| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 27, September 2024, pages 79-87
First in vitro insights into the digestive stability of polysorbate 80 micelles
Johanita Kruger*, Jan Frank
Department of Food Biofunctionality, Institute of Nutritional Sciences, University of Hohenheim, Garbenstraße 28, 70599 Stuttgart, Germany
*Corresponding author: Johanita Kruger, Department of Food Biofunctionality, Institute of Nutritional Sciences, University of Hohenheim, Garbenstraße 28, 70599 Stuttgart, Germany. Tel: +49-711-459 23376; Fax: +49-711-459 23386; E-mail: johanita.kruger@nutres.de
DOI: 10.26599/JFB.2024.95027389
Received: July 9, 2024
Revised received & accepted: September 18, 2024
| Abstract | ▴Top |
The improvement in bioavailability of various bioactives e.g. curcumin, digoxin and resveratrol, after polysorbate 80 (PS80) micellization, has been attributed, in part, to increased digestive stability of the bioactives. This research aimed to evaluate the role of the digestive stability of the PS80 micelles in increasing the in vitro bioaccessibility of bioactive compounds (curcumin, naringenin, Beta-carotene, CoQ10). This was done by comparing pre-micellization (solvent evaporation method) of bioactives in PS80 compared to co-digestion (simulated co-consumption) with PS80 (compared to that of olive oil). Additionally, the effects of relevant digestive compounds, lipase and bile extract, on the particle/micellar surface charge and curcumin bioaccessibility, were investigated. The solubility of the compounds in olive oil or PS80, played a substantial role in the modulation of their bioaccessibility. Co-digestion with PS80 resulted in similar or higher amounts of bioaccessible compounds, but pre-micellization was more efficient in increasing bioaccessibility, indicating some measure of digestive stability of the micelles. The modulating effect of lipase and bile extract on the bioaccessibility of curcumin however, suggests that not all micelles remain intact during the gastrointestinal digestion. While the extent of the digestive stability is still unclear, there is currently no available methods to quantify this.
Keywords: Surface charge; PS80; Curcumin; Naringenin; Beta-carotene; CoQ10
| 1. Introduction | ▴Top |
The effect of polysorbate-80 (PS80) micellization of lipophilic compounds on their oral bioavailability has been found to range from negligible for coenzyme Q10 (CoQ10) (1.1-fold) (Weis et al., 1994) to a very high increase of curcumin (185-fold) (Schiborr et al., 2014), with various bioactives in between: digoxin (2.6-fold) (Zhang et al., 2003), resveratrol (5-fold) (Calvo-Castro et al., 2018), astaxanthin (2- to 4-fold) (Odeberg et al., 2003) and progesterone (4- to 6-fold) (Potluri and Betageri, 2006). The reason for the varied effects and the mechanisms behind increased bioavailability have not been entirely elucidated. However, the most accepted theory is that the PS80 micellization increases the digestive stability, solubility, and bioaccessibility of lipophilic compounds in the intestinal lumen (Flory et al., 2021)(mechanisms not clear). This then results in increased cellular uptake and transepithelial transport (Frank et al., 2017), and ultimately bioavailability.
A number of studies have attempted to shed light on the physiochemical characteristics of compounds that play a role in their PS80 solubility (Persson et al., 2013), encapsulation efficiency by PS80 (Kruger et al., 2022) and the subsequent effects on bioaccessibility (Kruger et al., 2022) and bioavailability (Weis et al., 1994; Odeberg et al., 2003; Zhang et al., 2003; Potluri and Betageri, 2006; Schiborr et al., 2014; Calvo-Castro et al., 2018). From these studies, no single physiochemical characteristic could be identified that explained the variation in the PS80 solubility or the modulation of the in vitro solubility, bioaccessibility or resulting bioavailability.
One of the challenges in determining the mechanism of action of PS80 micellization, is that the digestive stability of the PS80 micellar structure itself is unknown. It is currently unknown if formulated micelles remain intact during the gastrointestinal digestion and cellular absorption or if they dissociate and their constituents are incorporated into physiological mixed micelles. In the case of the latter, expensive and time consuming micellization procedures (e.g. solvent evaporation, anti-solvent, fusion (Jin et al., 2021)) would not be required to increase the bioaccessibility and bioavailability of a compound, as the simple co-consumption with PS80 would presumably yield similar results.
However, similar bioaccessibility of a compound from a PS80 micelle and when co-consumed with PS80 is not definitive proof of micellar instability. Evaluating the effect of relevant digestive parameters (lipase activity and bile salt concentration) on the bioaccessibility of a compound from a PS80 micelle and when co-consumed with PS80 could be used to further estimate digestive stability. The hypothesis is, that if the PS80 micelles are stable during the digestion, then the presence or absence of lipase and bile salts would have no effect on the in vitro solubility and bioaccessibility of the micellized compound.
PS80 is composed of a hydrophilic head (sorbitan polyoxyethylene) linked by an ester bond to a hydrophobic tail (oleic acid). If a PS80 micelle was unstable during digestion, lipase could gain access to the PS80 and cleave the ester bond, producing free oleic acid and sorbitan polyoxyethylene, resulting in decreased micellization efficiency compared to intact PS80 (Khossravi et al., 2002). It has also been found that above their critical micellar concentration (CMC) of 2 to 4 mM (Golding and Wooster, 2010), bile salts can displace PS80 and other surfactants from oil in water interfaces, which would affect the stability and micellization efficiency of the PS80 micelles. The concentration of bile salts in in vitro digestions is approximately 10 mM (Brodkorb et al. 2019).
