| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 28, December 2024, pages 41-49
Microwave-assisted extraction of bioactives in fruits and vegetables: a comprehensive review
Kothakota Aravind Kumar, Saji Gomez*
Department of Postharvest Management, Kerala Agricultural University, College of Agriculture, Thrissur, Kerala, India
*Corresponding author: Saji Gomez, Department of Postharvest Management, Kerala Agricultural University, College of Agriculture, Thrissur, Kerala, India. E-mail: saji.gomez@kau.in
DOI: 10.26599/JFB.2024.95028394
Received: November 28, 2024
Revised received & accepted: December 18, 2024
| Abstract | ▴Top |
Fruits and vegetables are integral parts of the daily diet and consist of inedible peel, pomace, and kernel, which are rich in bioactive compounds (BACs). These BACs can be extracted by microwave-assisted extraction using microwave energy. Microwaves are non-ionising electromagnetic radiations with a frequency range of 300 MHz to 300 GHz. Its instrumentation includes a magnetron, waveguide, applicator, and circulator. Microwave extractors are of two types: open and closed. The former is used to extract thermoinsensitive compounds, and the latter to extract thermolabile compounds. Microwave extractors work with dual mechanisms called dipolar rotation and ionic conduction. They help to rupture the cell wall and release BACs into the solvent. The factors viz., solvent type and concentration, microwave power, extraction time, solvent-to-sample ratio, extraction temperature, and sample properties affect the extraction efficiency. Microwave-assisted extraction provides benefits such as higher yields, low extraction time, low solvent consumption, and compatibility with other methods.
Keywords: Fruit and vegetable waste; Bioactive compounds; Microwave-assisted extraction; Ionic conduction and dipolar rotation
| 1. Introduction | ▴Top |
Fruits and vegetables are enriched with nutritious compounds such as vitamins, minerals, fibres, phenolics and carotenoids, making them an integral part of the human diet. For consumption, some portions like peel, rind, core, seed, pomace, and kernel are removed, considered inedible, and waste. However, they are rich in bioactive compounds (BACs) like pectin, dietary fibres, tannins, phenols, carotenoids, and anthocyanins (Table 1). They are potential sources of antioxidant, antimicrobial, and anticancerous properties (Daduang et al., 2011).
 Click to view | Table 1. Bioactive compounds in food waste |
Banana peel, which is 30% of the total fruit weight, is rich in dietary fibres and phenolic compounds (Gonzalez-Montelongo et al., 2010). Mangosteen fruit rind, which accounts for two-thirds of its weight, is rich in anthocyanins (Netravati et al., 2024). Pineapple waste comprises of 70% of total fruit weight, which includes peel, core, trimmings, and crown, which are rich in bromelain, proteins, and peptides (Mala et al., 2021). Apple pomace 30% of raw material) is rich in polyphenols, triterpenes, fibres, and vitamins (Cristina-Gabriela et al., 2012). Watermelon rind accounts for one-third of total fruit mass and can be used as raw material for pectin preparation (Petkowicz et al., 2017). Avocado seeds constitute 13–17% of the fresh fruit and contain tannins, phenolic acids, and flavonoids (Araujo et al., 2020). These BACs from food waste can be extracted and incorporated into diets to overcome undernourishment, affecting around 735 million people globally (von Grebmer et al., 2023).
On the other hand, food waste disposal in the environment causes adverse effects to it. The incineration of food waste releases acid gases and furans, and landfills release methane, which is a significant greenhouse gas (Khan, 2021). In the above context, there is a dire need and sound scope for valorising food waste. Extraction is one sustainable way for the valorisation of food waste.
The extraction of BACs can be done by using different conventional methods. However, they have drawbacks like more time consumption (Carbone et al., 2020), higher solvent requirement, low efficiency, and hydrolytic degradation of some compounds (Sarfarazi et al., 2020). In this regard, we need an economical and eco-friendly technology that can give higher yields in a short time without degradation of BACs. One such technology is microwave-assisted extraction (MAE).
| 2. Microwaves | ▴Top |
These are non-ionising radiations that contain two oscillating perpendicular fields, viz., electric field and magnetic field. They lie between the frequency range of 300 MHz to 300 GHz (Airouyuwa et al., 2023), with a wavelength range of 1 mm to 1 m (Kaatze, 1995; Letellier and Budzinski, 1999; Pinto et al., 2021). The energy of a microwave photon is 0.037 kcal/mol (Gaba and Dhingra, 2011), which is very low compared to the energy required to break a molecular bond, and speed is way faster than the time required by a molecule to relax.
