| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 28, December 2024, pages 31-40
Analysis methods and food application of Gastrodia elata and related products: A review
Chang Liu*, Jianfeng Zhan, Weixin Wang, Ting Hu
Hubei Key Laboratory of Economic Forest Germplasm Improvement and Resources Comprehensive Utilization, Hubei Collaborative Innovation Center for the Characteristic Resources Exploitation of Dabie Mountains, Huanggang Normal University, Huanggang 438000, China
*Corresponding author: Chang Liu, College of Biological and Agricultural Resources, Huanggang Normal University, Huanggang 438000, China., E-mail: liuchang@hgnu.edu.cn
DOI: 10.26599/JFB.2024.95028393
Received: December 15, 2024
Revised received & accepted: December 28, 2024
| Abstract | ▴Top |
Gastrodia elata Blume (G. elata) widely reputed as an important traditional herbal medicine and food in China and other Asian countries, can manage headache, epilepsy, pediatric acute, and chronic convulsions in modern clinical practice. It has been revealed that G. elata contains a variety of chemical substances, which have attracted more and more attention from the food science community. Hence, a comprehensive search of published literature from the beginning to 2024 in Web of Science and CNKI, and the keyword of G. elata was used. This review systematically summarizes analytical methods of bioactive phytochemicals in G. elata. We also depicted known biological harmful constituents in this review. In addition, abundant food products made from G. elata have been developed and sold on market, and different processing methods of G. elata containing foods have significant effects on the bioactive phytochemical composition of G. elata. Hence, we further summarized the impacts of food processing on the development of G. elata related functional food products. In a nutshell, this review summarizes the bioactive phytochemicals, analysis methods, and application of G. elata and its associated food products, and aims to provide a valuable reference for the full utilization of resources of G. elata.
Keywords: Gastrodia elata Blume; Chemical substances; Gastrodin; Analysis method; Food application
| 1. Introduction | ▴Top |
Gastrodia elata Blume (G. elata) is a plant belonging to the orchid family, which has a wide geographical range in China and other Asian countries, especially preferring to grow in mountainous areas. Based on the traditional Chinese medicine theory, G. elata has been used as herbal medicine since ancient times in China because of its warm nature and suppressing excessive live-yang (Yang et al., 2024). In modern clinical practice, G. elata is employed for the treatment of headaches, dizziness, epilepsy, pediatric acute and chronic convulsions, etc. (Su et al., 2023). Currently, studies about the chemical components in G. elata and their pharmacological effects have attracted huge attention, due to their great medical value. Phytochemical research of G. elata has isolated or characterized varieties of compounds, including phenols, polysaccharides, organic acids, and glycosides among others. Some of these molecules have been proved that possess the function of inhibiting oxidative stress (Zhang et al., 2024), anti-depression (Jiang et al., 2024), anti-neuroinflammation (Li et al., 2020; Shao et al., 2018), and antidiabetic effects (Bai et al., 2021). For example, gastrodin, a phenolic glycoside identified in 1978, is one of the main active components in G. elata which has been systematically investigated on its pharmacological effects (Liu et al., 2018). Gastrodin has demonstrated neuroprotective effects and good efficacy in the treatment of Parkinson’s disease (Yan et al., 2019; Zhao et al., 2024), Alzheimer’s disease (Luo et al., 2022), and Tourette syndrome (Wang et al., 2021) in animal models, which has been approved as herbal drugs for the treatment of neurasthenia and migraine in China. For example, gastrodin at the concentration of 10–50 mg/kg could exert a neuroprotective effect on retinal ganglion cells in an acute glaucoma animal model via inhibiting microglia activation and microglial-mediated neuroinflammation (Wang et al., 2017). Except for medicinal applications, the tuber of G. elata can serve as raw food material for cooking or functional food development. In the literature review process, we noticed that barely any researcher has systematically reviewed the analysis method and acquisition route for types of chemical components in G. elata which is the fundamental techniques for pharmacology and food application. In this article, the developments in the analysis methods, acquisition routes, and food applications of important chemical substances in G. elata are summarized to provide guidelines for further research of bioactive in G. elata and its application in food.
| 2. Chemical constituents in Gastrodia elata Blume | ▴Top |
A number of chemical substances have been identified from G. elata since the 1950s, including phenolic compounds, organic acids, polysaccharides, peptides, and others. Among them, some molecules have been proven to exhibit varying pharmacological effects, such as sedative-hypnotic, neuroprotection, vascular protection, and antidiabetic.