The aim of the present study was to investigate the digestive stability of PS80 micelles and possible mechanisms behind the bioaccessibility modulation. This was done by first determining if pre-micellization (solvent evaporation method) with PS80 is necessary to increase the bioaccessibility of compounds. The effects of PS80 (co-digestion and two pre-micellized formulations) and olive oil (as lipid source to optimise formation of physiological mixed micelles) on the in vitro bioaccessibility of lipophilic compounds were compared. To further investigate the digestive fate of PS80 micelles, the effects of lipase and bile extract on the bioaccessibility and micellar surface charge of curcumin (model compound)/PS80 formulations were evaluated.
| 2. Materials and methods | ▴Top |
2.1. Reagents
For the preparation of the micelles, curcumin (95%), coenzyme 10 (CoQ10) (95%) from AQUANOVA AG (Darmstadt, Germany), naringenin (≥ 98%) from Carl Roth (Karlsruhe, Germany) and β-carotene from Sigma-Aldrich (Steinheim, Germany) were used. As solvents ethanol (ROTISOLV® HPLC Gradient Grade, Carl Roth), hexane (ROTISOLV® HPLC, Carl Roth) and dichloromethane (ROTISOLV® HPLC, Carl Roth) was used. The following extract and enzymes were used in the in vitro digestion; bile extract (Sigma B8631), pepsin (Sigma P7000; ≥250 U/mg labelled activity), pancreatin (Sigma P7545; 8 × USP labelled activity), and pancreatic lipase (Sigma L3126) all of porcine origin.
2.2. Micelle production
The micelles were produced using a solvent evaporation process as previously described (Kruger et al., 2022). PS80 (900 mg) and each bioactive (100 mg) were added to 20 ml of the respective solvents (curcumin & naringenin in ethanol, CoQ10 in hexane, Beta-carotene in dichloromethane) and dissolved in an ultrasonic bath at 25°C for 15 min. The solvent was evaporated overnight, using a rotary vacuum concentrator, after which 20 ml of H2Odd (distilled and deionized) was added and incubated (3 h, shaking at 150 rpm at 4°C). The H2Odd was then evaporated overnight, using a rotary vacuum concentrator, and the resulting micellar paste stored at 4°C until further use. One set of micelles was centrifuged (4,700 ×g, 10 min, 4°C) and filtered (Filtropur S, 200 nm, Sarstedt, Nümbrecht, Germany) before the final evaporation step, while the water from the second set was evaporated without filtering. The filtered micelles would only contain compounds and PS80 incorporated into smaller micelles (<200 nm), whereas the unfiltered samples would contain these smaller micelles, larger micelles (>200 nm) and all insoluble compound not incorporated into micelles.
2.3. In vitro digestion
A simplified version of the IFOGEST method (Rodrigues et al., 2017), consisting of an in vitro gastric and intestinal digestion, was carried out (n=6) as previously described (Kruger et al., 2022). For the gastric phase, the micelles (quantities according to Table 1) were diluted to a final volume of 8.5 ml, to which pepsin was added (1.5 ml 34 mg pepsin/ml 0.1 N HCl – final conc. 5 mg/ml), the pH adjusted to 2 and the digests incubated (in the dark at 37°C for 1 h, shaking at 120 rpm). For the small intestinal phase (final volume 15 ml), porcine lipase (9 mg/ml), porcine pancreatin (18 mg/ml) and bile extract (36 mg/ml) were prepared in 100 mM NaHCO3 and added to final concentrations of 0.6 mg/ml lipase, 1.2 mg/ml pancreatin and 7.2 mg/ml bile extract. The pH was adjusted to 6.5 using 1 N NaOH, the digest overlaid with nitrogen gas and incubated, as described above, for 2 h. The final digests were centrifuged (4,700 × g, 60 min, 4°C) and filtered (Filtropur S, 200 nm) to separate the soluble and bioaccessible fraction, respectively. All collected samples were overlaid with nitrogen gas and stored at −80°C for a maximum of two weeks before HPLC analysis.
 Click to view | Table 1. Preparation of bioactive + lipid/PS80 solutions for in vitro digestion |
For the first part of the study (Figure 1a), the effects of different formulations with a lipid source (olive oil) or PS80 on the in vitro solubility and bioaccessibility of coenzyme Q10, β-carotene, naringenin, and curcumin were determined. The sample preparations are summarised in Table 1. The contents of the compounds and PS80 were kept constant during preparation at 0.1 and 0.9 mg/ml final digestion volume, respectively. However, during the filtering step one set of micelles, the majority of the compound (Table 1), and possibly some PS80, were removed.