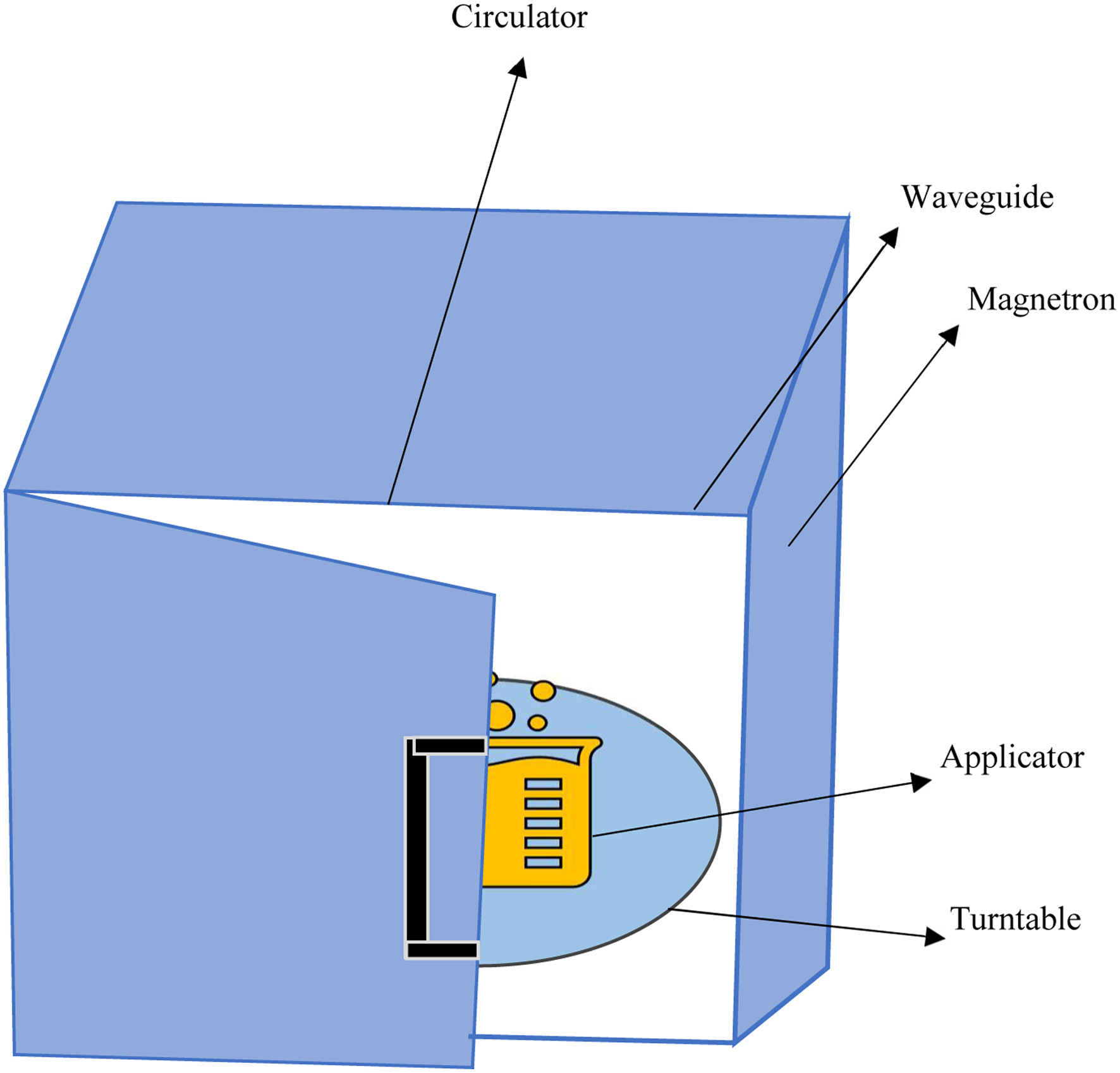 Click for large image | Figure 1. Microwave instrumentation. |
| 3. Applications of microwaves | ▴Top |
Microwaves can be used for diverse purposes like sterilisation (Potato, onion, carrot, and red pepper were sterilised at 100 °C for three min. to control Bacillus amyloliquefaciens, Cho and Chung, 2020), pasteurisation (Apple juice of Cv. Golden delicious was pasteurised at 80–90 °C (600–720 W) for 25 s, Mendes-Oliveira et al., 2020), drying (spinach leaves were dried at 750 W for 290 to 430 s, Ozkan et al., 2007), thawing (strawberry and mulberry frozen fruits thawed at 184 W, four °C preserved the antioxidant capacity, Le et al., 2018 ), blanching (Broccoli Cv. Empress blanched at 700 W for four min. retained its properties in long term storage, Brewer et al., 1995), and extraction (Optimum quality phenolic compounds were extracted from grape pomace at 1,000 W, 10 min (Da Rocha and Norena, 2020). This review emphasises the extraction of BACs using microwaves.
| 4. Microwave-assisted extraction | ▴Top |
The polar solvents that are in contact with the sample are heated by using microwave energy to extract the BACs present in the sample (Sharma and Dash, 2021).