Phenolic compounds are abundant in G. elata and exhibit remarkable pharmacological properties. They are compounds replacing hydrogen atoms on the benzene ring with one or more hydroxyl groups, including phenols, phenolic glycosides, phenolic ethers, phenolic aldehydes, nitrogen-containing phenols, and sulfur-containing phenols. Among them, gastrodin belongs to phenolic glycoside, chemically known as 4-(hydroxymethyl) phenyl β-D-glucopyranoside (C13H18O7), and its chemical structure is illustrated in Figure 1. The content of gastrodin of 4-hydroxybenzyl alcohol (HBA) is considered as the chemical marker in the quality standardization of G. elata, which should not be less than 0.25% according to the Chine pharmacopoeia. Parishins are a family of esters formed with different numbers of gastrodin or its derivatives and varying positions of citric acid, which have also been identified as a kind of bioactive ingredients in G. elata. Parishin C has multiple biological properties, such as antipsychotic (Shin et al., 2010), neuroprotective (Wang et al., 2021), and antidepressant effects (Jiang et al., 2024). Parishin J and B show significant protective effects in the treatment of myocardial hypoxia/reoxygenation injury (Wang et al., 2020). Organic acids have also been found in G. elata, including succinic acid, citric acid, palmitic acid, L-phenyllactic acid (Gong et al., 2024), cinnamic acid, and caffeic acid (Wu et al., 2022). In particular, citric acid can be formed by in vivo hydrolyzation of parishins, which have anti-oxidative (Abdel-Salam et al., 2014), neuroprotective and hepatoprotective effects (Abdel-Salam et al., 2016).
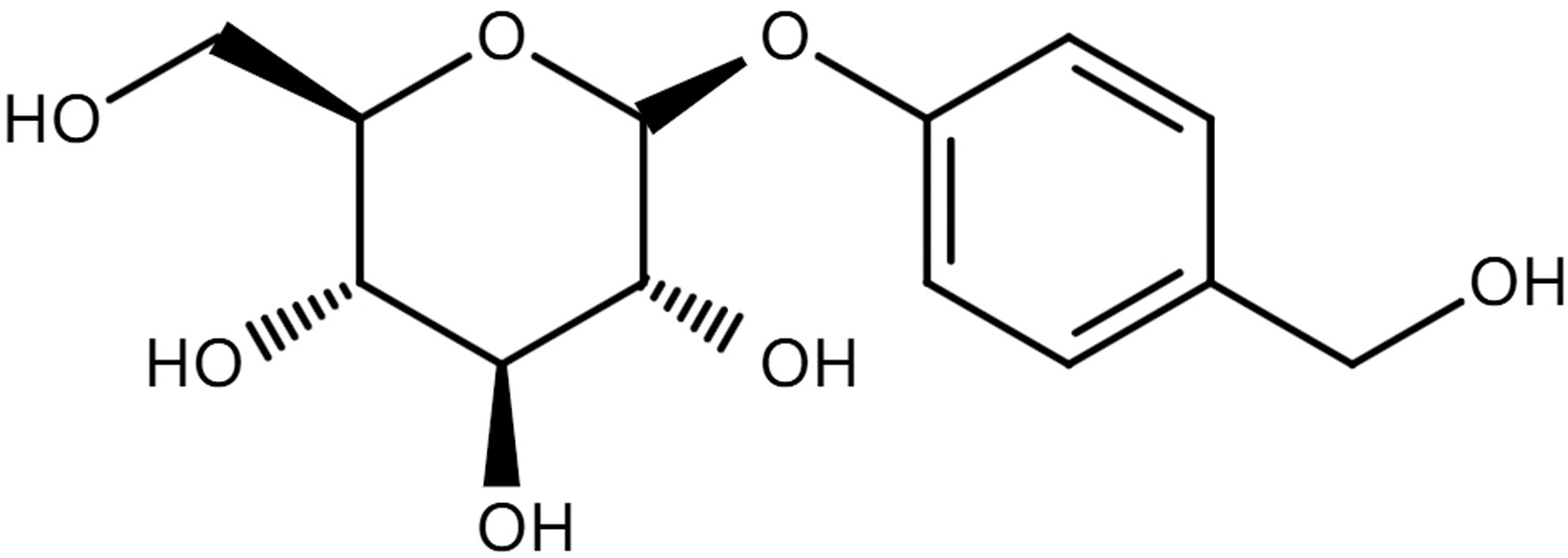 Click for large image | Figure 1. The chemical structure of gastrodin. |
In addition to small molecules, there is a diversity of large molecules in G. elata. Polysaccharides, as one of the most attractive active ingredients, have been reported to have anti-cancer, anti-oxidant, anti-virus, immunological, neuroprotective, and hypotensive effects ( Yang et al., 2024). An anti-fungal protein was found in G. elata, named GAFP-1 (Xu et al., 1998). Moreover, the polypeptide in G. elata extract was confirmed to have antimicrobial activity for gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa, the gram-positive bacterium Staphylococcus aureus and the fungus Candida albicans (Kong et al., 2019).