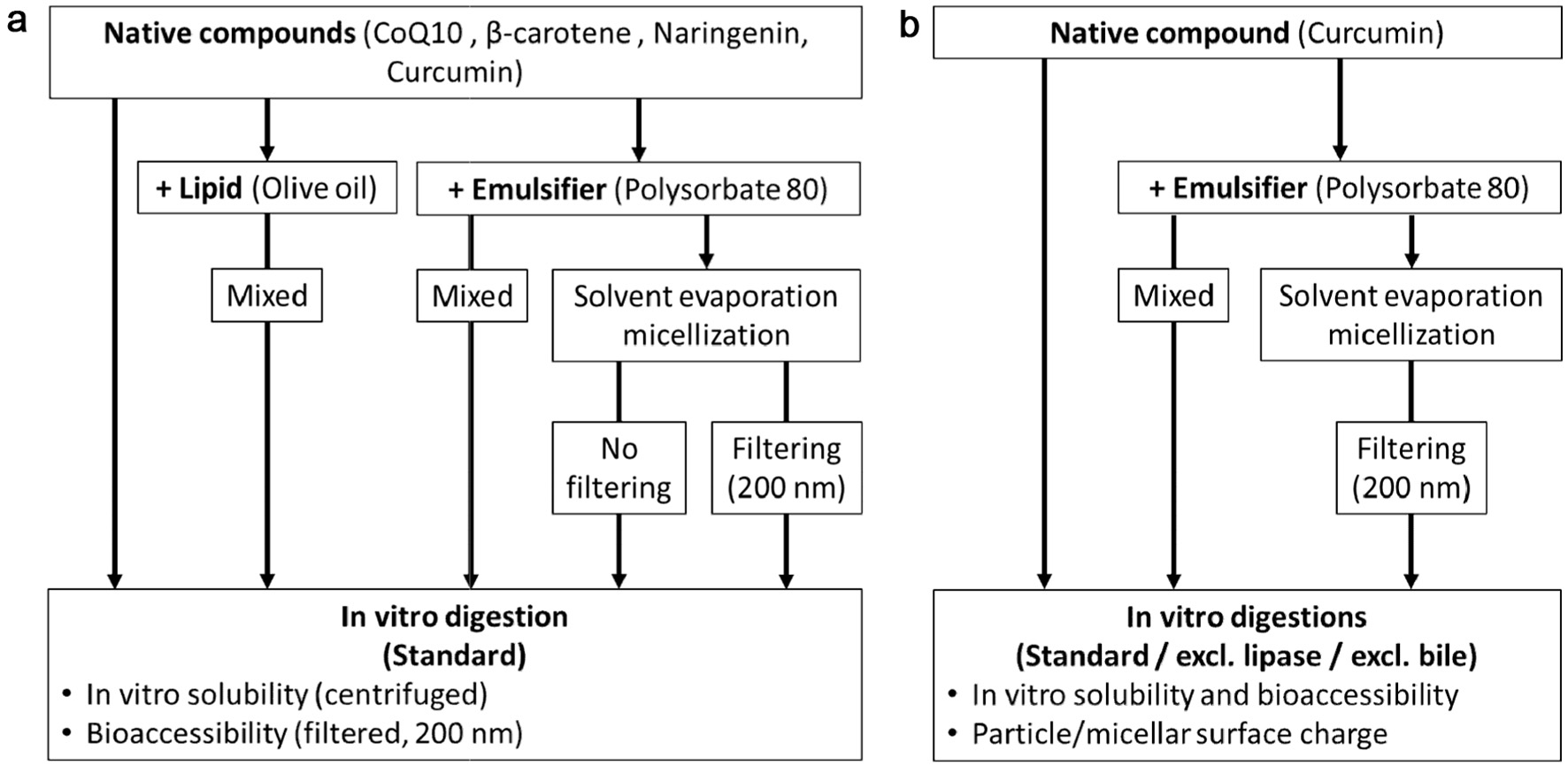 Click for large image | Figure 1. Experimental design with (a) all compounds digested with a lipid and different PS80 formulations and (b) Curcumin from PS80 formulations digested following different digestion protocols (excl. lipase or bile extract). |
For the second part of the study (Figure 1b), the in vitro digestion of the different curcumin/PS80 formulations was conducted three times: first using the standard digestion protocol and then without the addition of lipase or without the addition of the bile extract. The curcumin content of the different digestions, including the filtered micelles, was kept constant at 100 µg/ml final digestion volume and the amount of PS80 was kept constant at 1,370 µg/ml final digestion volume. For a total digestion volume of 10 ml, curcumin powder was added at 1 mg, PS80 at 13.7 mg and the PS80/curcumin micelles with a loading capacity (LC) of 6.8% at 14.7 mg.
In this paper, the in vitro solubility is defined as the amount of compound solubilised (supernatant after centrifuging) and bioaccessibility as the amount incorporated into small particles/micelles (supernatant after centrifuging and filtration, 200 nm) after in vitro gastrointestinal digestion (after both gastric and intestinal phase). Data are reported as the absolute amounts (µg/ml digest) and/or as the percentage (%) of the initial amount digested.
2.4. HPLC analyses
Coenzyme 10 (Kruger et al., 2022), β-Carotene (Stuetz et al., 2012), naringenin (Kruger et al., 2022) and curcumin (Schiborr et al., 2014) where analysed, before and after digestion, using HPLC methods as previously described.