Microwave energy is delivered through polar solvents to generate heat by converting electromagnetic radiation into thermal energy. Dielectric constants and dissipation factors are crucial for transforming electromagnetic radiation into thermal energy (Pimentel-Moral et al., 2018). Microwave-assisted extraction includes three sequential phases: desorption, internal diffusion, and external diffusion. In the first phase, the BACs present in the sample matrix are separated from the active sites of the sample. The second phase involves the diffusion of solvent into the sample matrix, and the last phase consists of the release of solutes from the sample matrix into the solvent (Thaiphanit et al., 2020).
4.1. Microwave extractors
Microwave extractors majorly include four major components: a magnetron (microwave generator), waveguide (propagates the microwaves into the microwave cavity), applicator (extraction vessel), and circulator (allows microwaves to pass forward movement only) (Figure 1) (De Castro and Priego-Capote, 2011). These are of two types, viz., closed type and open type extractors (De Castro and Castillo-Peinado, 2016; Li et al., 2004)
Closed vessel extractors (Figure 2) are usually multimode type, and the microwave treatment is done at high pressure (Pressurised extraction) with a random dispersion of microwaves inside the cavity. The turntable helps bring an even distribution of microwaves inside the cavity regardless of the position of the sample. Due to the elevated pressure levels inside the vessel, higher temperatures can be easily achieved. There is no significant loss of volatiles in this. Extraction can be done simultaneously for multiple samples. The disadvantage of this method is that it cannot be used to extract thermolabile compounds (Delazar et al., 2012).
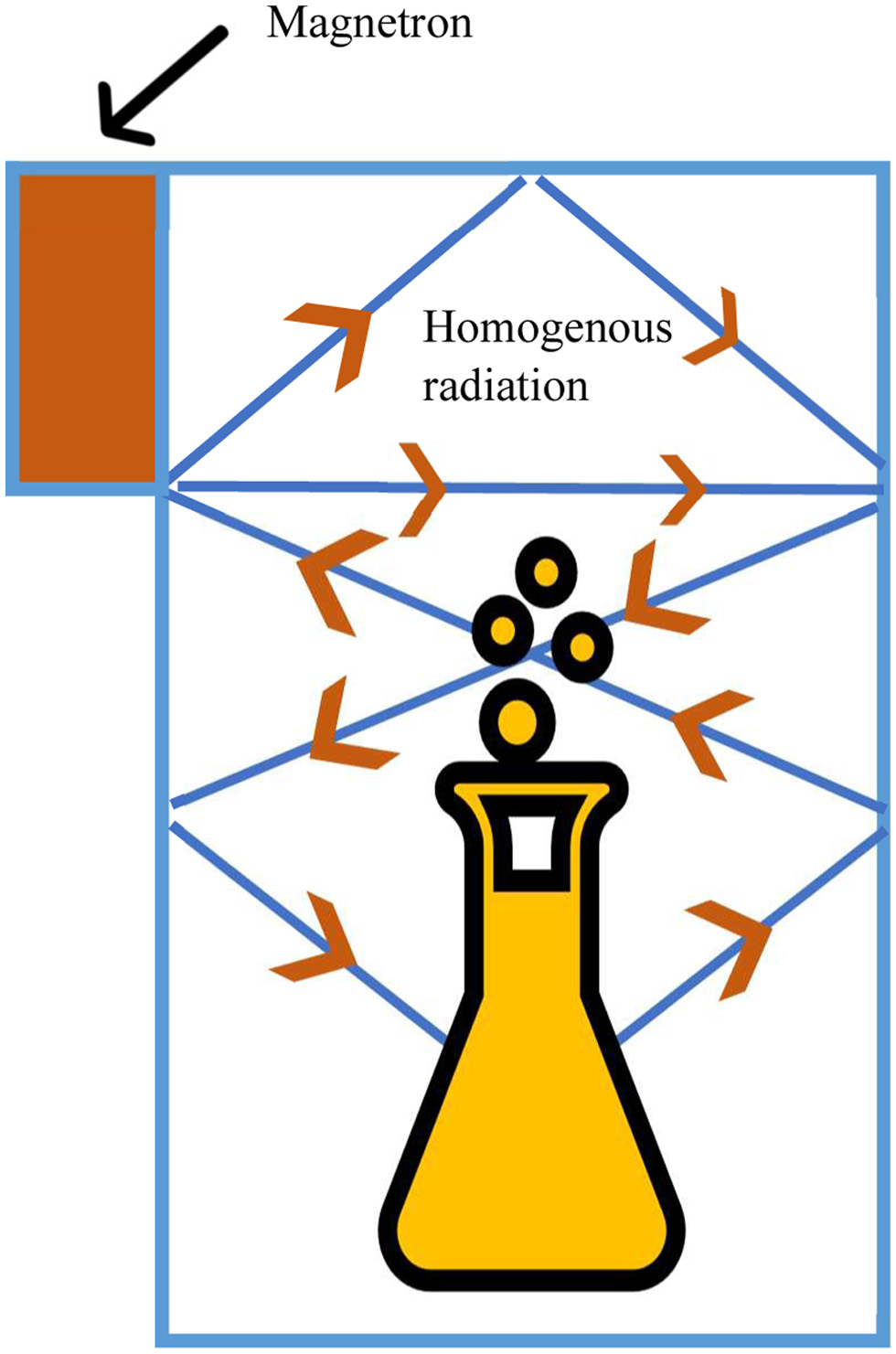 Click for large image | Figure 2. Closed type extraction. |
In open vessel extractors (Figure 3), the microwave treatment is done at atmospheric pressure and given only to a specified region (Focused extraction) where the sample is immersed in the solvent that absorbs microwaves (Li et al., 2004). The upper region of the flask remains calm as the glass is transparent to microwaves. Further cooling is brought by using a water condenser. This is safer to use for the extraction of thermolabile compounds as it is operated at atmospheric pressure and low temperature. The disadvantage of this method is that multiple samples can not be operated simultaneously (Delazar et al., 2012).
