| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 27, September 2024, pages 44-57
Integrated review of cardiometabolic biomarkers and dietary nutrients
Ravindra Vermaa, *, Prakash S Bisena, Mònica Bullób, c, d
aSchool of Studies in Biotechnology, Jiwaji University, Gwalior, India
bDepartment of Biochemistry & Biotechnology, University Rovira i Virgili (URV), Spain
cNutrition and Metabolic Health Research Group, Institute of Health Pere Virgili (IISPV), Spain
dCIBER Physiology of Obesity and Nutrition (CIBEROBN), Carlos III Health Institute, Spain
*Corresponding author: Ravindra Verma, School of Studies in Biotechnology, Jiwaji University, Gwalior, India. Tel: 91-7999212685; E-mail: vermaravindra917@gmail.com
DOI: 10.26599/JFB.2024.95027386
Received: June 25, 2024
Revised received & accepted: August 23, 2024
| Abstract | ▴Top |
There are many dietary options available to us in nature that can benefit our health and cure metabolic conditions. The aim of this study is to identify cardiometabolic biomarkers that can be modulated by dietary supplements. This study used data from PubMed, Web of Science, Scopus, Mendeley, and Embase from 2000 to Dec. 2023. Studies have found that certain fruits, foods, and species can treat cardiometabolic disorders. Low-density lipoprotein (LDL) cholesterol, total cholesterol, triacylglycerols, and reactive oxygen species (ROS) are some of the cardiometabolic biomarkers that can be affected by specific foods and additives. A wide array of clinical trials and scientific findings demonstrate that certain foods and additives can affect cardiometabolic biomarkers such as LDL cholesterol, total cholesterol, ROS, and reactive nitrogen species (RNS). Diet and lifestyle play an important role in lipid metabolism. Food ingredients interact with metabolism in a number of complex ways that will require further scientific research.
Keywords: Cardiometabolic; Cardiovascular; Foods; Lipid; Nutrients
| 1. Introduction | ▴Top |
A high death and morbidity rate has made heart disease research more relevant. Globally, cardiovascular disease (CVD) is estimated to cause 19.1 million deaths by 2020. Eastern Europe and Central Asia had the highest CVD mortality rates in 2020. They were followed by Oceania, North Africa and the Middle East, Central Europe, sub-Saharan Africa, South and Southeast Asia, and Oceania at similar levels. The lowest death rates were found in Asia Pacific, North America, Latin America, Western Europe, and Australasia, which are high income regions (Tsao et al., 2023). A number of factors contribute to heart disease and stroke, including high blood pressure, high LDL cholesterol and diabetes, smoking, passive smoke exposure, obesity, alcohol abuse, and tobacco use, unhealthy diet, and physical inactivity (Eyre et al., 2004). We have considered lipid profile to assess cardiometabolic disease risk and other chronic diseases based on excessive cholesterol and triacylglycerols. A change in lipid levels will cause inflammation (Azizi, et al., 2009). Inflammation causes oxygen radicals. An excess of oxygen radicals (called oxidative stress) damages lipids, proteins, and deoxyribonucleic acid (DNA), and can even lead to cell death. This condition results in diabetes in the liver and pancreas (Bhatti, et al., 2022). As cholesterol molecules and cellular waste accumulate in the arteries, plaques form. Plaques can block blood and oxygen supply to organs, resulting in heart attacks or death (Indumathy and Sudha, 2020). There is some evidence that medications and natural bioactive compounds may reduce plaque and in some cases reverse coronary disease (Kris-Etherton et al., 2002; Teodoro, 2019; Wahab et al., 2022). Evidence suggested that nuts may protect against metabolic disorders such as type 2 diabetes (T2D), dyslipidemia, and cardiovascular disease (Bibiloni et al., 2019). Nuts such as almonds, pistachios, and walnuts have different protective modulating properties, such as insulin resistance, glucose metabolism, and lipid profile. Avocados are considered a plant-based fat source rich in dietary fibres. However, avocado’s effects on cardiometabolic disorders have not fully been explored (Muralidharan et al., 2019). Functional foods are beneficial in the treatment and prevention of cardiovascular disease by several mechanisms: reducing blood lipid levels, improving arterial compliance, reducing LDL oxidation, decreasing plaque formation, scavenging free radicals, and inhibiting platelet aggregation (Hasler et al., 2000; Shahidi, 2004; Wang et al., 2011; Wang et al., 2021; Stolarczyk et al., 2022). Many foods, functional foods, and natural bioactive compounds are being investigated as potential therapies for cardiovascular disorders, but they must be tested scientifically first. As a result, our study seeks to identify foods with lipid-lowering properties and their effects on cardiovascular disease to remove structural barriers to healthy living.
| 2. Material and methods | ▴Top |
An extensive literature search was conducted from 2000 to Dec 2023 in PubMed, Web of Science, Scopus, Mendeley, and Embase (Figure 1). Our search also included Google Scholar and the official websites of cardiovascular and nutrition scientific societies and government organizations. The following keywords were considered: cardiometabolic disorders, metabolic factors, lipid profiles, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, triacylglycerols, food, functional food, bioactive, and/or. Only studies based on diagnosis and published in English dealing with observational and clinical trials were considered.