2.4.1. CoQ10
CoQ10 was analysed on a SHIMADZU HPLC system (autosampler SIL-20AC HT, degasser DGU-14A, column oven CTO-10AS VP, system controller SCL-10A VP, UV/VIS detector SPD 20A, liquid chromatograph LC -20AT) equipped with a Reprosil-Pur column (C18-AQ, 5 µm, 250 × 4.6 mm, Dr. Maisch GmbH, Ammerbuch, Germany) maintained at 35°C. After a 10 µl injection, chromatographic separation was achieved with a mobile phase consisting of 85% methanol and 15% 1,4-dioxane at a flowrate of 1 ml/min. The UV/VIS detector analysed CoQ10 at an excitation wavelength of 272 nm.
2.4.2. Curcumin
The different curcuminoids were analysed on a SHIMADZU HPLC system (see CoQ10), with the column maintained at 40°C. After a 20 µl injection chromatographic separation was achieved using a mobile phase of 55% H2Odd (pH = 3 with perchloric acid) and 45% acetonitrile at a flowrate of 1.4 ml/min. The analyses were carried out with the fluorescence detector, which quantified at an excitation wavelength of 426 nm and an emission wavelength of 536 nm. Peak integration was done with the LabSolution data management software and quantified against external standard curves of curcumin (purity >97%), demethoxycurcumin (purity >98%) and bis- demethoxycurcumin (purity >98%) standards (Chromadex, Irvine, USA).
2.4.3. Naringenin
Naringenin was analysed on a JASCO HPLC system (LC-Net II ADC, AS-2059-SF Plus, PU-2080 Plus, CO-2060 Plus, DG-2080-53, LG-2080-02 and a photodiode array detector PDA MD-2018; JASCO, Groβ-Umstadt, Germany) equipped with a Kinetex PFP column (100 A, 250 × 4.6 mm, 5 µm, Phenomenex, Aschaffenburg, Germany) maintained at 35°C. After a 20 µL sample injection, chromatographic separation was achieved using a multistep gradient method (0 min 0% B, 1 min 20% B, 4 min 20% B, 12 min 75% B, 14 min 75% B, 17 min 30% B) with mobile phase A (deionized water with 5% formic acid) and mobile phase B (acetonitrile with 10% deionized water and 5% formic acid) at a flow rate of 1.0 ml/min. Photodiode array detection was monitored at 280 nm for naringenin and catechin. Peak integration was done with the JASCO ChromNAV (version 1.19.1) data management software and quantified against external standard curves of naringenin (purity ≥ 98%, Carl Roth).
2.4.4. Beta-carotene
Beta-carotene was analysed on a SHIMADZU HPLC system (see CoQ10), with the column maintained at 40°C. After a 20 µl injection, chromatographic separation was achieved with a mobile phase consisting of 82% acetonitrile, 15% 1,4-dioxane and 3% methanol with 100 mM ammonium acetate and 0.1% triethylamine at a flow rate of 1.5 ml/min. The β-carotene was measured by the UV/VIS detector with an excitation wavelength of 400 nm. Peak integration was done with the LabSolution (SHIMADZU Corporation, Nakagyoku, Japan) data management software and quantified against external standard curves of β-carotene (≥97.0% purity, Sigma-Aldrich), retinyl palmitate (purity >97%, Sigmal Adrich) and retinol (purity >95%, Sigma-Aldrich (Merck Group KGaA), Darmstadt, Germany).
2.5. Particle surface charge
The surface charge of the particles/micelles from the soluble fractions of the in vitro digestion were prepared and analysed using a Zetasizer Nano (Malvern Instruments, Malvern, USA), as previously described (Kruger et al., 2022). In short; The surface charge (mV) was measured with laser Doppler microelectrophoresis, samples were diluted in H2Odd at a ratio of 1:10 and measurements were made at least 4 times with 20 runs each, at 25 °C.
2.6. Statistical analysis
Normality of data and equality of variance were assessed using the Shapiro-Wilk and Levene’s tests, respectively (GraphPad Prism 8.4.1, GraphPad Software Inc., La Jolla, USA). As all data were normally distributed, a one- and two-way ANOVA, followed by Tukey’s post-hoc test was performed and p < 0.05 considered statistically significant.
| 3. Results and Discussion | ▴Top |
3.1. The effects of olive oil and polysorbate-80 on the in vitro bioaccessibility of coenzyme Q10, β-carotene, naringenin and curcumin
The in vitro solubility (µg/ml) of the native compounds (Table 2), while low, was substantially higher than their respective water solubilities (Table 3). This indicates that these compounds were incorporated into physiological mixed micelles derived from the added bile acids, even when no lipid or surfactant was present. With the addition of a lipid (olive oil) or surfactant (PS80), it was expected that the number and/or size of the mixed micelles would increase, as there would be more ‘substrate’, which in turn would increase the amount of compound incorporated into the micelles. This was, interestingly, not consistently observed and the overall effects of olive oil and PS80 formulations could be grouped as follows: coenzyme Q10 and β-carotene, whose in vitro solubility and bioaccessibility were increased only when digested with olive oil (not PS80), and naringenin and curcumin, whose in vitro solubility and bioaccessibility were only increased with PS80 formulations (not olive oil).
