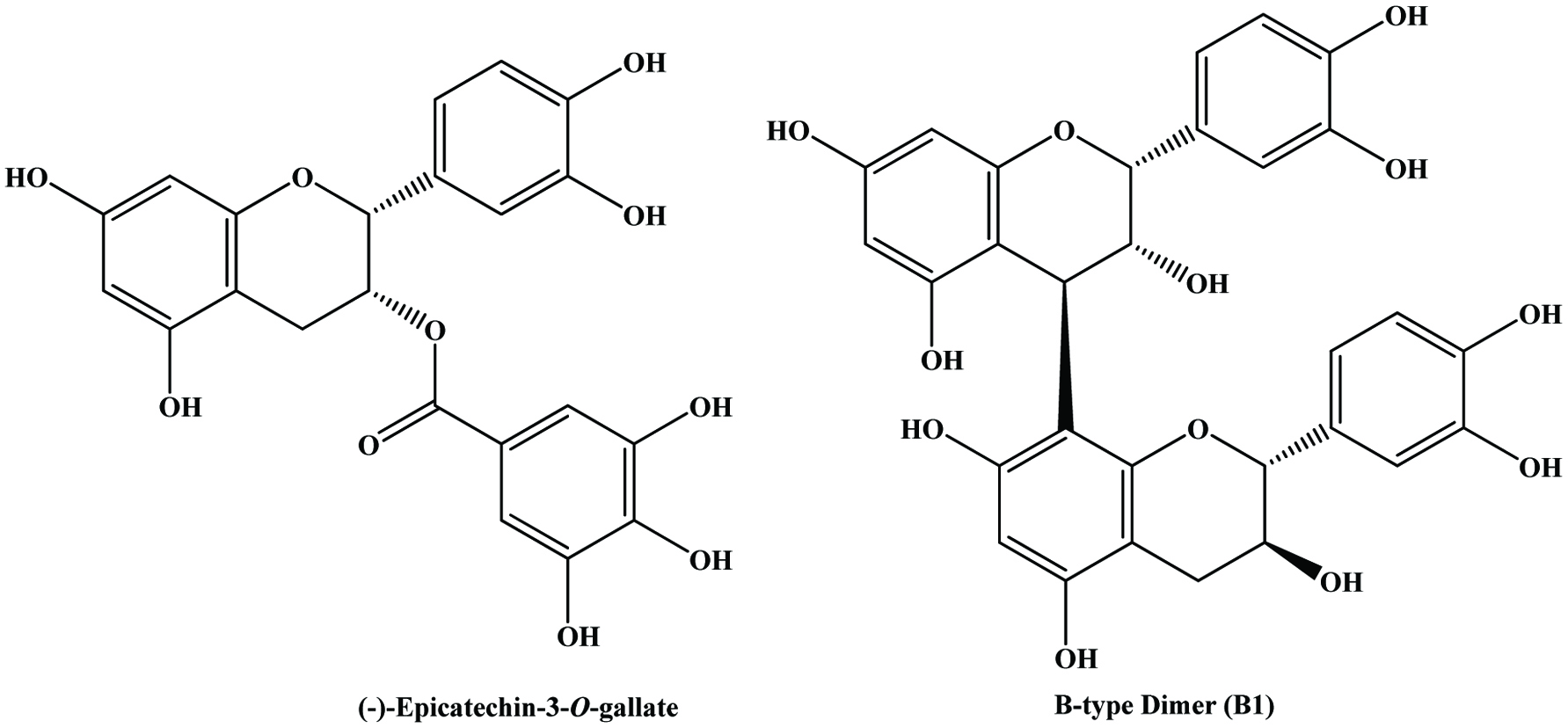
| Grape skin | Catechin, epicatechin, epicatechin gallate, epigallocatechin | Souquet et al. (1996) | |
| Grape seed | Catechin, epicatechin, epicatechin-3-O-gallate, dimeric procyanidins B2 and B3 | 325.37–811.95 mg/g (GAE) | Anastasiadi et al. (2009) |
| Grape stem | trans-Resveratrol, e-viniferin | 367.1–494.2 mg/g (GAE) | Anastasiadi et al. (2009), Püssa et al. (2006) |
| Grape pomace | Anthocyanins, catechins, flavonol glycosides, phenolic acids, trans-resveratrol | 107.12–376.71 mg/g (GAE) | Anastasiadi et al. (2009), Yi et al. (2009) |
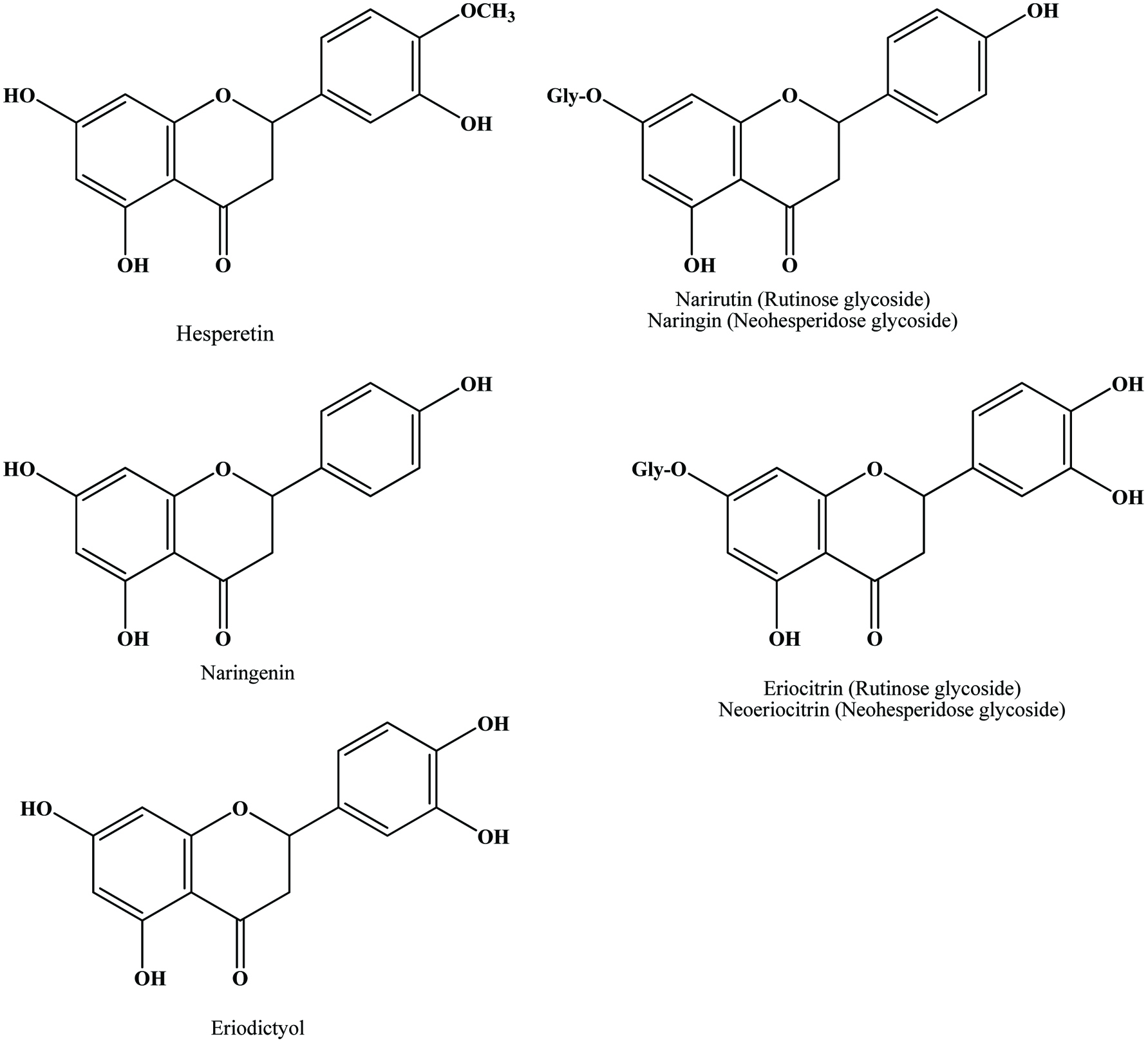
| Citrus by products (peel) | Hesperidin, narirutin, naringin eriocitrin and their glycosides, hydroxycinnamic acid | 24 mg/g (GAE) | Manthey and Grohmann (2001), Peterson et al. (2006), Sultana et al. (2008) |