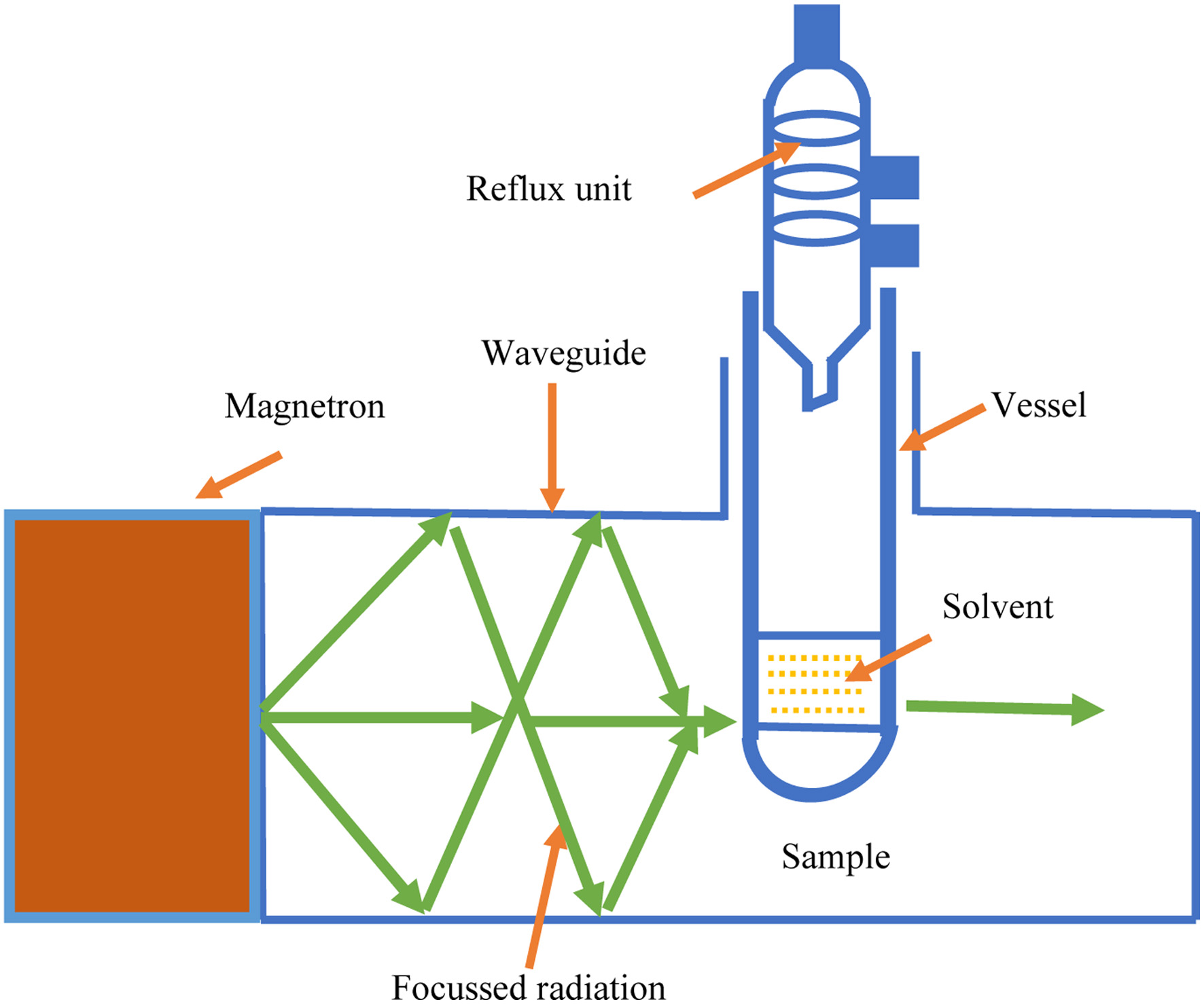 Click for large image | Figure 3. Open type extraction. |
4.2. Principle and mechanism of MAE
During MAE, microwaves pass through solvent and plant particles. The latter contains vacuoles with a certain moisture content (Chan et al., 2016). Moisture heating occurs due to dual mechanisms called ionic conduction and dipolar rotation (Gomez et al., 2020). Ionic conduction (Figure 4) refers to the electrophoretic migration of ions in accordance with the changing electric field, which generates friction between ions and the medium, resulting