 Click to view | Table 2. The in vitro solubility1 and bioaccessibility2 (µg/mL and percent of amount digested) of compounds from the native powder digested alone, with olive oil or polysorbate-80 (PS80) and from unfiltered/filtered PS80 micelles (solvent evaporation method) |
 Click to view | Table 3. Solubility of compounds1 in water, olive oil, or polysorbate-80 (PS80) compared to the changes in in vitro solubility2 after digestion with olive oil or PS80 |
No physiochemical characteristic has been found to predict the solubility of compounds in PS80 (Persson et al., 2013) or the effect of PS80 on their bioaccessibility (Kruger et al., 2022). Here, however, the solubility of the compounds in olive oil and PS80 (Table 3), in relation to the observed differences in bioaccessibility, was considered (Table 2). While all the compounds used were hydrophobic (high logP value), it does not necessarily mean that they all have high solubilities in specific oils or surfactants. The solubilities of the compounds in olive oil have been found to differ between 450 µg/ml for curcumin and 14 × 104 µg/ml for CoQ10, and in PS80 between 40 µg/ml for β-carotene and 7 × 104 µg/ml for naringenin (Table 3).
In general, high solubility of a compound in olive oil or PS80 (Table 3) corresponded with increased in vitro solubility and bioaccessibility (Table 2). This was, however, not the case for naringenin, which has high solubility in olive oil, but the digestion with olive oil did not significantly affect its bioaccessibility. While the solubility of naringenin in olive oil has been found to be pH dependent, with approximately 80% partitioning into the olive oil phase from a water phase at pH 5, compared to only 60% at a pH of 7, this does not fully explain the low bioaccessibility. It seems that increased bioaccessibility is unlikely without high solubility in a respective oil or surfactant, but also, that high solubility does not guarantee it. However, a much larger sample size of compounds, oils and surfactants would be required to substantiate this.
To answer the question if the micellization of PS80 is necessary, or if the co-digestion (simulated co-consumption) of a compound and PS80 could result in a similar increase in bioaccessibility, only naringenin and curcumin will be discussed based on their behaviour described above.
Across all formulations, there was no difference between the in vitro solubility and bioaccessibility of naringenin (Table 2). This means that, independent of what was added (olive oil or PS80) or how the PS80 was formulated (co-digestion or pre-micellization), that the soluble naringenin was incorporated into small (<200 nm) micelles. With curcumin, the in vitro solubility from the PS80 formulations were 2- to 4-fold higher than the respective bioaccessibility, indicating the incorporation into both small and larger (>200 nm) micelles/particles.
The unfiltered micelles had the highest amount of bioaccessible naringenin with no difference between the co-digested sample (native + PS80) and the filtered micelles. With curcumin, the bioaccessibility from the co-digested sample (native + PS80) and unfiltered micelles was higher than the filtered micelles. This suggests, that on top of the micelles formed during the solvent evaporation method, additional naringenin/curcumin was solubilised during digestion, through incorporation into physiological mixed micelles. It is however, not possible to know if the additional solubilised compound was that, which was initially not incorporated into the micelle, or from dissociated PS80 micelles, or more likely a combination of both. When the percentage of bioaccessible naringenin and curcumin was considered, the filtered micelles (lower amount of curcumin added to the digestion) had the highest percentage bioaccessibility (naringenin 82% and curcumin 32%), compared to the native + PS80 and the unfiltered micelles (naringenin 23–27% and curcumin 15–17%). While this does not necessarily mean that the PS80 micelles were 100% stable during the digestion, it does suggest that at least some of the micelles remained stable during the gastrointestinal digestion.
Simulated co-consumption (at the same concentration as the start of the micellization process) of PS80 and naringenin or curcumin resulted in similar or higher amounts of bioaccessible compound, whereas pre-micellization (solvent evaporation method), was more efficient in improving the bioaccessibility of both compounds.
3.2. The effects of lipase and bile on curcumin bioaccessibility and micellar surface charge
3.2.1. Bioaccessibility
The higher efficiency of the pre-micellized PS80 formulations in increasing the bioaccessibility of naringenin and curcumin suggested some digestive stability. This part of the work aimed to investigate the digestive stability further. This was done by evaluating the effect of different digestion conditions (excl. bile extract or lipase) on curcumin bioaccessibility and the surface charge of the resulting particles/micelles from different curcumin/PS80 formulations (simulated co-consumption and filtered pre-micellized). The hypothesis is, that if the PS80 micelles are stable during the digestion, then the presence or absence of lipase and bile salts would have no effect on the in vitro solubility and bioaccessibility of the micellized compound.
As observed in the first part of the work (Table 2), here (Figure 2) the curcumin bioaccessibility from all formulations was also much lower than their respective in vitro solubility. Also, both the in vitro solubility and bioaccessibility from the native curcumin powder, was much lower compared to the PS80 formulations. Overall, the in vitro curcumin solubility from the pre-micellized PS80 and when co-digested with PS80 was similar, while the percentage curcumin bioaccessibility from the filtered micelles was higher than that of the native curcumin + PS80. In the first part of the work, the curcumin and PS80 concentrations across all formulations were kept constant to the concentrations with which the solvent evaporation process started, here the concentrations were normalised to that in the curcumin/PS80 micelles. This would mean that when the same amount of curcumin from the different formulations was digested, that the amount of bioaccessible curcumin would be highest from the filtered pre-micellized formulation.