| Mango peel | Flavonol-O-glycosides, xanthone-C-glycosides | 55–110 mg/g (GAE) | Ajila et al. (2007), Schieber et al. (2003b) |
| Mango seed | Gallic acid, ellagic acid, gallates | 117 mg/g (GAE) | Puravankara et al. (2000), Soong and Barlow (2004) |
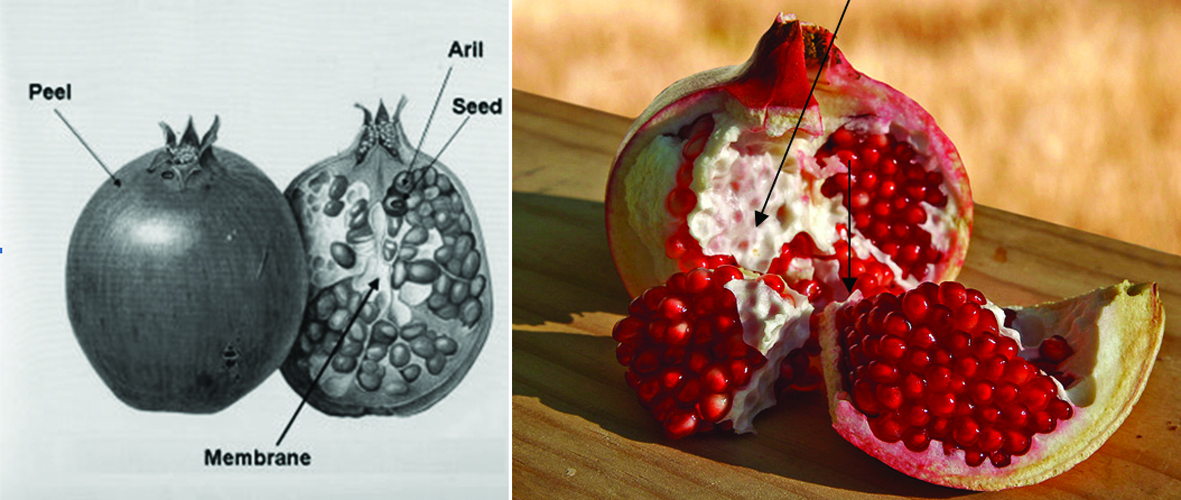
| Pomegranate peel | Punicalagin, punicalin, ellagic acid, gallagic acid, quercetin, kaemferol, myricetin | 364 mg/g (GAE) | Sultana et al. (2008) |
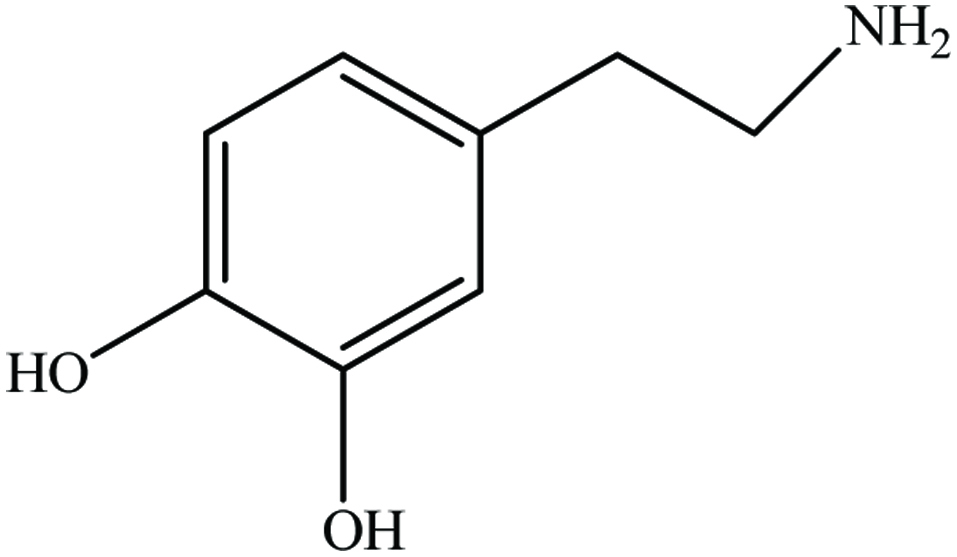
| Banana peel | Dopamine, flavonone glycoside, naringin, rutin | 11 mg/g (GAE) | Kanazawa and Sakakibara (2000), Sultana et al. (2008) |
| Banana bracts | Anthocyanin | Pazmino-Duran et al. (2001) | |
| Longan seeds | Gallic acid, ellagic acid | Soong and Barlow (2005, 2006) | |
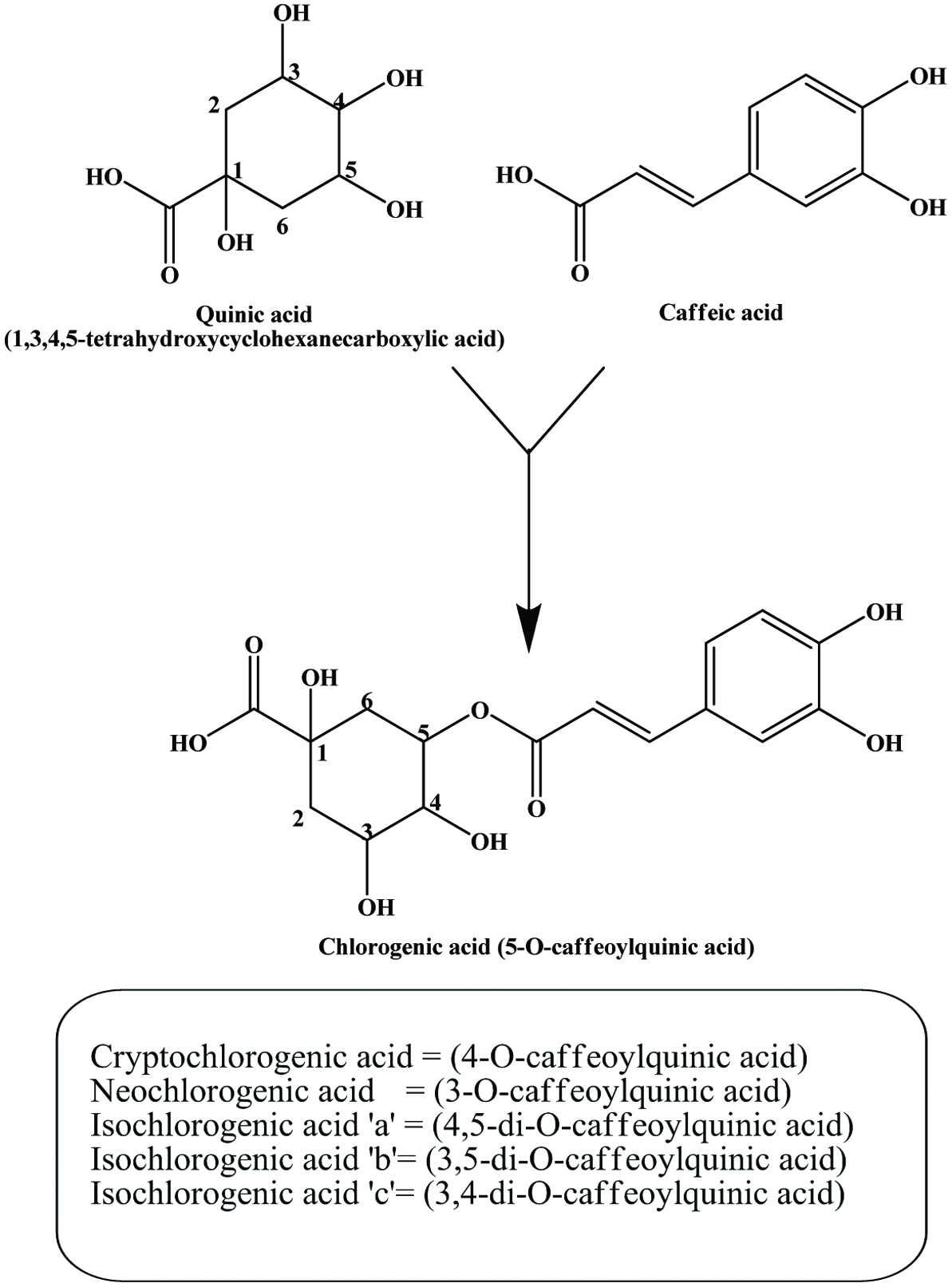
| Almond hull | Catechin, procatechuic acid, chlorogenic acid, cryptochlorogenic acid, prenylated benzoic acid | 35.9–166.7 mg/g (GAE) | Sang et al. (2002a), Sfahlan et al. (2009) |