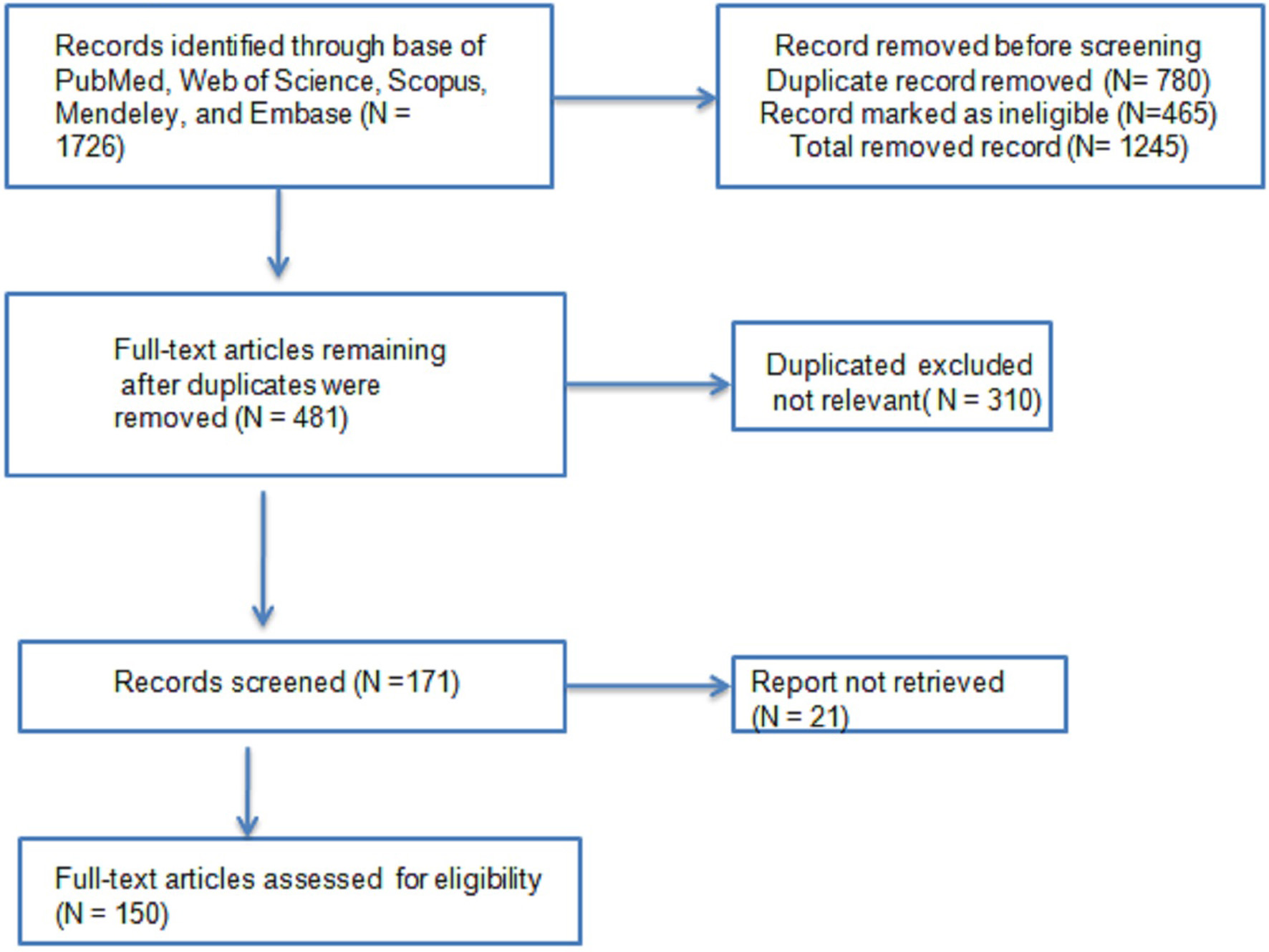 Click for large image | Figure 1. Literature Search Strategy and Selection Criteria. |
| 3. Results and discussion | ▴Top |
3.1. Lipid oriented cardiometabolic disorders
Cardiometabolic risk factors are closely related to obesity, dyslipidemia (unbalanced cholesterol or fat content in lipids), and hypertension. An inexpensive screening tool could use simultaneous measurement and interpretation of waist circumference and fasting TAG to identify men with atherogenic metabolic factors (hyperinsulinemia, high apo B, dense LDL) and CAD risk (Lemieux et al., 2000; Keirns et al., 2021). Many studies have observed the strong relationship between CVD and small dense low-density lipoproteins (sd-LDL) rather than large, buoyant LDLs (Moon et al., 2007; Yoshida et al., 2004; Yokoyama et al., 2018). The central mechanism of action of statins is to increase LDL receptors, which suggests that they should not significantly affect very-low-density lipoprotein (VLDL) triacylglycerol levels, but clinical trials have indicated that statins lower VLDL levels significantly (Table 1) (Ginsberg, 2006). Statin therapy has an uncertain effect, even though the exact extent of its impact is unknown (Parhofer, 2021; Shoamanesh and Selim, 2022).
 Click to view | Table 1. - Lipid-Lowering Drugs and Their Working Mechanism |
Free radicals such as reactive nitrogen species (RNS) and reactive oxygen species (ROS) cause and propagate oxidative stress in human cells. During metabolism, oxygen can be part of potentially harmful cellular molecules termed “free radicals.” These free radicals can act against normal cells, leading to structural and functional damage. Oxidative stress damages large molecules like proteins, lipids, and Deoxyribonucleic Acid (DNA), increasing several risks like heart disease (Cojocaru et al., 2023; Venditti and Di Meo, 2020; Sies et al., 2022). Several natural antioxidants, such as polyphenols and carotenoids, inhibit lipid, protein, and nucleic acid oxidation and prevent oxidising chain reactions (Demirci-Çekiç et al., 2022; Pisoschi et al., 2021).
Doctors commonly recommend cholesterol levels ((LDL-C, HDL-C, and TAG (triacylglycerols)), homocysteine levels, alanine aminotransferases, aspartate aminotransferases (AST), uric acid, fasting insulin, fasting glucose, and haemoglobin A1C (HbA1C) levels. In order to prevent cardiovascular disease, it is more effective to know how much LDL particles are present in the blood (LDL-P) rather than how much LDL cholesterol is present in the blood (LDL-C). There are a number of tests to measure small, dense LDL, including the vertical auto profile (VAP) cholesterol test, LDL gradient gel electrophoresis and Nuclear Magnetic Resonance (NMR) Lipoprofile test. These tests can be fairly expensive, and are not available in all medical facilities. TAG: HDL (high density lipoprotein) ratio is the most accurate biomarker of small dense LDL, the most accurate biomarker of cardiovascular disease. In general, an HDL level of 60 or above indicates healthy cardiovascular health, regardless of the other fractions. A small crystalline fraction of LDL cannot be harmful if LDL is less than 100.