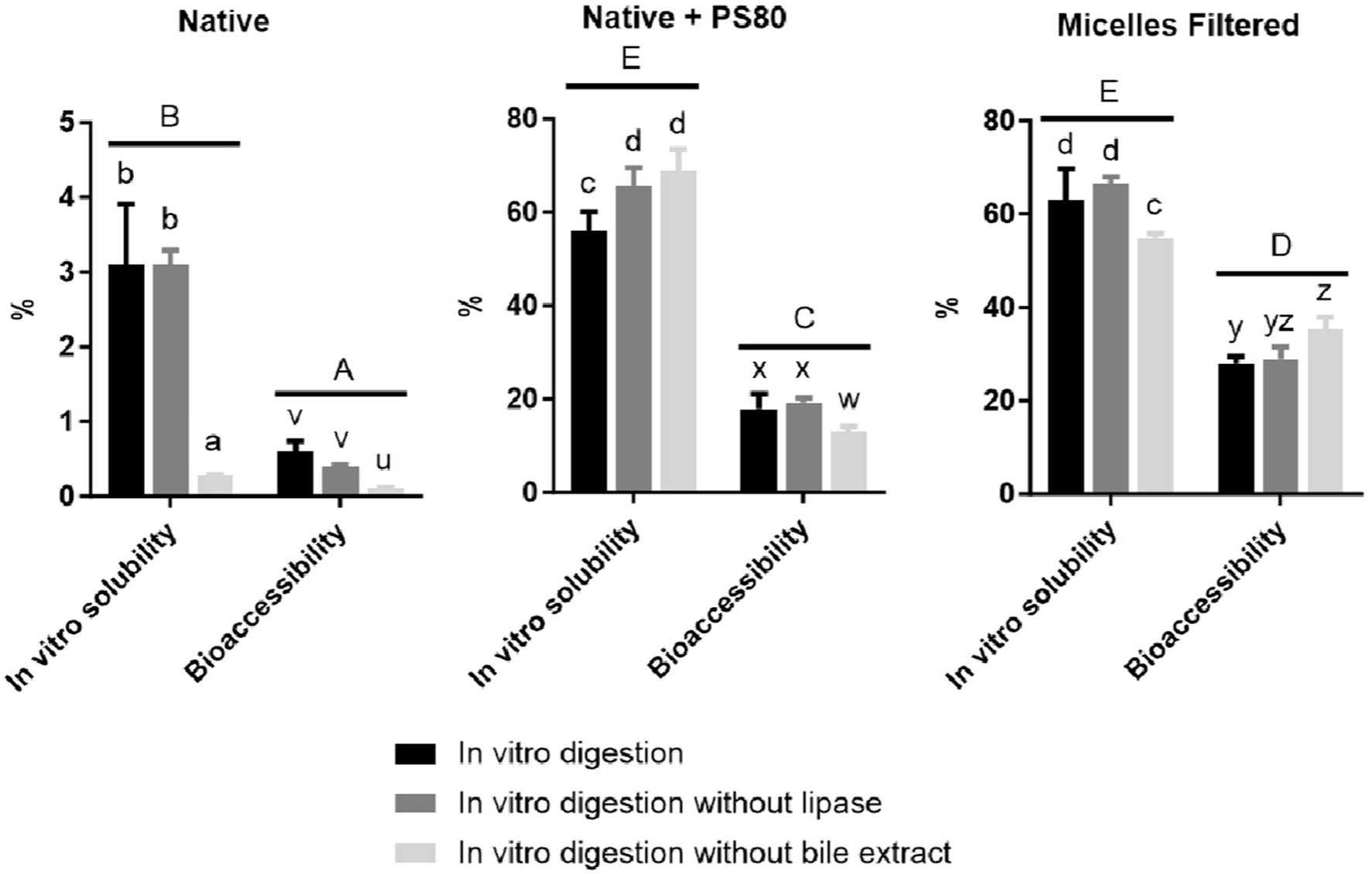 Click for large image | Figure 2. The in vitro solubility (%) and bioaccessibility (%) of curcumin from native curcumin powder digested alone (Native), co-digested with PS80 (Native + PS80) or as pre-micellized PS80/curcumin (Micelles filtered) (n = 6). In vitro digestive conditions were standard or conducted without lipase or bile extract, respectively. Bars with different lowercase letters (abc – in vitro solubility and uvw – bioaccessibility) differ significantly (p < 0.05). Different uppercase letters (ABC) indicate significant differences between overall in vitro solubility and bioaccessibility between different formulations. |
Excluding lipase had no effect on the in vitro solubility and bioaccessibility of the native curcumin, but after digestion without bile extract, both were substantially reduced. This again supports the theory that native curcumin was incorporated into physiological mixed micelles when digested without an oil or surfactant (Figure 2). The effects of excluding lipase and bile extract were different for curcumin from the co-digested native + PS80, compared to the filtered pre-micellized curcumin/PS80. While digestion without lipase increased in vitro solubility of curcumin when co-digested with PS80, the smaller increase in in vitro solubility from the filtered pre-micellized curcumin was not significant. Lipase omission had no effect on the curcumin bioaccessibility. Digestion without bile increased the in vitro solubility of curcumin when co-digested with PS80 and decreased it from the filtered micelles. The effect on curcumin bioaccessibility, however, was vice versa.