in the liberation of heat. Dipole rotation (Figure 5) arises when the permanent dipole tries to align its phase in line with the changing electromagnetic field (Veggi et al., 2012). The continuous randomised forced movement results in heating (Mendes et al., 2016). These mechanisms result in the vaporisation of moisture and a tremendous increase in internal pressure inside the cell matrix, which leads to rupture of the cell wall and allows active leach out of phytoconstituents into the solvent (Figure 6) (Dhobi et al., 2009).
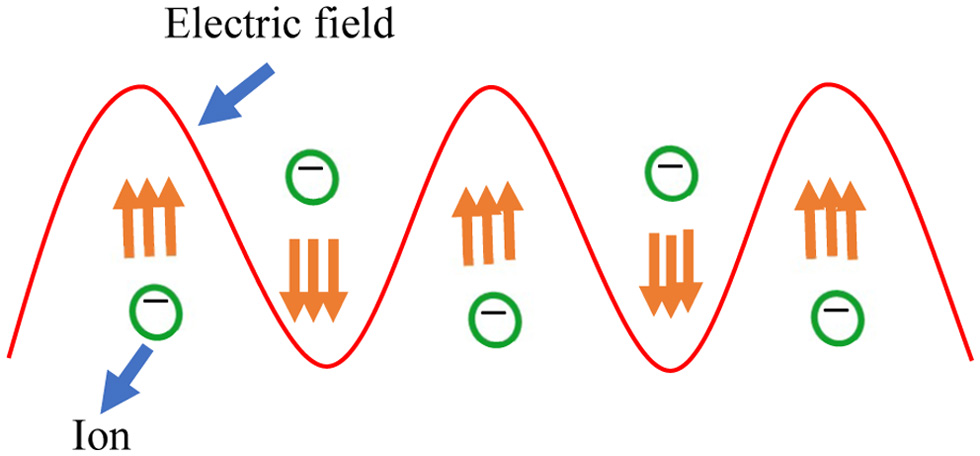 Click for large image | Figure 4. Ionic conduction. |
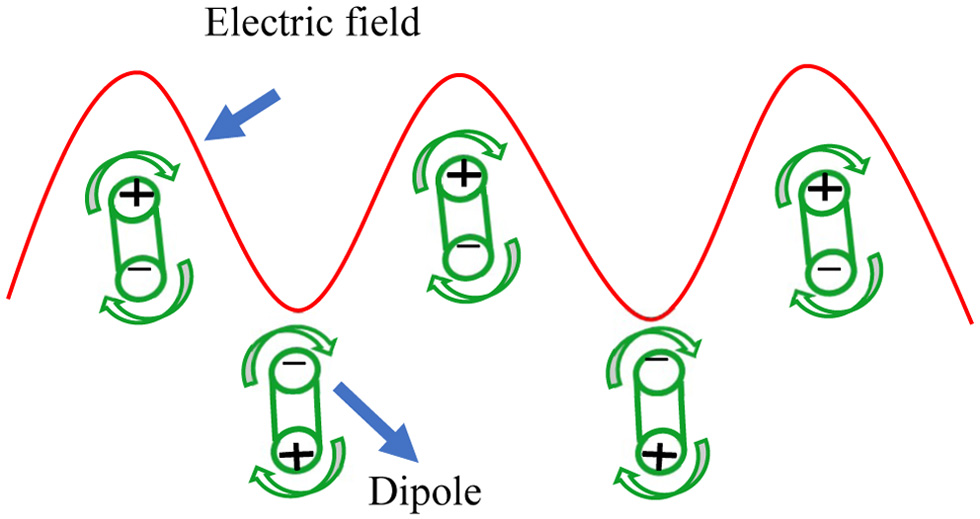 Click for large image | Figure 5. Dipolar rotation. |
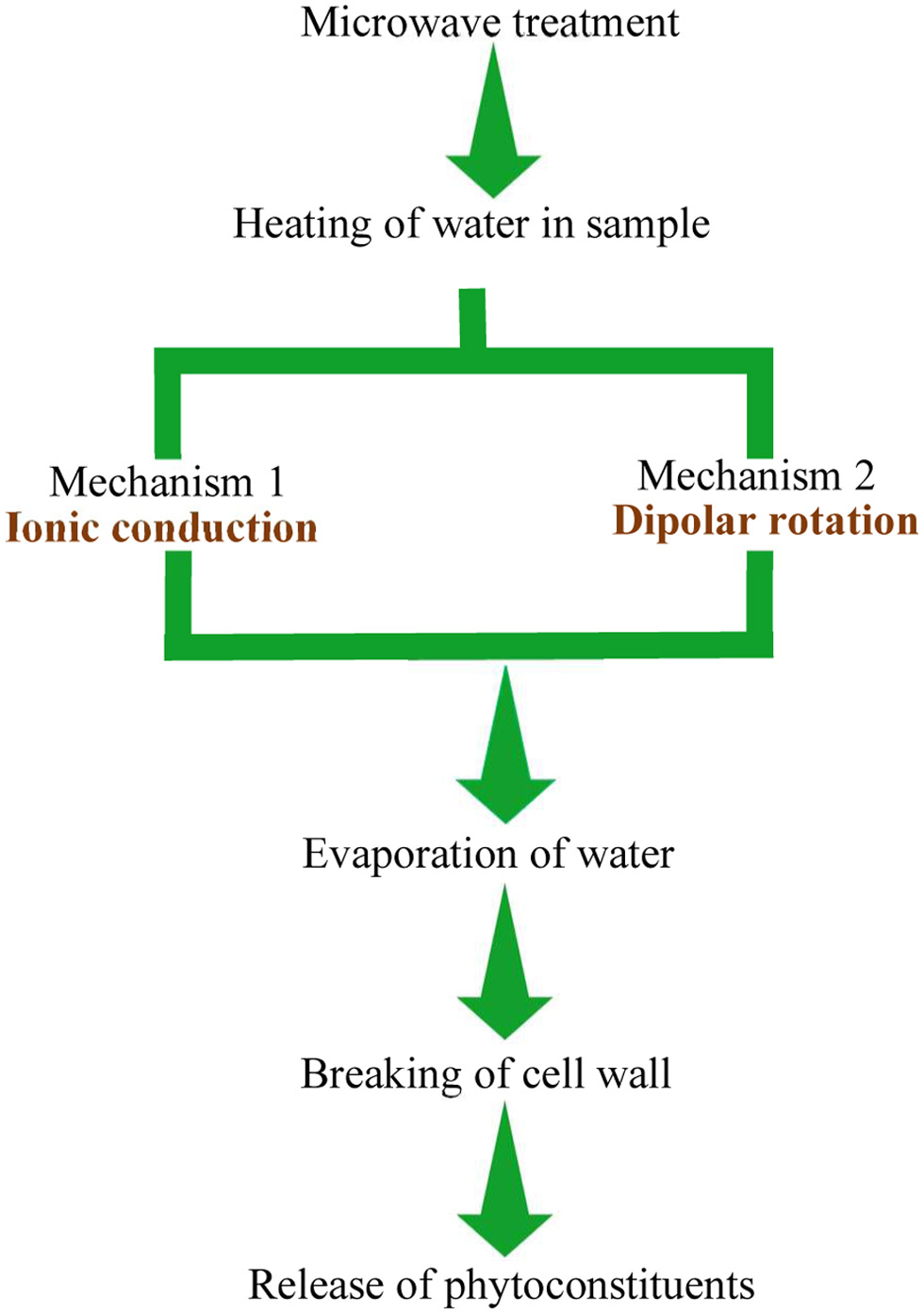 Click for large image | Figure 6. Flow chart of microwave assisted extraction. |
| 5. Factors affecting MAE | ▴Top |
Solvent type and concentration, microwave power, extraction time, solvent-to-feed ratio, extraction temperature, and sample properties affect the efficiency of MAE (Figure 7) (Xie et al., 2014; Bachtler and Bart, 2021; Daliri Sosefi et al., 2024; Elakremi et al., 2022).