| Almond green shell cover | Caffeic acid, p-coumaric acid, ferulic acid, sinapic acid | 18.5–62.7 mg/g (GAE), 71 mg/g (CE) | Sfahlan et al. (2009), Wijeratne et al. (2006), Siriwardhana and Shahidi (2002) |
| Almond skin | Flavan-3-ols, flavonol glycosides, dihydroflavonols, flavonones, phenolic acid | 88 mg/g (CE) | Siriwardhana and Shahidi (2002) |
| Hazelnut skin | Phenolic acids | 577.7 mg/g (CE) | Shahidi et al. (2007) |
| Hazelnut shell | 214.1 mg/g (CE) | Shahidi et al. (2007) | |
| Hazelnut green leafy cover | 127.3 mg/g (CE) | Shahidi et al. (2007) | |
| Hazelnut leaf | 134.7 mg/g (CE) | Shahidi et al. (2007) | |
| Cashew nut shell liquid | Anacardic acids, cardols, cardanols | 353.6 mg/g | Trevisan et al. (2006) |
| Cashew fibre | Alkyl phenols | 6.1 mg/g | Trevisan et al. (2006). |
| Cashew skin | Epicatechin, catechin | 243 mg/g | Kamath and Rajini (2007) |
| Pistachio hull | Gallic acid | 32.8–34.7 mg/g (TAE) | Goli et al. (2005), Vahabzadeh et al. (2004) |
| Walnut skin (pellicle) | Juglone, syringic acid, ellagic acid, hydrolysable tannins, condensed tannins | 230–490 mg/g (GAE) | Colaric et al. (2005), Labuckas et al. (2008) |
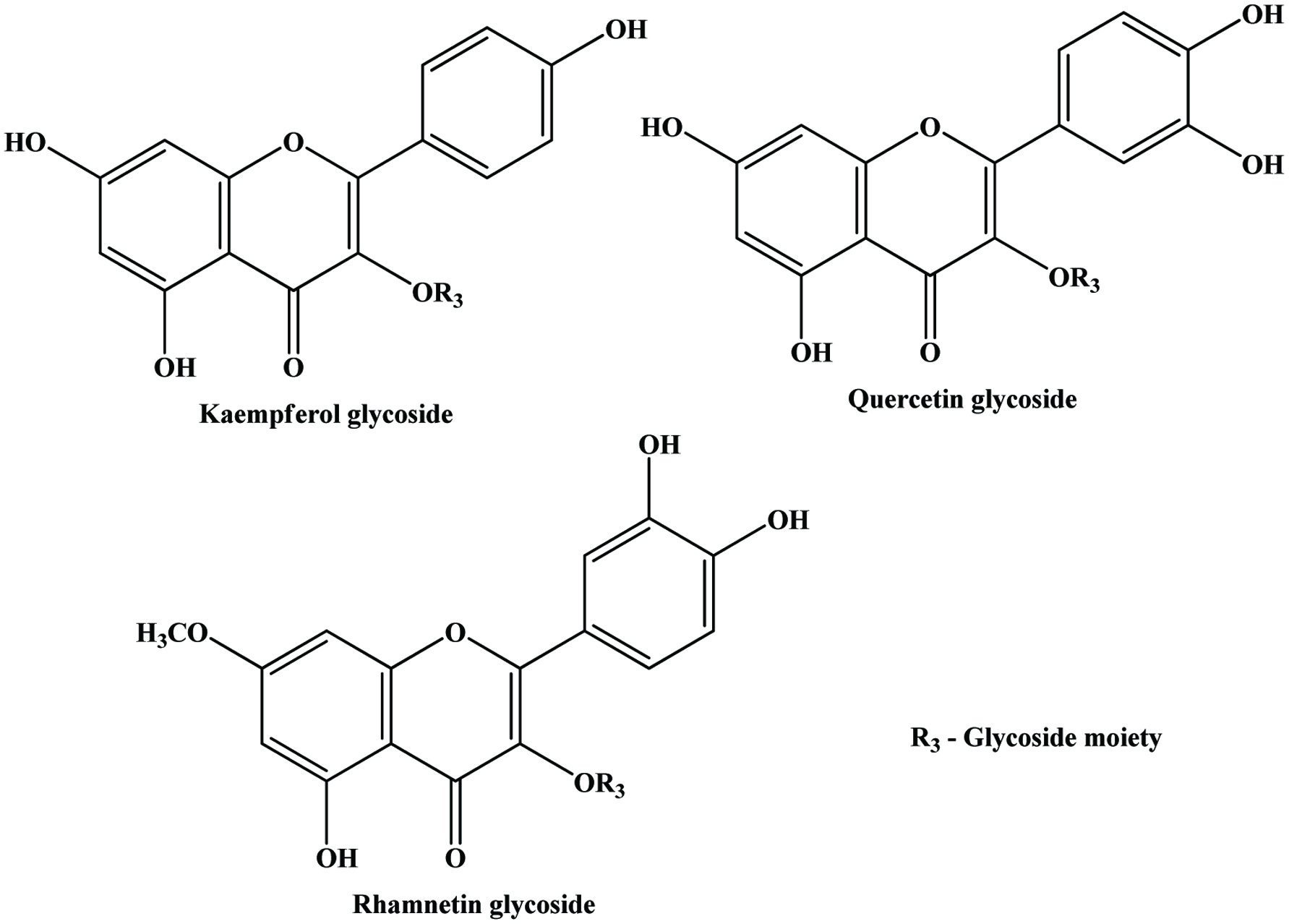
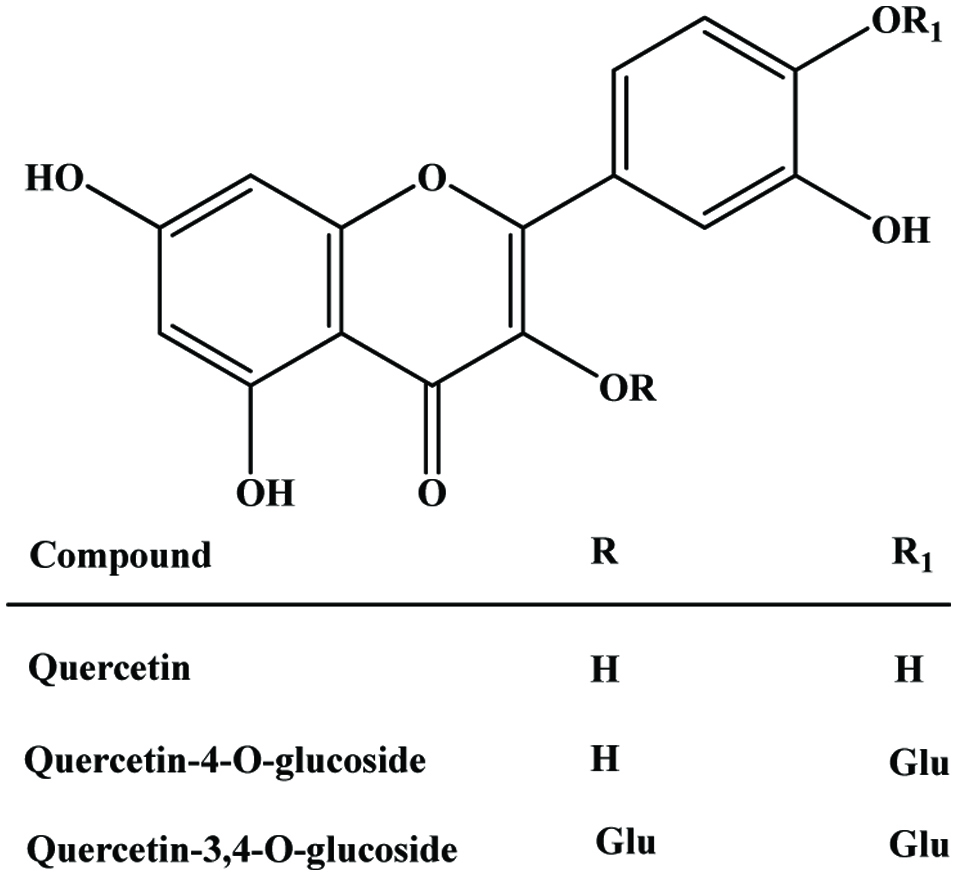
| Red onion dry peel | Quercetin, quercetin glycoside | 384.7 mg/g (GAE) | Singh et al. (2009) |
| Tomato skin | Quercetin, kaempferol, rutin, phenolic acids, naringenin | 0.29 mg/g (GAE) | Toor and Savage (2005) |
| Potato peel | Chlorogenic acid, gallic acid, protocatechuic acid, caffeic acid | 2.9–4.2 mg/g | Singh and Rajini (2008) |
| Red Beet pomace | l-Tryptophan, p-coumaric acid, ferulic acid | 87–151 mg/g (GAE) | Peschel et al. (2006) |