In cardiology, cardiovascular diseases describe disorders of the heart and blood vessels. These disorders include coronary heart disease, cerebrovascular disease, peripheral arterial disease (PAD), and deep vein thrombosis (DVT). CVD risk factors include dyslipidemia, hyperglycemia, and hypertension which are related to lipids. An abnormally high level of blood lipids is characterized by hyperlipidemia. It refers to high triacylglycerols, total cholesterol, low-density lipoprotein cholesterol, or low high-density lipoprotein cholesterol levels (Dixit et al., 2014). Due to blood pumping, blood flows through the circulation in a forward direction. Blood viscosity plays a minor role in flow resistance, but vessels’ diameter, and specifically their arterioles, plays a more significant role (Zhou et al., 2023). Blood red blood cells, white blood cells, and platelets are suspended in plasma. Nutrition, oxygen, and metabolic products are distributed through the cardiovascular system. Major lipids cannot be dissolved in aqueous solutions and do not circulate freely. As albumin carries free fatty acids (FFAs), lipoproteins carry cholesterol, triacylglycerols, and phospholipids. Among its many functions, cholesterol plays a crucial role in cell membranes and is the precursor to steroid hormones and bile acids. Cholesterol often forms plaque deposits in arteries when low-density lipoproteins are elevated, a condition known as atherosclerosis, which significantly contributes to coronary heart disease and other types of cardiovascular disease (Abela, 2010; Libby, 2021; Stanciulescu et al., 2023). The term “cerebrovascular disease” refers to a group of conditions affecting blood flow through the brain and its blood vessels. It can occur due to narrowing blood vessels (stenosis), clot formation (thrombosis), artery blockage (embolism), or blood vessel rupture (haemorrhage). Stroke, Aneurysms, Arteriovenous malformations (AVM), Carotid-Cavernous Fistulas, Carotid Stenosis, transient ischemic attack (TIA) and Stroke are all cerebrovascular issues (Dong et al., 2017). PAD is the narrowing or blocking of vessels in the legs that carry blood from the heart. In most cases, it is caused by fatty plaque buildup in the arteries. Deep vein thrombosis (DVT) occurs when a blood clot (thrombus) develops in one or more deep veins in the legs. Legs are usually swollen or painful with this condition. A high blood lipid level leads to all diseases mentioned above. Maintaining a healthy lipid profile can prevent these diseases.
3.2. Mechanisms of plaque formation in arteries
Cardiovascular disorders are metabolic disorders, a byproduct of food conversion into energy (Khera and Kathiresan, 2017). The disease without a name is metabolic dysfunction, which affects all organs. Metabolic dysfunction manifests itself as increased levels of LDL, cholesterol, and blood pressure in specific tissues (Grundy et al., 2004; Pahwa et al., 2021; Oishi and Manabe, 2020). Glucose is broken down through a series of metabolic steps called glycolysis to release pyruvic acid, which in turn releases adenosine triphosphate (ATP) as energy. There are two options for pyruvic acid from there. A cell’s mitochondria (the powerhouse of energy) allow you to produce much more ATP by breaking it down metabolically, a process known as the Krebs cycle (and making carbon dioxide, another waste product). (b) If mitochondria are not functioning properly, pyruvic acid is diverted to a process known as de novo lipogenesis (increased fat creation) where it is converted into palmitic acid, which is bound to glycerol molecules and transported from the liver in triacylglycerols (Miranda-Silva et al., 2021; Liang et al., 2022). Compared to typical LDL cholesterol, small LDL cholesterol is smaller, heavier, and associated with an increased risk of atherosclerosis. This is believed to be due to small, dense LDL penetration artery walls. In addition, it is more susceptible to oxidation and stays in the bloodstream longer (Hirayama and Miida, 2012; Browning et al., 2017).
LDL cholesterol migrates into a dysfunctional endothelium, which results in decreased nitric oxide (NO) production, and is oxidized by macrophages and smooth muscle cells. Activated adhesion molecules, released by growth factors and cytokines, attract more monocytes. A plaque forms when lipid-laden macrophages accumulate foam cells and smooth muscle cells proliferate (Figure 2). During plaque formation, extracellular inflammatory cells infiltrate, smooth muscle cells die through apoptosis, and the matrix is degraded by proteolysis (by matrix metalloproteinases-MMPs). A thin fibrous cap surrounds an ultra-necrotic core rich in lipids. A ruptured plaque may cause thrombosis, which may lead to the occlusion (or blockage) of the vessel (Messner and Bernhard, 2014; Naseem, 2005; Bhargava et al., 2022).
