| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 26, June 2024, pages 52-60
Potential of clove, cinnamon and honeysuckle in suppressing the SARS-CoV-2 spike protein-ACE2 binding, inhibiting ACE2 activity, and scavenging radicals
Huan Wua, b, Nora Baustiana, Yanfang Lia, Ethan Leea, Yinghua Luoc, Margaret Slavina, *, Liangli (Lucy) Yua
aDepartment of Nutrition and Food Science, University of Maryland, College Park, Maryland 20742, United States
bKey Laboratory of Xin’an Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei 230038, China
cCollege of Food Science and Nutritional Engineering, National Engineering Research Center for Fruits and Vegetables Processing, Key Laboratory of Storage and Processing of Fruits and Vegetables, Ministry of Agriculture, Engineering Research Centre for Fruits and Vegetables Processing, Ministry of Education, China Agricultural University, Beijing 100083, China
*Corresponding author: Margaret Slavin, Department of Nutrition and Food Science, University of Maryland, 0112 Skinner Building, College Park, Maryland 20742, United States. Tel: 301-405-4533; E-mail: mms@umd.edu
DOI: 10.31665/JFB.2024.18380
Received: April 12, 2024
Revised received & accepted: June 16, 2024
| Abstract | ▴Top |
Water and ethanol extracts of clove (Syzygium aromaticum (L.) Merr. & L.), cinnamon (Cinnamomum verum) and honeysuckle (Lonicerae japonicae) were examined for their chemical compositions, inhibition of SARS-CoV-2 spike protein-ACE2 binding, ACE2 inhibition, and free radical scavenging activity. UHPLC-MS/MS analysis tentatively identified 34, 36, and 27 compounds in clove, honeysuckle, and cinnamon water (WE) and ethanol extracts (EE), respectively. The extracts dose-dependently suppressed SARS-CoV-2 spike protein-ACE2 binding activity and ACE2 activity. Clove and cinnamon WEs at 33.3 mg dry botanical equivalents (BE)/mL, and cinnamon and honeysuckle EEs at 3.3 mg BE/mL showed almost 100% inhibition of S-protein-ACE2 binding activity. TPC values ranged from 5.3 mg GAE/g (honeysuckle EE) to 180.0 mg GAE/g (clove EE). The highest HO•, DPPH•, ABTS•+ scavenging activity were 2,181 (clove EE), 536.91 (clove WE), and 3,525.1 (clove WE) µmol TE/g respectively. These data suggested the potential of these botanicals in reducing the risk of SARS-CoV-2 infection and COVID-19 severe symptom development.
Keywords: Clove (Syzygium aromaticum (L.) Merr. & L.); Cinnamon (Cinnamomum verum); Honeysuckle (Lonicerae japonicae); COVID-19; SARS-CoV-2 spike protein; angiotensin-converting enzyme 2 (ACE2)
| 1. Introduction | ▴Top |
Coronavirus disease 19 (COVID-19) is a human disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus infection. By April 2024, SARS-CoV-2 has infected over 775 million people and has caused over 7 million deaths according to the World Health Organization (WHO, 2024). Vaccines have been developed to protect against the virus infection and the development of severe COVID-19 symptoms and death, but they do not completely prevent infection, especially for newly emerging variants (Ciotti et al., 2022). To date, 67% of the total population has received a complete primary series of a COVID-19 vaccine, with 32% global population having at least one booster dose of a COVID-19 vaccine. It was also noted that vaccines are not widely available in some parts of the world. Thus, alternative preventive or treatment methods are still attractive for reducing the spread of SARS-CoV-2 infection and its associated serious illness or death.
When the SARS-CoV-2 infection rapidly spread throughout the world, great efforts were put into identifying the mechanism of pathogenesis for the resulting COVID-19. SARS-CoV-2, a member of the Coronaviridae family, has four structural proteins: the nucleocapsid, membrane, envelope proteins, and the spike (S) protein, which has been identified as the key for its entry into the host cells (Satarker and Nampoothiri, 2020). The SARS-CoV-2 S protein is divided into two subsections called the S1 and S2 sites. The S1 subunit, which represents the “tip” of the S protein, binds to its major receptor angiotensin-converting enzyme 2 (ACE2) (Lamers and Haagmans, 2022). The S2 subunit, which makes up the “shaft” of the S protein, facilitates the fusion of the viral and host cell membranes (Huang et al., 2020). ACE2 is a common enzyme that is found in several organs, but is primarily found in the lungs, kidney, and intestines (Kuba et al., 2021). While this seems to be the consistent mechanism for entry into host cells, the immune system response seems to be what differentiates severe COVID-19 from mild and moderate cases. In severe cases, the immune system overproduces cytokines, which are glycoproteins responsible for controlling inflammation in the body (Figure 1). In severe cases, overproduction of cytokines, called cytokine release syndrome, becomes inflammatory and can lead to potential tissue and cell damage. Major pro-inflammatory cytokines involved in this process may include tumor necrosis factor (TNF-α), interleukin 6 (IL-6), interleukin 1β (IL-1β) and interleukin 17 (IL-17) (Darif et al., 2021). These cytokines are intertwined in that an overproduction of one causes an overproduction of others. The production of pro-inflammatory cytokines has been shown to be responsible for the generation of radical oxygen species (ROS) (Borys et al., 2019). An imbalance between ROS and the host’s ability to detoxify these free radicals can lead to oxidative stress, which if left uncontrolled can induce several diseases such as cancers and cardiovascular disease, as well as accelerate the development of severe COVID-19 symptoms which may cause death (Pizzino et al., 2017).