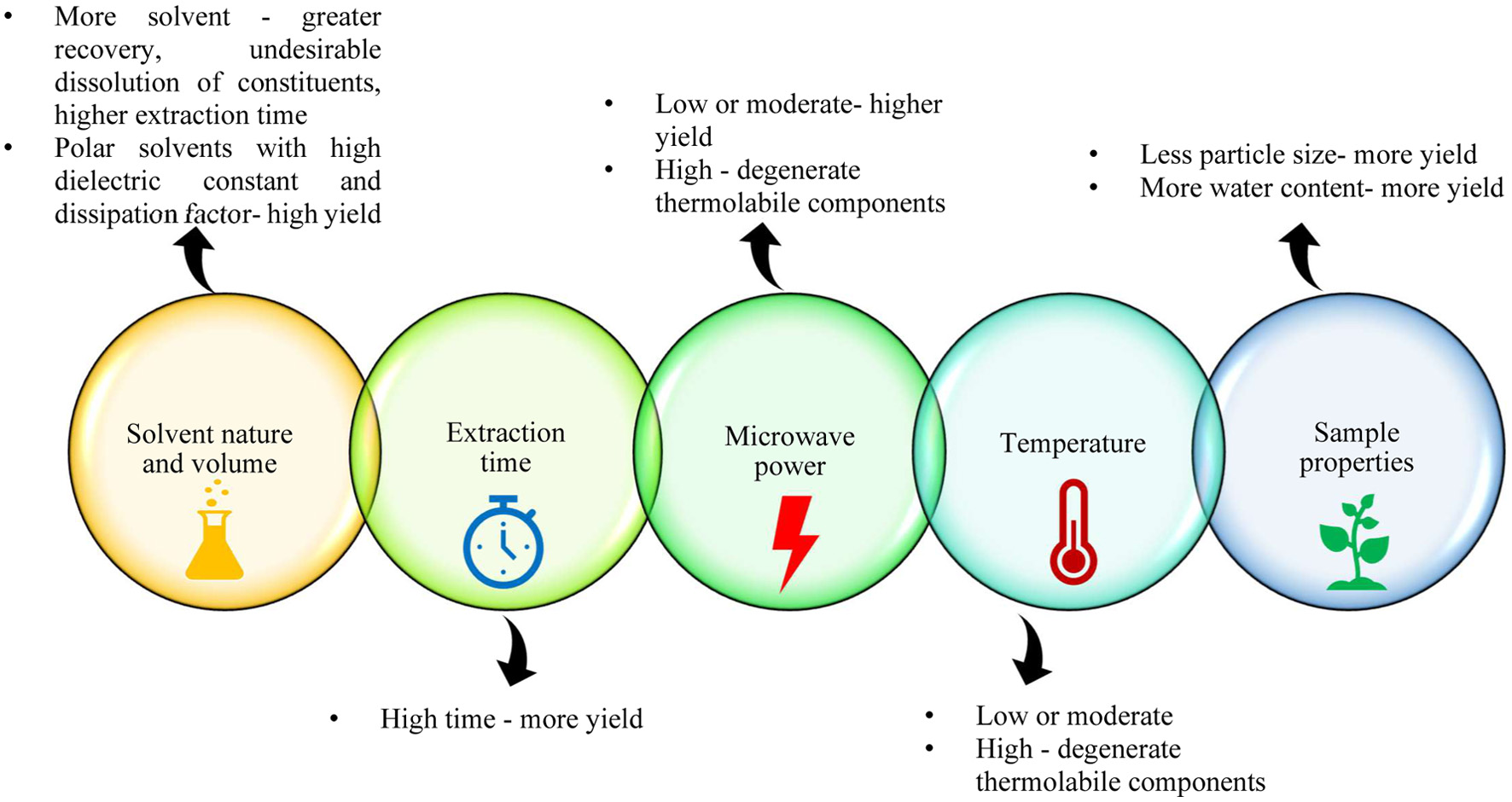 Click for large image | Figure 7. Factors affecting MAE. |
5.1. Solvent nature and volume
The Dielectric constant, solvent penetration and its interaction with the sample matrix, molecular size, and solubility of the compound of interest are considered for improving extraction efficiency. A high dielectric constant of the solvent will enhance the extraction process more rapidly (Ihsanpuro et al., 2022). The solvent volume must be sufficient to immerse the sample throughout the extraction process (Veggi et al., 2012). Solvents with lower molecular size and higher polarity will enhance extraction yields by improving heating and making it easy to penetrate into the solvent. Methanol gives a higher extraction yield, having a lower molecular size and higher polarity, but ethanol is the widely used solvent because of safety concerns (Chan et al., 2017). Karami et al. (2015) investigated the effect of solvents (ethanol 80%, methanol 80% or water) on the yield of phenolic compounds from liquorice roots. From the study, they inferred that the higher extract was obtained by ethanol; it also improved the extract’s total phenolic content and antioxidant activity.