| Carrot peel | Chlorogenic acid, caffeic acid | 13.8 mg/g (GAE), 9.79 mg/g (GAE) | Chantaro et al. (2008), Zhang and Hamauzu (2004) |
| Pumpkin peels | Vanillic acid, p-coumaric acid, sinapic acid | Schmidtlein and Herrmann (1975) | |
| Pumpkin seed hull | p-Hydroxybenzoic acid, protocatechuic acid, vanillic acid, trans-p-coumaric acid, ferulic acid, trans-sinapic acids, syringic acid, p-hydroxybenzaldehyde | Peričin et al. (2009) | |
| Pumpkin oil cake | p-Hydroxybenzoic acid, protocatechuic acid, vanillic acid, trans-p-Coumaric acid, ferulic acid, trans-sinapic acids, caffeic acid, p-hydroxybenzaldehyde | Peričin et al. (2009) | |
| Artichoke by-products | Caffeoylquinic acid derivatives, luteolin and apigenin glycosides, cynaroside | Liorach et al. (2002), Zhu et al. (2004) | |
| Peanut skin | Caffeic acid, chlorogenic acid, ferulic acid and coumaric acid, catechins, procyanidins, resveratrol | 90–125 mg/g, 144.1–158.6 mg/g | Nepote et al. (2002), Yu et al. (2005) |