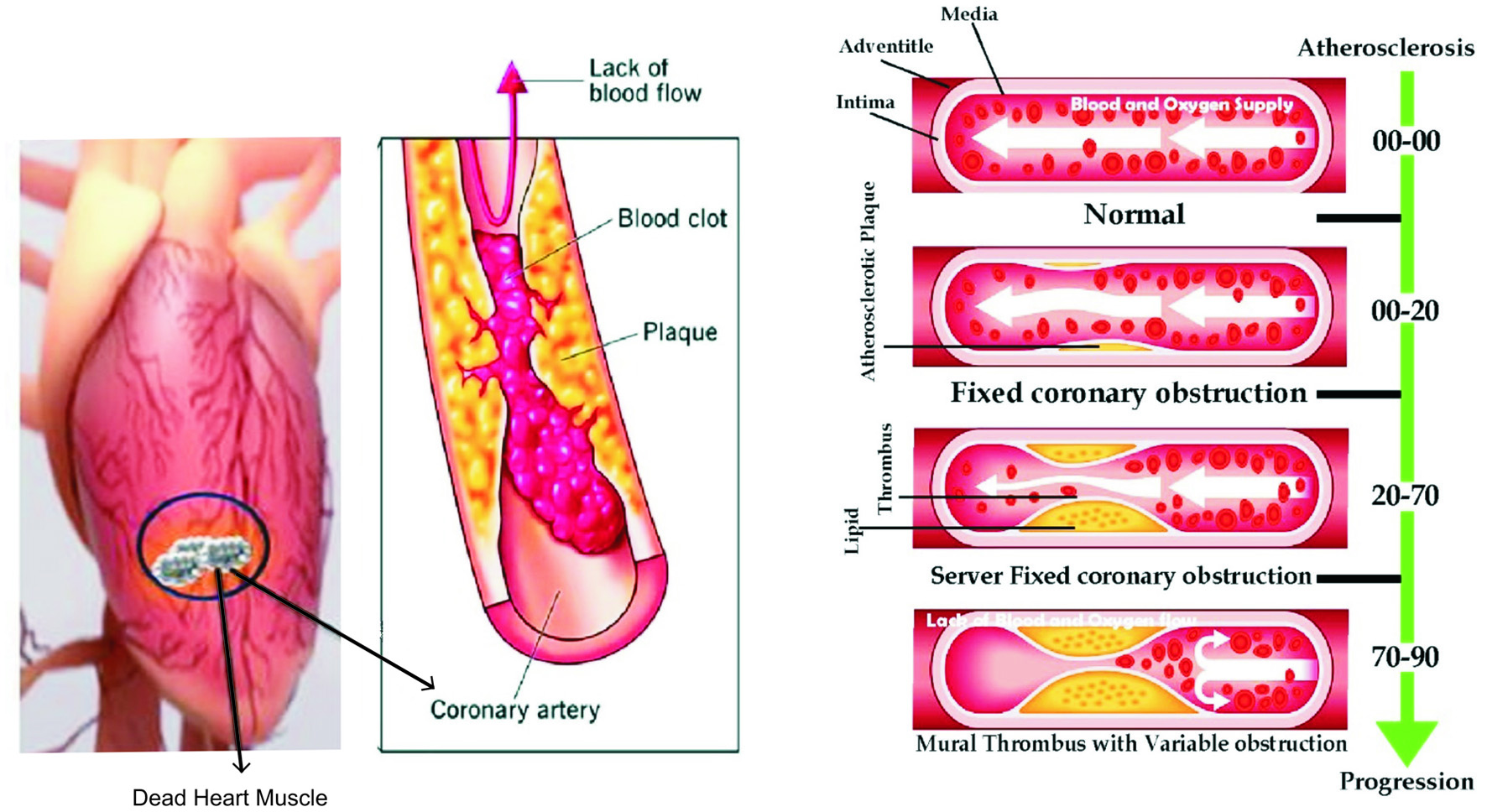 Click for large image | Figure 2. Systematic Development of Plaque in arteries. |
3.3. Interaction of food bioactive compounds with molecular biomarker of cardiovascular diseases
Food and cardiovascular disease have a complex relationship. There is evidence that bioactive compounds from natural sources reduce plaque growth in addition to statins (Noce et al., 2021). Nuts and dried fruit consumption has been associated with decreases in waist circumference and body mass index (BMI) (Carughi et al., 2015). It has been shown that certain metabolites in a diet may help prevent cardiovascular disease since they inhibit platelet aggregation and atherosclerosis (Fernández-Rojas et al., 2022). GALIAT [Galicia Atlantic Diet] investigated lipid profiles, glucose metabolism, inflammation markers, and adiposity as intervention units and found positive effects on all four (Calvo-Malvar et al., 2016). In a randomized trial, walnuts lower cholesterol and modify lipoprotein profile along with normal diet (Guasch-Ferré et al., 2018). Another observational cohort study and a secondary prevention trial have demonstrated a lower incidence of heart attacks among people with a Mediterranean diet that includes extra-virgin olive oil or nuts (Estruch et al., 2013). A recent In-vitro and Ex-vivo study confirmed that raw garlic juice and allicin inhibited the gut microbiota’s production of γ-butyrobetaine (γ-BB) and trimethylamine-N-oxide (TMAO). By modulating the gut microbiota, raw garlic juice and allicin may protect against cardiovascular disease (Panyod et al., 2022).Through the biosynthesis of endogenous lipid mediators; macrophages play a vital role in cardiac repair after injury. This ensures tissue is repaired quickly while preventing chronic inflammation. Cardiovascular repair activates cytokines and adhesion molecules. These include TNF-α and Interleukin-1, to 6. According to research on their receptors, TNF-α is one of the most significant inflammatory cytokines (Halade and Lee, 2022). Omega-3 fatty acids reduce inflammation, which reduces heart disease risk; and they reduce serum triacylglycerol levels, which reduces plaque buildup (Ruscica et al., 2022). A plant-based diet that contains more healthy foods, such as whole grains, fruits, vegetables, nuts, legumes, oils, tea, and coffee, is linked to a decreased CVD risk (Table 2) (Hemler and Hu, 2019). The study examined only functional foods that contain bioactive ingredients with specific biological properties, which could have specific health benefits in CVD. Scientific evidence suggests that selected foods are a dense source of antioxidants, minerals, vitamins and other nutrients. These foods modulate lipoprotein metabolism and TAG, reducing CVD risk factors.
 Click to view | Table 2. - Interaction of Foods Bioactive Compounds with Cardiometabolic Biomarker |
A combination of Ginger, Garlic, Lemon, and Apple Cider Vinegar extract is used traditionally in India to treat cardiovascular disease. Despite small impacts on some blood parameters, herbal extracts (apple cider vinegar, honey, garlic, ginger and lemon) could have cardioprotective effects (Naseem et al., 2016). An additional study concluded that an apple cider vinegar mixture with ginger, garlic, lemon, and honey reduced hyperlipidemia, hypertension, and diabetes. However, the waist-to-hip ratio and BMI did not change significantly (Aslam et al., 2021). Besides these, we have examined a number of functional foods that may help maintain healthy lipid profiles to prevent cardiovascular disease (Table 3).