| 3. Analysis method | ▴Top |
3.1. Gastrodin and other phenolic compounds
Gastrodin is one of the most significant phenolic constituents in G. elata since it has been regarded as the main active ingredient in herbal medicine and functional food. Thus, many methods have been developed for the analysis of gastrodin in G. elata. Moreover, various detection approaches in biological samples were proposed to support the pharmacokinetic studies on G. elata. Besides its medicinal application, G. elata has been consumed as a food for a long history in China and was added to the catalogue of “substances traditionally considered as both food and herbal medicine” in 2023, allowing it to be processed and sold as a food in the legal sense. Hence, there are few methods applied for phenolic compounds detection in food made from G. elata. Different methods used for quantitative analysis of phenolic compounds in varied types of samples are shown in Table 1.
 Click to view | Table 1. Summary of quantitative methods for gastrodin and other phenolic compounds |
3.1.1. Plant material
Previous studies have found that G. elata, most notably the tuber part, contains a variety of phenolic compounds, including gastrodin, 4-hydroxybenzyl alcohol, 4-hydroxybenzaldehyde, and parishins. According to the Chinese Pharmacopoeia, the minimal content of gastrodin and 4-hydroxybenzyl alcohol is 0.25% for the quality control of G. elata. As to parishins, their contents in G. elata are relatively low and in the range of 0.03–0.13% (Shan et al., 2021). The phenolic compounds possess conjugated structure. Thus, as shown in Table 1, high-performance liquid chromatography with ultraviolet detection (HPLC-UV) was the most commonly applied assay for the quantitative determination of phenolic compounds in G. elata. The detection wavelength is usually set at either 220 or 270 nm. A reversed-phase C18 column is used with water (phase A) and methanol or acetonitrile (phase B) as mobile phases. To improve the resolution, formic acid, phosphoric acid, and acetic acid can be added to adjust the pH of mobile phase. Besides HPLC-UV, high-performance liquid chromatography with fluorescence detection (HPLC-FLD) was also used for the determination of phenolic compounds in G. elata. The excitation and emission wavelength are set to 275 nm and 295 nm, respectively. Compared with the HPLC-UV, HPLC-FLD is of great sensitivity. Noteworthy, few methods applied near-infrared (NIR) spectroscopy for the determination of the phenolic compounds in G. elata. NIR relies on the absorption of electromagnetic radiation with a wavelength range spanning from 800–2,500 nm (Pasquini, 2018). The main advantage of NIR is non-destructive analysis of samples without sample preparation. However, the intrinsic complexity of NIR spectra spells trouble for its application, which made it significant to develop a robust and reliable calibration model to analyze the spectra data.
The sample preparation of plant material is quietly simple and involves mainly liquid extraction with polar solvents. Prior to the extraction, the initial step in the sample preparation is drying the plant and grinding it into powder, which could improve the kinetics of analytic extraction and the contact of the sample surface with the solvent (Sasidharan et al., 2011). Due to the high polarity of phenolic compounds, the plant samples were extracted with methanol, ethanol, or a mixture of alcohol solvents and water. During the solvent extraction process, varied methods were employed to obtain satisfied recovery rates of compounds, including microwave-assisted extraction, pressurized extraction, sonication-assisted extraction, and reflux extraction. Aside from solvent extraction, solid-phase extraction with molecularly imprinted polymers can be performed as an approach of purification process.
3.1.2. Biological sample
The quantitative analyses of analytes in biological samples are the crucial part of pharmacokinetic studies, including plasma, urine, and tissues. Different from plant sample analysis, the biological sample analysis is of a grand challenge because of low abundant analytes and complex matrix. Thus, high-performance liquid chromatography-mass spectrometry (HPLC-MS) or high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) are widely used in the quantitative analysis of phenolic compounds in plasma due to their excellent sensitivity. The chromatographic separation is performed on a C18 column with water (phase A) and acetonitrile or methanol (phase B). Similarly, formic acid can be used to adjust the pH of mobile phase. As for mass detection, multiple reaction monitoring (MRM) or full-scan analysis is carried out for the quantitation of analytes. In general, the HPLC-MS/MS is of better sensitivity than HPLC-MS and HPLC-UV. Taking gastrodin as an example, lower LOQs were attained for the plasma sample (1.32–50 μg/L) as illustrated in Table 1.