| Peanut hull | Luteolin | 7.3–41.8 mg/g | Duh et al. (1992) |
| Bean seed coat | Condensed tannins, anthocyanins, quercetin, kaempferol glycosides | 6.7–270 mg/g (CE) | Madhujith and Shahidi (2005a) |
| Corn bran | Ferulic acid p-coumeric acid and vannilin | 50 mg/g (GAE) | Ou and Kwok (2004) |
| Corn tassel | 0.74–1.57 mg/g (GAE) | Mohsen and Ammar (2009) | |
| Corn cob | 12–42 mg/g (GAE) | Sultana et al. (2007) | |
| Wheat bran | Ferulic, syringic, p-hydroxybenzoic, vanillic, coumaric, caffeic, salicylic, trans-cinnamic acids | 4 mg/g (GAE) | Kim et al. (2006), Ou and Kwok (2004), Sultana et al. (2008) |
| Rice bran | 4 mg/g (GAE) | Sultana et al. (2008) | |
| Rice hull | Phytic acid, vannillic acid, syringic acid, ferulic acid, iosvitexin | 2 mg/g (GAE) | Sultana et al. (2008) |
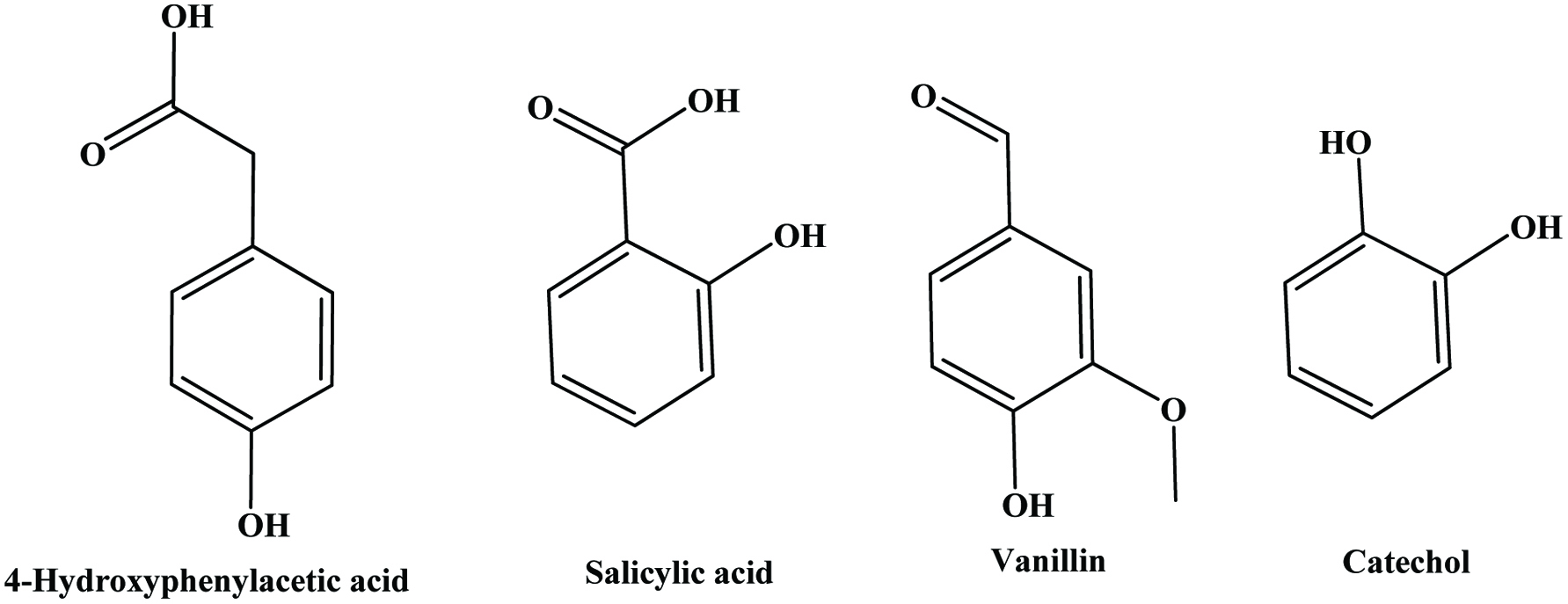
| Oat hull | Ferulic, p-coumaric, p-hydroxybenzoic, vanillic, o-coumaric, sinapic, 4-hydroxyphenylacetic, salicylic acids, vanillin, catechol | 0.56 mg/g | Xing and White (1997) |
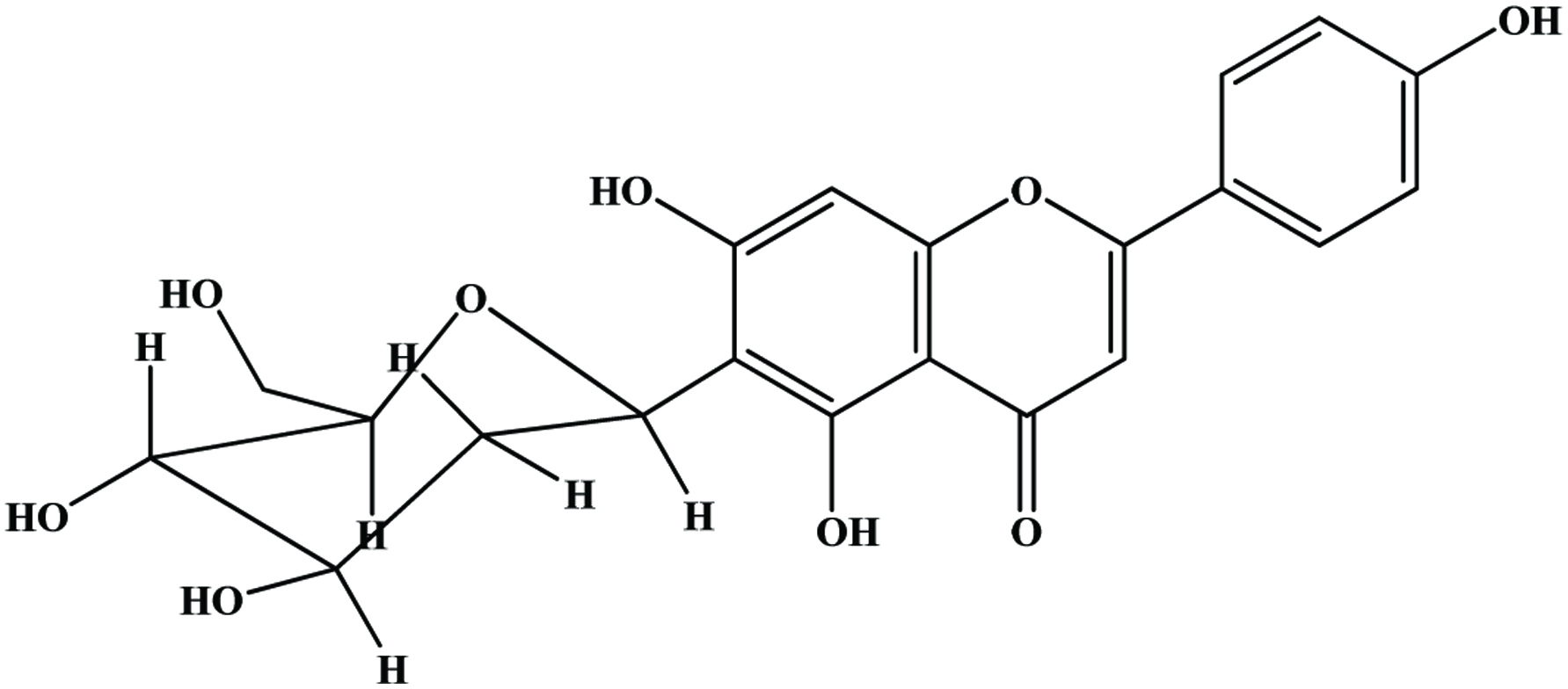
| Buckwheat hull | Quercetin, rutin, hyperin, vitexin, isovitexin, protocatechuic acid, 3,4-dihydroxybenzaldehyde, proanthocyanidins | 39 mg/g | Watanabe et al. (1997) |
| Canola Hull | Phenolic acids, condensed tannin | 94.3–296 mg/g (SE) | Amarowicz et al. (2000a), Naczk et al. (2005) |
| Olive leaves | Oleuropein, hydroxytyrosol, luteolin-7-glucoside, apigenin-7-glucoside, verbascoside | Benavente-Garcia et al. (2000) | |
| Olive mill waste water | Hydroxytyrosol, tyrosol, oleuropein, dimethyloleuropein, verbascoside, catechol, hydroxycinnamic acids, 4-hydroxybenzoic acid | Bianco et al. (2003), Casa et al. (2003), Feki et al. (2006), Lesage-Meesen et al. (2001), Ramos-Cormenzana et al. (1996) | |