The increased in vitro solubility of curcumin co-digested with PS80 (Native + PS80), when digested without bile and lipase, suggest that both these compounds interfered with the ability of PS80 to micellize curcumin. Lipase possibly cleaved the ester bond, producing free oleic acid and sorbitan polyoxyethylene side chain, with decreased micellization efficiency compared to PS80 (Khossravi et al., 2002). Christiansen et al. evaluated the effects of pancreatic lipase on the PS80 and found that in 90 mins, approximately 14% of the PS80 was hydrolysed (released fatty acids) (Christiansen et al., 2010). They also evaluated the effect of increasing concentrations of PS80 on the digestion different concentrations of olive oil. The PS80 was added as a solution (no specific micellization step) at concentrations ranging between 0.0 to 0.5 mmol/l. They found that PS80 inhibited olive oil digestion in a concentration-dependent manner, with a IC50 of 0.13mM. Interestingly they found that at low concentrations (0.01mM), below the CMC of PS80 that the inhibition was competitive, but at higher concentrations that the type of inhibition was unclear, but likely uncompetitive. This supports the current data that the PS80, when not in micellar form (below the CMC), is succeptable to lipase hydrolysis, but when in a micellar form, it (and its contents) is somewhat protected from gastrointestinal digestion.
The bile salts probably displaced PS80 from the oil in water interfaces (Golding and Wooster, 2010). On the other hand, the reduced curcumin bioaccessibility when digested without bile, indicates that the bile salts also contributed to the assembly of smaller (<200 nm) physiological mixed micelles containing curcumin. Imai et al. compared the CMC of PS80 and bile salts to micellize tocopherol (Ilai et al., 1983). They found that while the CMC of PS80 (0.4 mM) was similar to that of sodium glycodeoxycholate (0.35 mM) and sodium taurodeoxycholate (0.48 mM). The CMC concentration of the rest of the bile salts evaluated ranged between 0.5 and 3.3 mM. While tocopherol was not evaluated in this study, it is possible that the CMC of bile salts in micellizing the bioactives used in this study was also similar and considering that the concentration of bile salts from the bile extract is higher that that of the PS80, it is very possible that that the bile salts displaced the PS80.
The smaller and insignificant effect of lipase omission on the in vitro solubility of pre-micellized curcumin suggests that the PS80 in the micelles, formulated with the solvent evaporation method, was to some extent protected from enzymatic degradation. Interestingly, the increased in vitro solubility of pre-micellized curcumin, when digested without bile, was accompanied by decreased bioaccessibility. This could be due to the bile salts resulting in dissociation of the preformed micelles (decreased bioaccessibility), but then also incorporation of the curcumin from these dissociated PS80 micelles into larger physiological mixed micelles (increased in vitro solubility). Then, when digested without the bile salts, more small (<200 nm) PS80 micelles remained intact, resulting in a higher concentration of bioaccessible curcumin.
3.2.2. Surface charge
The surface charge of the undigested curcumin-loaded and -unloaded PS80 micelles were close to zero at −7.0 mV and −3.6 mV, respectively (Figure 3), as PS80 is a non-ionic surfactant. Across all samples, the surface charge of the micelles (particles) digested with bile extract, was the lowest (−22 to −48 mV). Bile extract had the biggest impact on the surface charge of the micelles, with an approximately 35–60% higher surface charge when digested without bile extract, probably due to the anionic nature of the bile salts (Wickham et al., 1998). The interactions of bile salts with PS80 formulations are relevant, as it has been found that PS80 elicits a biliary response in humans (Andren and Theander, 1960). The surface charge of the micelles/particles from the digestions that included PS80 was 24 to 70% higher, compared to the curcumin powder digestions, indicating that the PS80 was incorporated into the physiological mixed micelles, possibly decreasing the concentration of the negatively charged bile salts.