In terms of biological sample treatment, one of the similarities is protein precipitation for both plasma and urine samples. Two choices of solvents were applied for protein precipitation during the analysis of gastrodin and other phenolic compounds in biological samples, which were methanol and a mixture of methanol and acetonitrile. After the protein removal, the residue solution can be directly injected into the analytical instrument. In addition, the internal standards were applied in biological samples to help account for variability in sample preparation (Tan et al., 2009), including bergenin, tyrosol, and geniposide, which are similar to the gastrodin or other phenolic compounds.
3.1.3. Food and medicinal products
Food and medicinal products made from G. elata have been developed, including liquor, enzyme-containing beverages, tablets, capsules, and granules. The most commonly detected ingredient in these products is gastrodin. HPLC-UV method is currently the primary analytical tool for detecting gastrodin in food and drug products. As for sample preparation, the liquid sample was concentrated and redissolved in appropriate solvents followed by instrument analysis. The pretreatment procedures of solid product samples are similar to the plant sample, which are solvent extraction with methanol, ethanol, or a mixture of an organic solvent with or without water.
3.2. Polysaccharide
The commonly used methods for the analyses of G. elata polysaccharides encompass total content, molecular weight, monosaccharide composition, surface morphology, chemical structure, and so on. The total content of G. elata polysaccharide is usually determined by colorimetric methods, such as the phenol-sulfuric method and the anthrone-sulfuric acid method (Ji et al., 2022; Zhu et al., 2019). The molecular weight of polysaccharide varies from 7.64×104 to 8.75×106 Da, which can be determined using size-exclusion-chromatography (SEC) and high-performance gel permeation chromatography (HPGPC) ( Yang et al., 2024). The composition of monosaccharides can be analyzed by high-performance anion exchange chromatography (HPAEC) with the anion-exchange column. A polysaccharide from G. elata (named GEP-1) was analyzed via HPAEC coupled with pulsed amperometric detector and was found mainly composed of glucose, galactose, and arabinose(Guan et al., 2022). Moreover, high-performance ion chromatography (HPIC) was also used to detect the monosaccharide composition of G. elata polysaccharide, and the results showed it was mainly composed of glucose, with small amounts of galactose and galacturonic acid(Gan et al., 2024).
Since the bioactivity of polysaccharides is generally correlated with their structure, the surface morphology of G. elata polysaccharide is often examined by scanning electron microscope (SEM) to study its microstructure (Chen et al., 2024; Ji et al., 2022). The ultraviolet (UV) spectroscopy, infrared (IR) spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy can provide the chemical structure information of G. elata polysaccharide, which are robust approaches for G. elata polysaccharide structure analysis (Chen, et al., 2024). In addition, the thermal characteristics of G. elata polysaccharide can be revealed through differential scanning calorimetry (DSC) analysis or thermos gravimetric analysis (TGA) (Guan et al., 2022; Ji et al., 2022). The zeta potential and particle size of G. elata polysaccharide play important roles in its application in food and biomedical areas, which can be determined with phase analysis light scattering (PALS) analyzer (Ji et al., 2022).
3.3. Polypeptide and protein
The polypeptide and protein in G. elata have been proven to have antibacterial activity (Cai et al., 2019). However, compared with gastrodin and parishins, the function of polypeptide and protein in G. elata has not been systematically studied. Hence, it is significant to establish analysis methods of polypeptides and proteins in G. elata to explore the potential correlation of G. elata polypeptides and proteins with their bioactivities. Prior to an analysis, polypeptides are extracted from the product matrix with water solution, and enriched through an enzymolysis approach. Generally, the rhizomes of G. elata were extracted by saline, and papain was added to the extracted solution. Subsequently, the solution was passed through an ultrafiltration membrane to attain the polypeptide solution. The concentration of polypeptide can be detected by the bicinchoninic acid (BCA) method or Lowery’s method (Cai, et al., 2019; Kong, et al., 2019). Similar to polysaccharides, the molar mass distribution of polypeptide can also be determined using HPGPC. For protein analysis, the sample preparation procedure is simpler, and the enzymolysis step is not required. The qualitative analysis can be performed by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry (Chen et al., 2018; Zeng et al., 2018). A typical SDS-PAGE figure of protein in G. elata was demonstrated in Figure 2.