| Sunflower seed shell | Chlorogenicacid, o-cinnamic acid, protocatechuic acid, caffeic acid, ferulic acid, syringic acid | 0.4–0.86 mg/g | Leonardis et al. (2005), Weisz et al. (2009), |
| Sesame seed coat | Sesamin, sesamolin | 146.6–29.7 mg/g (CE) | Chang et al. (2002), Shahidi et al. (2006) |
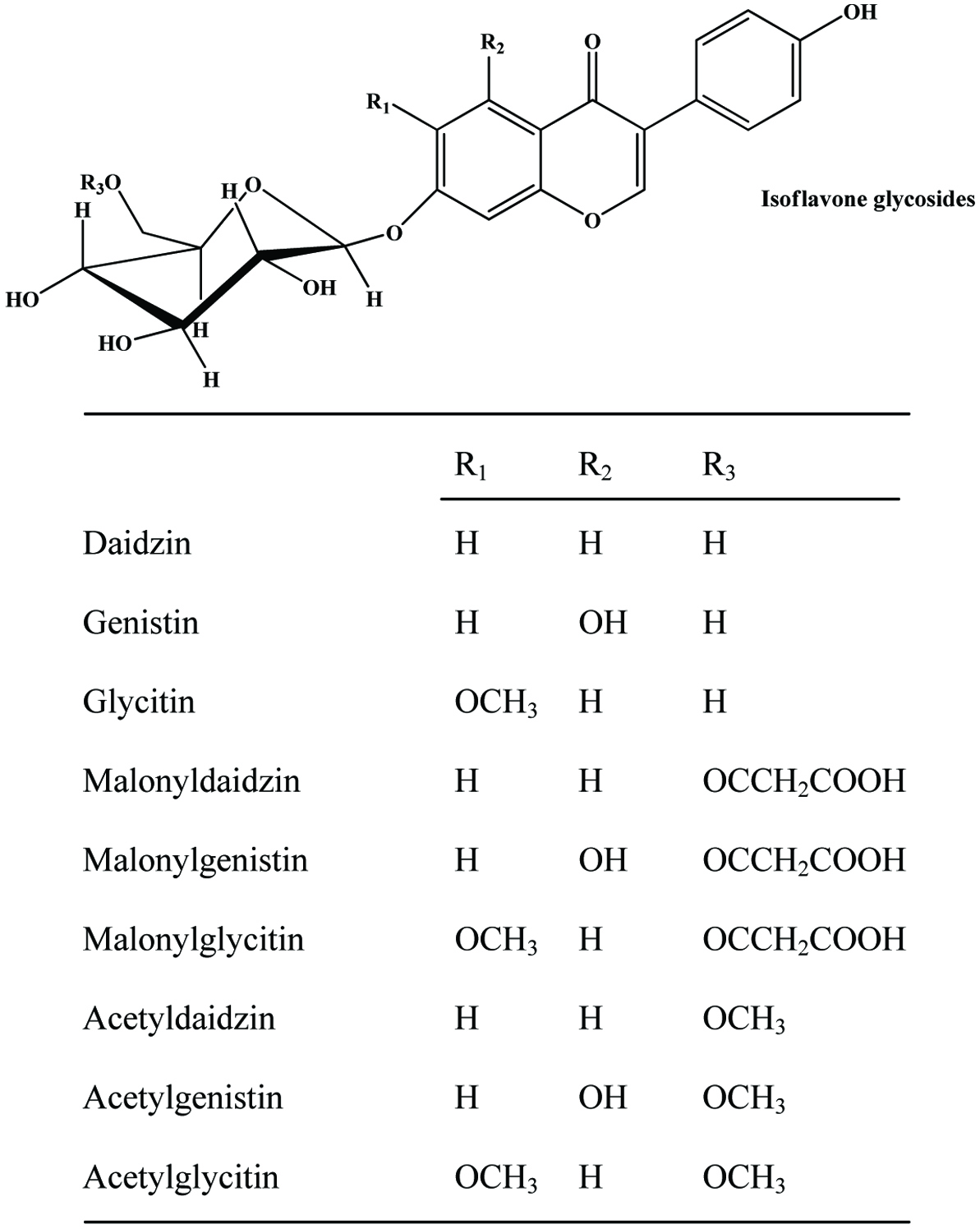
| Soybean cake | Daidzein, genistein and glycitein and their glucosides, acetylglucoside, malonylglucoside | Kao et al. (2008) | |
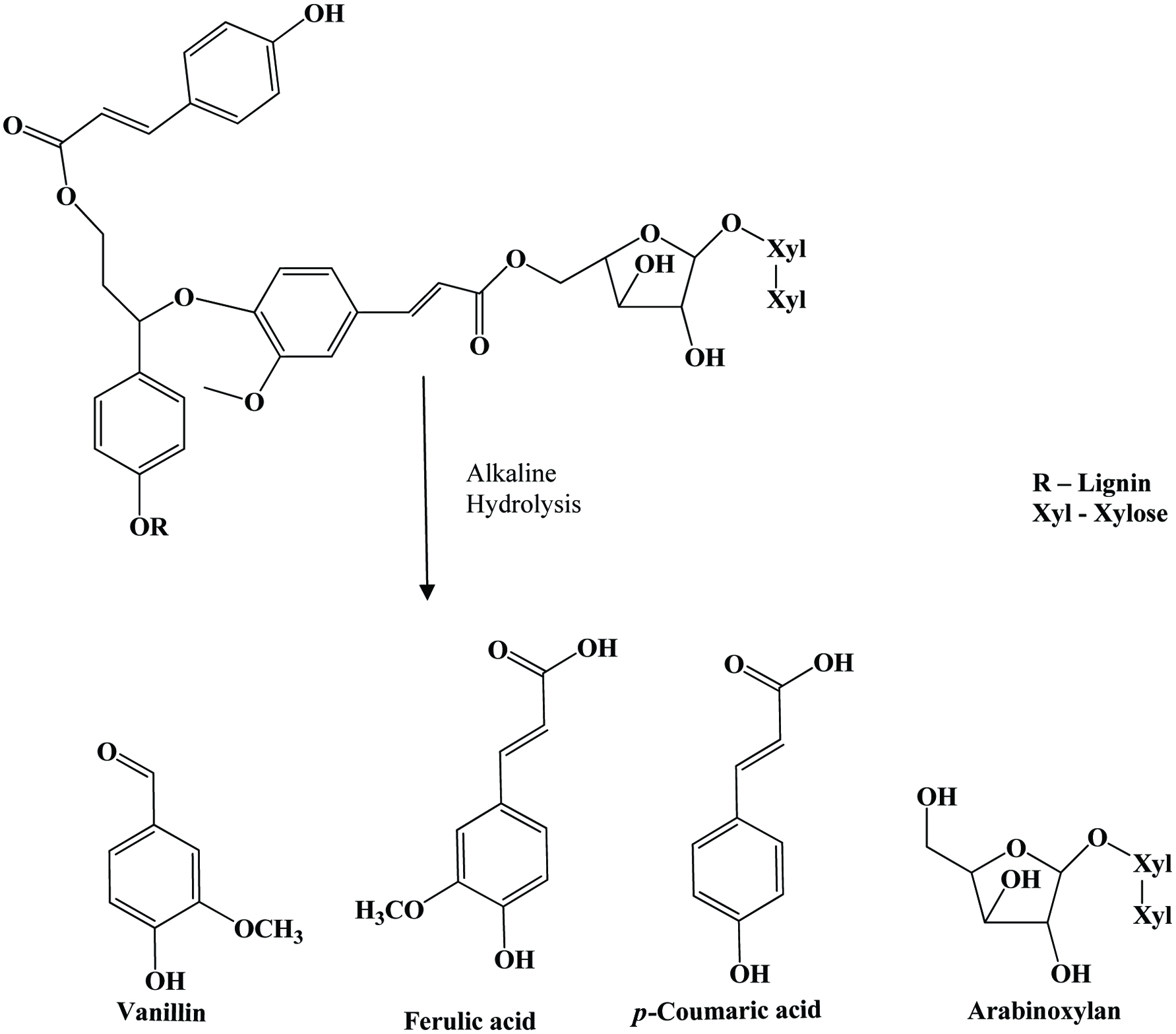
| Flax shive | p-Coumaric acid, ferulic acid, p-hydroxybenzaldehyde, vanillic acid, syringic acid, vanillin, acetovanillone | Buranov and Mazza (2009) | |
| Cocoa leaves | Epicatechin, epigallocatechin gallate, epigallocatechin, gallic acid, epicatechin gallate | 284 mg/g | Osman et al. (2004) |