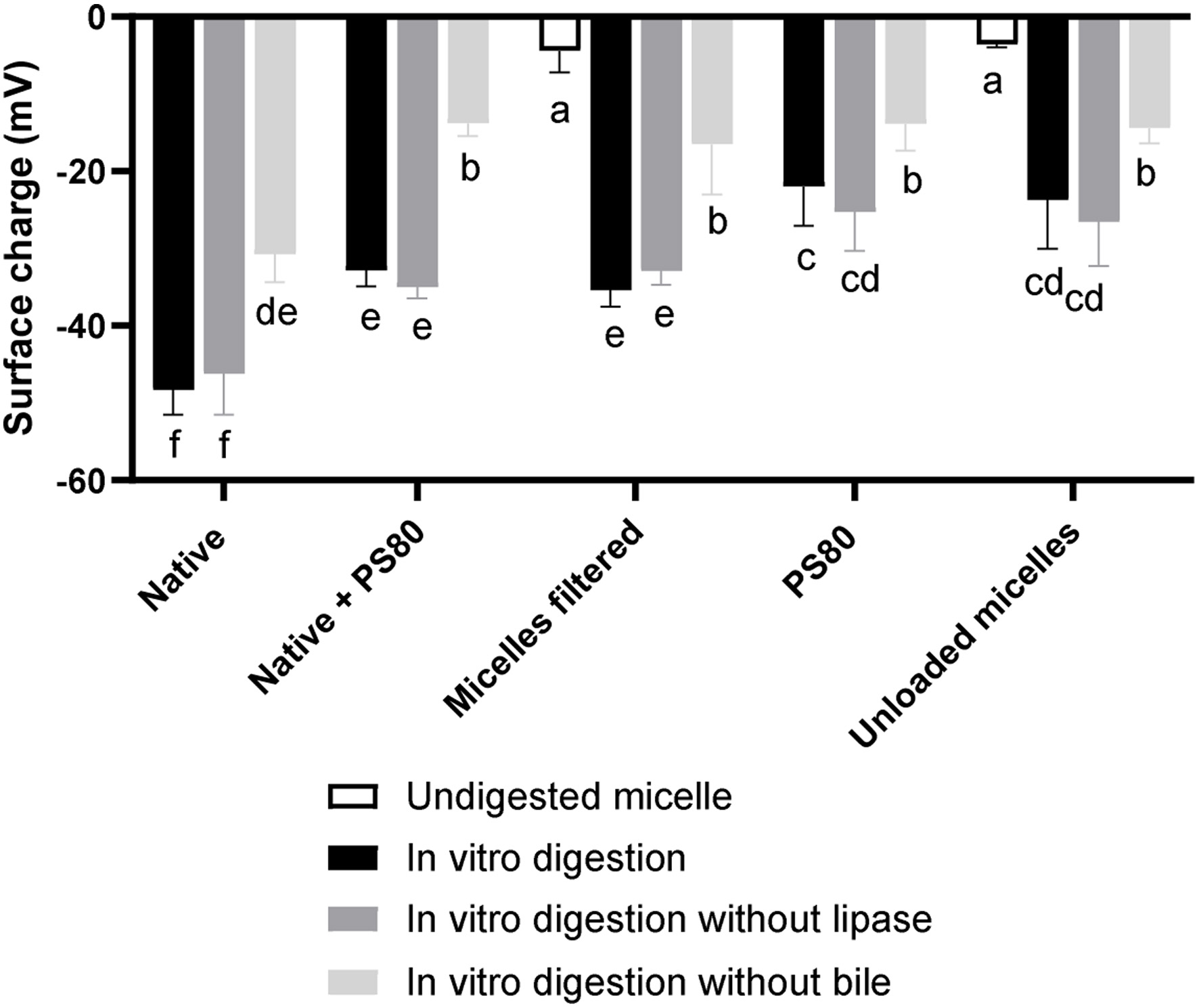 Click for large image | Figure 3. The surface charge (mV) of polysorbate 80 (PS80) and curcumin/PS80 micelles and particles from the in vitro solubility fraction* of digestions of curcumin and PS80 (alone and combined) and PS80/curcumin micelles, digested with and without lipase and bile extract. *Supernatant taken after the in vitro digested micelles were centrifuged (4,700 ×g, 60 mins, 4°C). |
Micelles from the digestions that contained curcumin (PS80 + curcumin and filtered micelles) had a 30–50% lower surface charge than those that did not (PS80 and PS80 micelles), probably because curcumin is a weak Brönsted acid (Priyadarsini, 2014) with a negative charge. The differences in the surface charge (ΔmV) between the formulations containing curcumin and not (Filtered micelle vs. PS80 micelle and Native + PS80 vs. PS80), under the different digestive conditions, were compared in Table 4.
 Click to view | Table 4. Difference (ΔmV) in surface charge of particles/micelles of digestions of curcumin loaded and blank formulations |
The difference between the surface charge of the undigested micelles containing curcumin and not (Filtered micelle vs. PS80 micelle) was small (3.4 mV), but increased to 6.8–11.7 mV after the in vitro digestions containing bile extract. However, after the digestion without bile extract, the difference remained small (2.1 mV). The same was observed for the native + PS80 vs. PS80; after the in vitro digestions with bile, the difference in surface charge was large (9.7–10.9 mV), but after the digestion without bile extract, there was no difference (−0.1 mV). Decreased surface charge could have been caused by curcumin being located closer to the surface of the micelles, which might be explained by a weakened micellar structure resulting from the digestion (lipase activity), causing curcumin to relocate more towards the outer part of the micelle and/or exposing more of the inner hydrophobic core. This also supports the higher bioaccessibility of curcumin from PS80 micelles digested without bile salts. This suggests that when digested without bile salt, the micellar structure was more stable, resulting in more, small bioaccessible PS80 micelles remining intact. This again, supports the notion that the PS80 micelles were not entirely stable during the gastrointestinal digestion, the extent of which is not quantifiable at present.
| 4. Conclusions | ▴Top |
The solubility of a compound in olive oil or PS80 appears to play a substantial role in the modulation of its in vitro solubility and bioaccessibility. Simulated co-consumption (co-digestion) of compounds with PS80 can result in similar or higher amounts of bioaccessible compounds, compared to the native compound. Micellization is, however, more efficient in increasing bioaccessibility, indicating a certain degree of digestive stability. The modulating effect of the lipase and bile on the in vitro solubility and bioaccessibility of curcumin, however, suggest that not all pre-micellized PS80 micelles remain intact during the gastrointestinal digestion. It is not clear to which extent the micelles are stable. While further experiments are warranted to investigate the stability of pre-micellized PS80 during digestive processes, there is currently no available methodology with which to determine this. When measuring the digestive stability, solubility and bioaccessibility of bioactive compounds during and after the in vitro digestion, it is not possible to ascertain if the compounds remain in intact PS80 micelles, or if the bioactives and the PS80 are incorporated into physiological mixed micelles.
| References | ▴Top |