 Click to view | Table 3. Clinical trials on Cardiovascular Associated Disorder and Dietary Nutrients |
3.3.1. Apple
Apple consumption has been linked to a reduced risk of cardiovascular disease (Sesso et al., 2003). Apples contain flavonoids, anthocyanins, dihydrochalcones, quercetin, catechins, tannins, and dietary fiber, especially pectin. Researchers have observed that apples lower plasma cholesterol (Aprikian et al., 2001; Leontowicz et al., 2002). It has been also observed that gallic acid inhibits immunoproteasome activity, attenuating PTEN degradation and activating downstream signaling. It may represent a promising candidate for treating hypertensive atrial fibrillation (Han et al., 2020). It has been reported that chlorogenic acid modulates glucose-6-phosphatase activity and reduces the risk of cardiovascular disease (CVD) by decreasing the oxidation of LDL cholesterol and total cholesterol (Karthikesan et al., 2010; Cho et al., 2010; Naveed et al., 2018). Apple antioxidants reverse oxidative damage to nerve cells and reduce diabetes risk (Ajayi et al., 2020). A clinical trial examined the effects of daily Gala apples consumption over six weeks on obesity-associated inflammation, which escalates CVD risk without weight loss (Liddle et al., 2021a). An intervention study evaluated the effect of three whole Gala apples (200 grams) on the postprandial metabolic activity and caloric content of a high fat meal providing 1 g fat per kilogram of body weight when consumed immediately after a meal with 1 g fat. It was demonstrated that apples modulated postprandial plasma IFN-γ and reduced its peak concentration. Compared to unstimulated and LPS-stimulated PBMC, apples increased IL-4 in unstimulated PBMC and decreased GM-CSF and IL-17 in LPS-stimulated PBMC. These results suggest that acute whole apple consumption can mitigate postprandial inflammation associated with high fat meals in overweight and obese individuals (Liddle et al., 2021b).
3.3.2. Almond
Almond consumption significantly reduced diastolic blood pressure, total cholesterol, triacylglycerol, low-density lipoprotein, non-high-density lipoprotein (HDL), and very low-density lipoprotein (VLDL) (Morvaridzadeh et al., 2022; Ruisinger et al., 2015). Almonds prevent obesity, hyperlipidemia, hypertension and hyperglycemia. According to a study, almonds can decrease the risk of cardiovascular disease in patients with type 2 diabetes mellitus by reducing adiposity, glycemic control, and lipid profile (Li et al., 2011). Diabetes patients worry about postprandial glucose and insulin levels. There is evidence that almond consumption decreases insulin secretion in hyperlipidemia (Gayathri et al., 2023). Nut consumption may not increase postprandial glucose levels in diabetics (Nishi et al., 2023). In diabetic patients, almonds can improve glucose control by lowering the glycemic index of their diets and providing a rich source of oleic acid and tocopherols (Chen et al., 2017). Almonds in healthy diets can help diabetic patients control blood sugar levels to better glycemic control (Liu et al., 2013; Li et al., 2011). In a recent meta-analysis study, almond consumption reduced LDL-C in T2D patients, but had no positive effect on other cardiometabolic outcomes (Moosavian et al., 2022).
3.3.3. Avocado
Avocados have both fats and fibres in addition to various micronutrients and phytochemicals that provide health benefits. In two prospective cohort studies of men and women followed for 30 years, avocado consumption was inversely associated with CVD and CHD cases. Avocado consumers had a lower risk of CVD and CHD than nonconsumers, but no association with stroke (Pacheco et al., 2022). Avocado consumption was associated with an improved dietary pattern and trend toward improved glucose control and reduced cardiometabolic risk in free-living adults with insulin resistance and obesity when replacing avocado energy with carbohydrate energy (Zhang et al., 2022). According to a study, a diet consisting of one avocado a day for five weeks decreased plasma LDL cholesterol and small dense LDL particles significantly (Wang et al., 2015). A postprandial challenge in middle-aged, overweight/obese adults showed beneficial effects on glycemic and vascular markers when carbohydrate energy was replaced with avocado energy rich in monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), fiber, and bioactive phytochemicals (Park et al., 2018; Pacheco et al., 2022).
3.3.4. Cinnamon
There is some evidence that cinnamon supplementation has a small effect on patients with diabetes or impaired glucose tolerance; however, cinnamon supplementation decreased TAG and total cholesterol levels significantly (Maierean et al., 2017). Cinnamon significantly reduced blood glucose (FBG) and homeostatic model assessment for insulin resistance (HOMA-IR) levels. The weighted mean difference in glycosylated haemoglobin A1C (HbA1c) % and lipid profiles (mmol/L) did not change substantially (Deyno et al., 2019). An intervention with 3 grams of cinnamon for 16 weeks resulted in substantial improvement of all metabolic syndrome components in an Asian Indian sample from north India. The study showed significant reductions in glycemia, abdominal obesity, lipids, blood pressure, and the percentage of individuals with metabolic syndrome. This was after a single nutrient intervention of cinnamon. This included a significant decrease in glycemia, adiposity, lipids, and blood pressure (Gupta Jain et al., 2017). There are several molecular mechanisms involved in this phenomenon, including inhibition of carbohydrate metabolism enzymes (pancreatic amylase and glucosidase) and stimulation of cellular glucose uptake by membrane translocation of glucose transporter protein type-4 (GLUT-4). It also stimulates glucose metabolism and glycogen synthesis. It inhibits gluconeogenesis, stimulates insulin release and potentiates insulin receptor activity, and inhibits gluconeogenesis through key regulatory enzyme effects (Adisakwattana et al., 2011; Mohamed Sham Shihabudeen et al., 2011; Anand et al., 2010). Diabetes is an important risk factor for cardiovascular disease, and cinnamon improves insulin sensitivity and insulin secretion. It also regulates glucose-metabolizing enzyme activity; regulates liver, fat, and muscle glucose metabolism; ameliorates oxidative stress and inflammation; and improves diabetes complications (Shang et al., 2021).