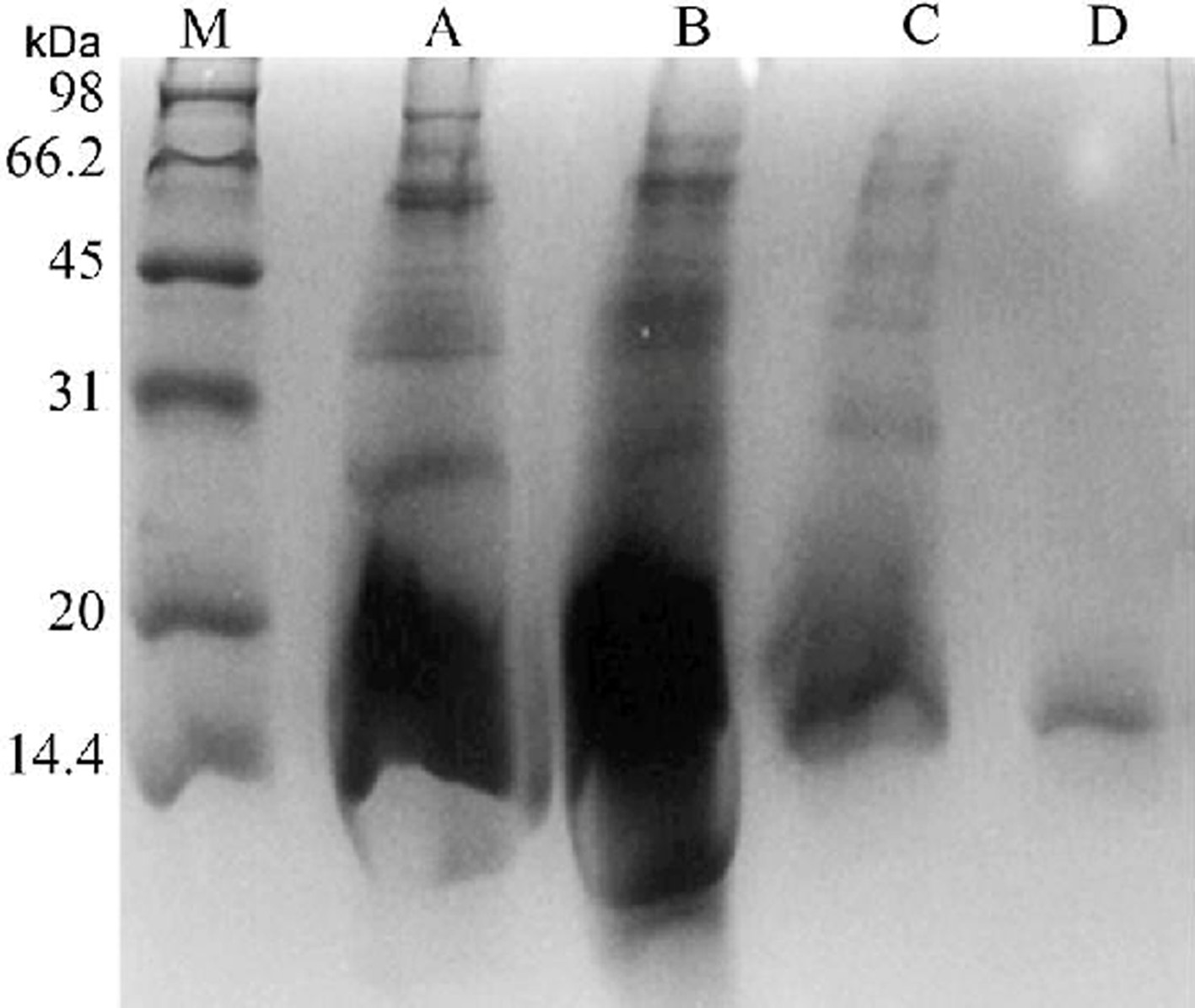 Click for large image | Figure 2. SDS-PAGE analysis of antibacterial protein during successive purification steps. M, protein marker. A, crude extract of G. elata tubers. B, dialyzed proteins (precipitated by ammonium sulfate). C, purified proteins after chromatography on DEAE-52 (ion-exchange). D, purified protein after chromatography on Sephadex G-50 (gel-filtration) (Chen, et al., 2018). |
3.4. Others
Aside from bioactives, the harmful compounds in G. elata should also be paid attention to guarantee its edible safety. Heavy metals are highly and cumulatively toxic, which possess severe adverse effects on human health. They would be absorbed from soil or water by herbal medicines. For example, G. elata has an absorptive tendency for Hg, Cu, and Zn (Tu et al., 2016). The analytical methods of heavy metals have been reported by various instrumental approaches, such as atomic absorption spectrometry (AAS), inductively coupled plasma-mass spectrometry (ICP-MS), and X-ray fluorescence spectrometry. The low concentration of heavy metals in samples makes it difficult to be directly detected, and the sample preparation techniques are required to extract and enrich the target analytes from samples, including solid-phase extraction (Khan et al., 2020), liquid-liquid microextraction (Sharifi et al., 2016), and solid-phase microextraction (Baghaei et al., 2023). A method using magnetic solid-phase extraction (MSPE) coupled with AAS was established for quantitative analysis of Cu, Pb, and Cd in G. elata. The proposed method showed good linearity in the range of 1.0–100.0 μg/L, and the recoveries of the spiked samples varied from 90.0 to 102.0% (Tu et al., 2016).