5.2. Microwave power
Microwave power influences the quantity of extracted compounds and extraction time. Elevated power levels provide a higher yield. It causes localised heating in the sample, which helps destroy the plant matrix so that the BACs can be diffused into the solvent. The increase in power level will increase the extraction yield in a shorter time. Conversely, a high-power level decreases the extraction yield by degrading the thermolabile compounds (Veggi et al., 2012). Luo et al. (2021) conducted a study on antioxidant activity and total phenolic content of Akebia trifoliate peel extracts at different power levels from 300–800 W. From the study, they inferred that the total phenolic content and antioxidant showed a positive trend from 300–500 W, above this power levels they are decreased significantly. Doulabi et al. (2020) conducted a study to evaluate MAE’s effect on eggplant peel by-products’ bioactive compounds. They reported that extraction yield was increased with an increase in microwave power from 100–300 W. Bioactive active alkaloids were extracted from lotus plumules using microwave-assisted extraction (Xiong et al., 2016). They reported that an increase in the liquid-to-solid ratio from 5:1 to 20:1 increased the extraction yield, and thereafter, there was no rise in yield.
5.3. Extraction time
Extraction time usually correlates with microwave power and shows an inverse relationship. At each power level, an optimum extraction time will give a better extraction yield (Chan et al., 2017). Dielectric properties of the solvent also influence it. The solvents with higher dielectric constant heat up highly under overexposure, thus risking the yield of thermolabile (Veggi et al., 2012). A study was conducted to determine the effect of extraction time on antioxidant activity and total phenolic content of Akebia trifoliate peel extract and inferred that an increase in time enhances the yield until optimum conditions; later on, it decreased (Luo et al., 2021). A study by Alara et al. (2021) reported that the phenolic content increased with an increase in time from two to four minutes, and after that, there was a rapid decline in the phenolic content in C. papaya leaf.
5.4. Extraction temperature
Temperature is a crucial factor in extraction. Increasing the extraction temperature will lead to higher diffusion, improving the release of BACs into the solvent. It also decreases the solvent’s viscosity, resulting in easy penetration into the cells and enhancing solute desorption into the solvent. Higher temperature leads to the loss of thermolabile compounds (Vladić et al., 2020), increased extract impurities, and poor stability of the final extracted compound (Bachtler and Bart, 2021). Zheng et al. (2011) extracted polysaccharides from pumpkins and studied the effect of extraction parameters on extraction yield. From the study, they reported that a temperature of 70 °C is suitable for breaking analytic matrix bonds and gives a higher yield of polysaccharides. At a temperature of more than 70 °C, the yield of polysaccharides declined and gave scorched extract.
5.5. Sample properties
Extraction of BACs from an intact plant part is complex and inefficient. The sample’s particle size characterises the amount of disruption and influences the extraction yield. Smaller particle sizes give higher extraction yields, as the diffusivity of the BACs increases with smaller particles due to the larger contact surface area (Chan et al., 2017). Particles of large and tiny sizes will reduce the extraction yield because of smaller surface area and easy agglomeration, respectively (Xiong et al., 2016). Smaller particle size gave a higher yield of seed oil (32%) in pomegranate compared to the larger particles (11%) under the same extraction conditions, i.e., 238 W, 6:1 solvent to sample ratio, and 5 minutes extraction time (Keskin Cavdar et al., 2017). some BACs extracted through MAE are listed in Table 2.
 Click to view | Table 2. Bioactive compounds extracted through MAE |
| 6. Benefits of MAE | ▴Top |
Microwave-assisted extraction has the advantages such as low extraction time (Garrido et al., 2019; Bener et al., 2022) and solvent consumption (Chumnanpaisont et al., 2014; González-de-Peredo et al., 2022), higher extraction yields (Thaiphanit et al., 2020), low cost (Dahmoune et al., 2015; Mellinas et al., 2020), better potential for automation (Weremfo et al., 2020), low energy consumption (Sarfarazi et al., 2020), high quality extracts (Olalere et al., 2021; Vélez-Erazo et al., 2021), and compatibility with other methods.
Despite these benefits, it is adopted only in laboratories due to the difficulty in scale-up and optimisation of the process parameters for extraction of BACs from different samples (Chan et al., 2016).
| 7. Conclusion | ▴Top |
Microwave-assisted extraction is a sustainable technology for extracting bioactive compounds from fruit and vegetable waste. The extraction process involves the open type and closed type extractors. Ionic conduction and dipolar rotation are the two critical mechanisms involved in the extraction process. The extraction parameters are specific to each BAC based on the matrix properties. This technology gives more extraction yields quickly, with less solvent and energy consumption. Microwave-assisted extraction plays a crucial role in the extraction of thermolabile compounds due to the lower exposure times to heat. Further research has to be done to determine the combined abilities of this technology with other methods to achieve synergistic effects from both technologies. Optimisation of extraction parameters, i.e., microwave power, extraction time, temperature, and solvent-to-feed ratio is required to avail the maximum BACs from the sample.
Acknowledgments
Not applicable.
Conflict of interest
The authors declare that there is no conflict of interest among them.
| References | ▴Top |