3.3.5. Flaxseed
Flaxseed reduces cholesterol in healthy subjects with mild cardiovascular disease biomarkers. In peripheral artery disease patients, milled flaxseed reduces total and LDL cholesterol. When combined with cholesterol-lowering medications, it can decrease LDL cholesterol even further (Khandouzi et al., 2022; Edel et al., 2015; Hadi et al., 2020; Adegbola et al., 2022). Patients with metabolic syndrome and related disorders who were supplemented with flaxseed oil showed lower levels of IL-6 and MDA and higher levels of TAC (Tamtaji et al., 2020). The study suggests that women with postmenopausal lipid profiles may benefit from flaxseed supplementation by lowering total cholesterol, non-HDL cholesterol, and apo B, risk factors associated with coronary heart disease (Lucas et al., 2002). Participants in a randomized, double-blinded, controlled clinical trial consumed 30g of milled flaxseed/d for 6 months with peripheral arterial disease (75% hypertensive). Compared to the control group, the flaxseed group showed significant reductions in systolic (−10 mm Hg) and diastolic (−7 mm Hg) blood pressure. It may be that the linolenic acid in flaxseed inhibits soluble epoxide hydrolase, resulting in altered oxylipin concentrations that reduce hypertension in peripheral artery disease patients (Caligiuri et al., 2014). As a result of the Flax PAD clinical trial (Flaxseed for Peripheral Artery Disease) conducted over a year, dietary flaxseed significantly decreased brachial systolic and diastolic blood pressure. Oxylipins are implicated as mediators (Caligiuri et al., 2016). Flaxseed modestly reduces total and low-density lipoprotein cholesterol concentrations, reduces glucose absorption postprandially, decreases inflammation markers, and increases omega-3 fatty acid levels. Cardioprotective long-chain n-3 fatty acids are naturally derived from alpha-linolenic acid (Katare et al., 2012).
3.3.6. Garlic
There is evidence that aged garlic extract (AGE) with the active ingredient S-allylcysteine can reduce inflammation and prevent atherosclerosis as well as blood pressure, perfusion, and lipids in patients with intermediate CVD risk (Ried, 2020; Ahmadi et al., 2013). Garlic is believed to possess cardioprotective properties by lowering blood pressure and LDL. IL-6 is known to play a role in the inflammatory processes. Among females with low cardiac risk profiles, the study was designed to examine whether AGE could influence inflammatory biomarkers (IL-6 and CRP), lipid profile, and blood pressure. According to a study, AGE lowers IL-6 in females at risk of cardiovascular disease (Wlosinska et al., 2021). Garlic (Allium sativum) and other natural products from it have been shown to improve vascular endothelial and platelet function, both crucial to arteriosclerosis and cardiovascular disease (Gadidala et al., 2023). In addition to decreasing cytokine levels in endothelial cells, modifying adipocyte metabolic profiles, and activating anti-inflammatory genes, garlic extract may have anti-inflammatory effects. According to a study, garlic might be beneficial in selected patients due to the significant role inflammation and dyslipidemia play in heart disease mortality risk factors among hemodialysis patients (Asgharpour et al., 2021).
3.3.7. Ginger
Ginger significantly reduces triacylglycerols and LDL cholesterol, but not total cholesterol. Both triacylglycerols and total cholesterol seem to be lowered more effectively at doses lower than 2 g/day of dairy ginger powder (Pourmasoumi et al., 2018). A double-blind, placebo-controlled clinical trial enrolled 70 T2D patients, who were randomized to the ginger and control group. A dose of 1,600 mg of ginger was administered daily for 12 weeks compared to an equivalent dose of wheat flour placebo. Serum sugar, lipids, CRP, PGE2 and TNFα were measured before and after the intervention. Ginger significantly reduced fasting blood glucose, HbA1C, insulin, HOMA, triacylglycerols, total cholesterol, CRP and PGE2 compared to placebo. In type 2 diabetic patients, ginger improved insulin sensitivity and some lipid fractions, as well as reduced CRP and PGE2 (Arablou et al., 2014). A number of cellular processes and signaling pathways associated with cardiovascular diseases are modified by ginger (Roudsari et al., 2021). A dose of 1.8 grams of ginger daily showed significant reductions in triacylglycerols, total cholesterol, and LDL cholesterol in obese metformin patients (El Gayar et al., 2019). A study by Verma and Bisen, (2022) found that ginger inhibited reactive oxygen species, inducible nitric oxide synthase, superoxide dismutase, glutathione, heme oxygenase, and inducible nitric oxide synthase. Studies have shown that ginger has positive effects on cardiovascular disease, including preclinical and clinical studies. In addition to its powerful antioxidant and anti-inflammatory properties, ginger inhibits VSMC proliferation, blocks voltage-dependent Ca2+ channels, inhibits platelet aggregation, enhances cholesterol efflux from macrophages, inhibits angiogenesis, and promotes autophagy (Verma and Bisen, 2022; Li et al., 2021; Enayati et al., 2021). Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide. Inflammation and oxidative stress play critical roles in the progression of various CVD types. A number of studies have confirmed ginger’s anti-inflammatory and antioxidant properties, consistent with its rich phenolic content. Based on their broad pharmacological properties, ginger and its bioactive components might be an effective clinical choice. Ginger modifies many cellular processes and in particular was shown to have potent inhibitory effects against nuclear factor kappa B (NF-κB); signal transducer and activator of transcription; NOD-, LRR-, and pyrin domain-containing proteins; toll-like receptors; mitogen-activated protein kinase; and mammalian target of rapamycin signaling pathways. A study concluded that GSE alters the lipoprotein profile by increasing fecal cholesterol excretion and inhibiting intestinal NPC1L1, ACAT2, and MTP mRNA expression (Lei et al., 2014).