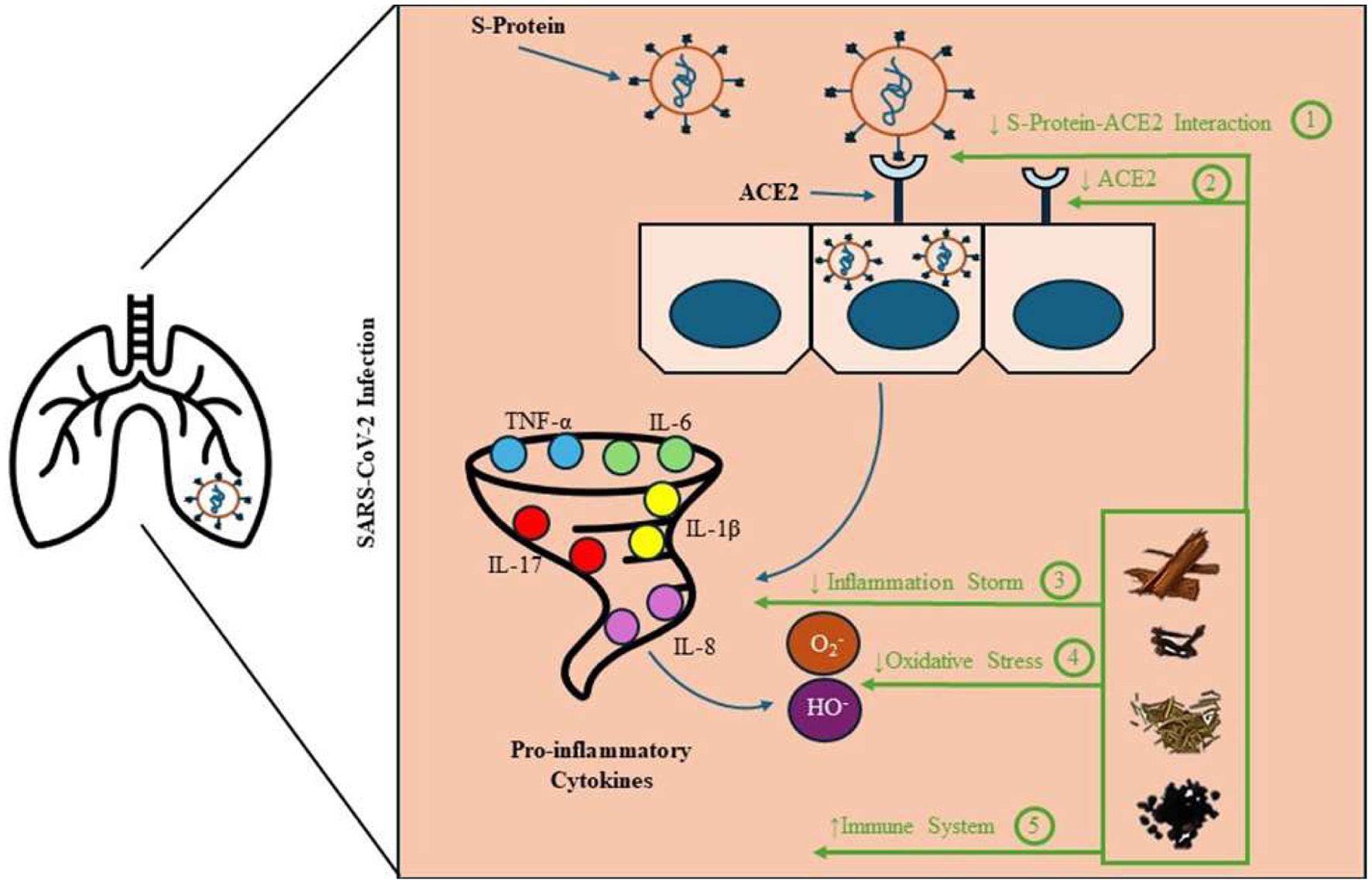 Click for large image | Figure 1. Edible botanical approaches to reducing the risk of COVID-19. Several biological mechanisms of COVID-19 infection and symptom exacerbation may be amenable to modification by edible botanicals, including: (1) prevention of SARS-CoV-2 and ACE2 binding, (2) ACE2 inhibition, (3) modulation of inflammatory response by phenolic compounds, (4) reduction of oxidative stress through free radical scavenging, and (5) overall support of appropriate immune function. |
It could be hypothesized that plant extracts, which are known to have free radical scavenging compounds, may be able to help mitigate the severe symptoms and mortality risk of COVID-19. Plant extracts also contain high levels of phenolic compounds, which have been shown to be potentially effective in supporting numerous immune functions such as modulation of macrophages, increasing the proliferation of B & T cells, and modulation of inflammatory responses (Shakoor et al., 2021). Additionally, many phenolic compounds have been shown to disrupt spike-mediated receptor-binding by binding to various portions of spike protein (Goc et al., 2021). Clove, cinnamon and honeysuckle were investigated for their potential to inhibit ACE2 activity and the binding between SARS-CoV-2 spike protein and ACE2, as well as their total phenolic contents, and scavenging capacities against HO•, DPPH• and ABTS•+ radicals. These botanicals were selected due to their potential to modulate immune responses through their anti-inflammatory and anti-virus activities, and for their traditional use in anti-inflammatory treatments. The research results can be used as the scientific foundation for the potential utilization of clove, cinnamon and honeysuckle in preventing SARS-CoV-2 infection and alleviating COVID-19 disease risk.
| 2. Materials and methods | ▴Top |
2.1. Chemical reagents and materials
Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), gallic acid, fluorescein, 2,2-diphenyl-1-picrylhydrazyl (DPPH radical), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt (ABTS), Folin & Ciocalteu’s phenol detection reagent (FC reagent), hydrogen peroxide (H2O2), and ferric chloride (FeCl3) were obtained from Sigma-Aldrich (St. Louis, MO, USA). LC-MS grade acetonitrile and formic acid were purchased from Merck (Darmstadt, Germany). The SARS-CoV-2 Spike-ACE2 interaction screening assay kit (Catalog Number 502050) and the ACE2 inhibitor screening assay kit (Catalog Number 502100) were purchased from the Cayman Chemical Company (Ann Arbor, MI, USA). Commercial dry clove (Syzygium aromaticum (L.) Merr. & L.), cinnamon (Cinnamomum verum) and honeysuckle (Lonicerae japonicae) samples were obtained from Frontier Co-op (Norway, IA, USA).