Among herbal medicine processing methods, the Sulfur Fumigation of herbs is an important post-harvest processing method that has been used in Asian countries for a long history (Kan, Ma and Lin, 2011). Inappropriate sulfur fumigation operations can lead to excessive SO2 in herbs such as G. elata, which places potential danger to human health (Li et al., 2024). The fluorescence analysis method is one of the most attractive techniques due to its simple operation, high sensitivity, and time-saving. A mitochondrial fluorescent probe indole-incorporated-benzoeindolium was synthesized and was successfully applied to detect SO2 and its derivatives in G. elata with recovery rates from 96.14% to 108.3% (Zheng et al., 2022). It is noteworthy that G. elata is found to contain naturally occurring bisphenol F (bis(4-hydroxyphenyl) methane), which is structurally similar to bisphenol A belonging to endocrine disrupting chemicals (Huang et al., 2019). Bisphenol F can be extracted from foods/herbs and their products by aqueous methanol solution, but limited specific quantitative methods have been reported till now.
| 4. Application in functional food | ▴Top |
G. elata has been applied as a food for a long history, and there are many herbal cuisine recipes and food products using G. elata in China and other southeast Asia countries. With systematical research about the biological function of G. elata, more and more relative food products have been developed, including alcohol drinks, tea, biscuits, pastries, noodles, and beverages among others.
Food processing methods have impacts on the bioactives and flavor substances of G. elata, which is shown in Table 2. For example, the fermentation of G. elata by lactobacilli and yeast can significantly change its flavor composition and improve the concentration of pleasant flavor compounds (Tan et al., 2024). Moreover, the fermentation process can also raise the content of 4-hydroxybenzyl alcohol and 4-hydroxybenzaldehyde (Zhao, et al., 2022). Besides small molecules, the polysaccharides are also affected by fermentation, which would become fragmented and loose. Steaming and boiling can increase gastrodin content and decrease 4-hydroxybenzyl alcohol content, but lead to hydrolysis of polysaccharide and gelatinization of starch granules.
 Click to view | Table 2. Summary of effects of food processing on the chemical substances in G. elata |
| 5. Conclusion | ▴Top |
This review summarized the research of chemical substances in G. elata in the scope of analysis method and food application, which can help readers to systemically capture the knowledge about how to analyze different kinds of compounds in G. elata and their application in the food area. G. elata has been proven to contain a variety of chemical substances, including phenolic compounds, organic acids, polysaccharides, peptides, and others. Gastrodin and 4-hydroxybenzyl alcohol are markers for the quality control of G. elata. Some compounds exhibiting excellent pharmacological effects make it valuable to conduct deep research. Thus, many methods have been developed for the analysis of these compounds in plant, biological, food, and drug samples. In addition, there are a few methods for the analysis of harmful substances, which should be given more attention, such as bisphenol F.
As for its application in the food area, G. elata has been applied as food for a long history, and abundant relative products have been developed including liquor drinks, tea, biscuits, noodles, beverages, and so on. It is noteworthy that, different food processing methods have a varied effect on the chemical composition of G. elata, which can provide the guide for the development of functional food of G. elata.
This research was funded by the Youth Talent Project of Education Department Scientific Research Plan of Hubei Province (GRANT number Q20232904).
| References | ▴Top |