3.3.8. Green tea
Epidemiological studies show that green tea consumption protects against hyperlipidemia. There is considerable evidence that green tea can delay the onset or progression of numerous ailments, including cardiovascular disorders, metabolic disorders and hypertension (Santos and Sinha, 2021; Lange et al., 2022). A meta-analysis of 31 trials with 3,321 subjects revealed that green tea intake significantly lowered total cholesterol (TC). Despite reduced triacylglycerols, green tea consumption did not affect high-density lipoprotein (HDL) cholesterol (Xu et al., 2020). Studies have demonstrated green tea’s efficacy in preventing obesity and cardiovascular diseases related to lipid reduction (Zheng et al., 2011). It is believed that polyphenols, which are the most abundant components of green tea, may have antioxidant effects on the cardiovascular system. They regulate intermediary outcomes like blood pressure, body fat, lipids, and improve glycemic control, all of which may contribute to overall health (Zheng et al., 2011; Dinh et al., 2019; van Dam et al., 2013; Neyestani and Nikooyeh, 2022; Landini et al., 2021).
3.4. Reversal of coronary arteries diseases
Researchers examined the relationship between vessel wall shear stress and the progression of atherosclerosis. Low shear stress significantly increased progression (Tziotziou, et al., 2023). Reverse cholesterol transport removes excess cholesterol from peripheral tissues and is delivered to the liver. Cholesterol will be redistributed to other tissues or removed from the body by the gallbladder. HDL-C plays an essential role (Figure 3). Muralidharan et al., (2022) analyzed baseline data from a case-control study within the PREDIMED study and found several HDL structural and functional parameters were related to blood cell membrane composition. A protein Apo A-1 (70 percent of HDL-C’s protein content) is synthesized in the intestines and liver, which makes its way into the bloodstream and out to peripheral tissues (e.g., the heart). Blood vessel walls have receptors called ATP-Binding Cassette, Sub-Family A (ABC1), Member 1 (ABCA1) that interact with Apo A-1 in veins and arteries (Chapman et al., 2010; Yu et al., 2019; Juhl and Wüstner, 2022). It has not been conclusively proven that bioactive compounds increase HDL-C. In the absence of strong evidence, supplementing these compounds alone or using functional foods does not improve HDL-C levels. The combination of diet and physical exercise increases HDL-c not only due to one factor but also both factors (Marques et al., 2018).
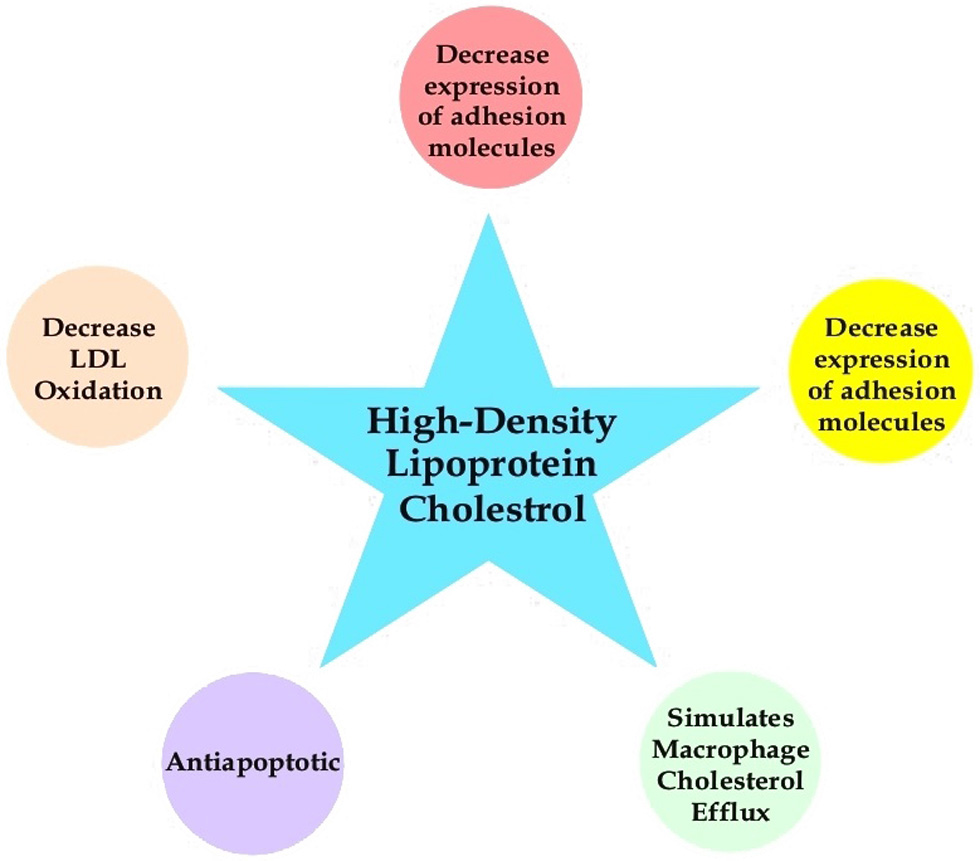 Click for large image | Figure 3. Role of High Density Lipoprotein in cardiometabolic diseases. |
An association has been found between HDL levels circulating in the blood and muscle mitochondrial function. There has been evidence that mitochondrial ATP synthase is a high affinity receptor for apoA-I, which is a major component of high density lipoproteins (HDL). In reverse cholesterol transport and vascular endothelial protection, the F1-ATPase pathway/s may play a significant role and its regulation might represent a potential therapeutic target for HDL-related therapies for cardiovascular disease (Giacona et al., 2022; Vantourout et al., 2010). According to another study, HDL-related biomarkers like circulating serum inhibitory factor 1 (IF1) are strongly and independently associated with mortality in CAD patients (Genoux et al., 2016). Dietary supplements may prevent and reverse vascular diseases by forming micelles from bile salts containing cholesterol (Houston, 2022). The focus of pathology is serum cholesterol and LDL-C in relation to cardiovascular disease (Figure 4). Stellaard (2022) found that dietary methods that reduce dietary cholesterol absorption and intake are effective in reducing serum cholesterol levels (Stellaard, 2022). They also neutralise radicals with antioxidants, and reduce artery plaques with fibrinolytic activities (Kaliora et al., 2006). Coagulation breaks down fibrin clots. The plasmin enzyme cuts the fibrin mesh at different points, resulting in fragments removed by the kidneys and liver. Plasma HDL binds to peripheral cholesterol molecules and transports them to the liver, lowering plasma cholesterol levels. Exercise and a balanced diet increase HDL levels (Man et al., 2020).
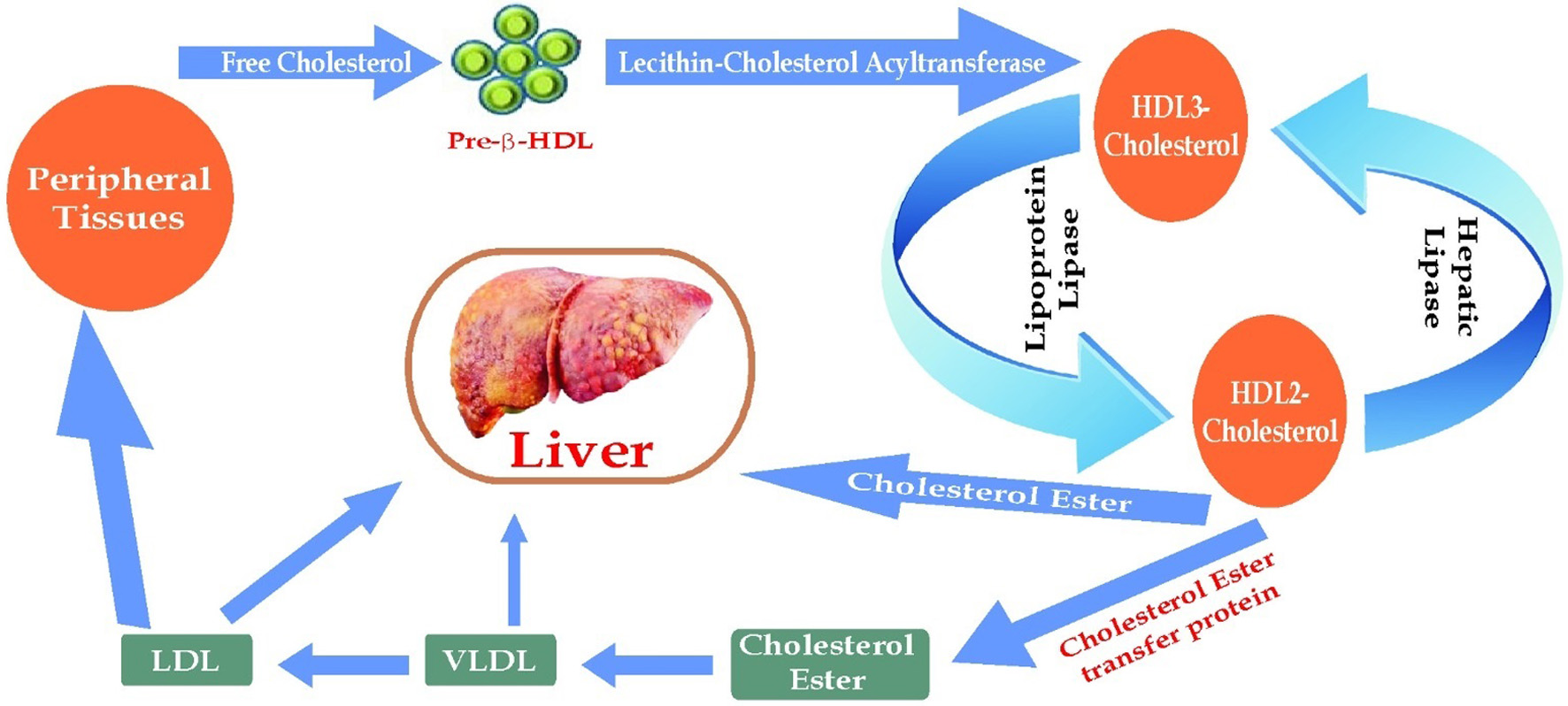 Click for large image | Figure 4. Reversal Mechanism of Cardiometabolic Disease. |
| 4. Conclusion | ▴Top |
There have been significant advancements in the diagnosis and management of acute coronary syndromes. CVD and complications risk increases in individuals with elevated plasma cholesterol levels. In many studies, it has been established that controlling plasma cholesterol through diet and medication slows and even reverses the progression of cardiometabolic disorders and their complications. It has been shown that functional ingredients in apples, almonds, avocado, cinnamon bark, flax seeds, garlic, ginger, and green tea can treat cardiovascular diseases caused by lipids. The potential of these foods to treat cardiometabolic biomarkers is supported by scientific research. Eating a healthy diet, changing lifestyle reduces the biomarker of lipid oriented cardiometabolic diseases. It is necessary to conduct further scientific investigation to better understand the mechanisms that interact between food ingredients and the cardiometabolic system.
| 5. Future perspectives and challenges | ▴Top |
The relationship between nutrition and biomarkers has been the subject of significant research, but the mechanisms underlying these interactions are unclear. A lot remains to be understood about how specific dietary components contribute to cardiovascular disease and how their interactions affect health outcomes. Further research is needed to elucidate these mechanisms. In cardiometabolic biomarker studies, the methods and techniques are not standardized. Study results may vary depending on the way data is collected, sequenced, and analyzed. To ensure consistency and reliability in cardiometabolic research, standard protocols and approaches must be developed. The majority of studies that examine the relationship between diet and metabolism are limited in scope and are often limited to a specific population. It is necessary to conduct controlled trials over extended periods of time in order to assess the effectiveness of dietary interventions over the long-term.
Acknowledgments
None.
The authors did not receive support from any organization for the submitted work. The authors have no relevant financial or non-financial interests to disclose.
Conflict of interest
The authors report there are no competing interests to declare
All authors truly contributed to the development of this study. RV participated in study concept and design, acquisition of data, interpretation of data and critical revision of the manuscript for important intellectual content. PSB and MB participated in drafting the manuscript, and critical revision of the manuscript.
| References | ▴Top |