2.2. Sample extraction
One gram of clove or a different botanical sample, ground to pass through a 40-mesh sieve, was extracted with 10 mL ultrapure water in a water bath at 85 °C for 2 h, followed by an additional 22 h at ambient temperature; or extracted with 10 mL of 100% ethanol at ambient temperature for 24 h. After extraction, the mixture was centrifuged, and the supernatant was collected and stored at −20 °C until further analysis. Triplicate extraction was performed for each botanical sample.
2.3. Identification of chemical compounds
An ultra-high-performance liquid chromatography (UHPLC) paired with an Orbitrap Fusion ID-X Tribrid mass spectrometer was utilized to analyze the chemical components in the water and ethanol extracts of clove, cinnamon and honeysuckle (Li et al, 2022). The compound identification was accomplished based on their molecular weights, mass fragmentation patterns, data from the literature, and/or references to natural product databases including SciFinder and PubChem.
2.4. Inhibition on SARS-CoV-2 S-protein and ACE2 interaction
The inhibition of the binding between the SARS-CoV-2 S protein and ACE2 was measured using a SARS-CoV-2 Spike-ACE2 Interaction Inhibitor Screening Assay Kit described in a previous publication by our group (Li et al., 2022). Following the manufacturer’s instructions, solutions at various botanical concentrations were prepared and incubated with reagents at ambient temperature with shaking for one hour. The absorbance was measured at a wavelength of 450 nm. The level of inhibition was determined using the following equation:
2.5. ACE2 Activity inhibition
The inhibition on ACE2 activity was measured using an ACE2 Inhibitor Screening Assay Kit also described in a previous publication by our group (Li et al., 2022). Following the manufacturer’s instructions, solutions at various botanical concentrations were prepared and incubated with reagents in the dark at room temperature for 0.5 hours. The absorbance was measured at an excitation wavelength of 320 nm and an emission wavelength of 405 nm. The level of inhibition was calculated as:
2.6. Total phenolic contents (TPC)
The TPC of the water and ethanol extracts of each botanical sample were examined according to the laboratory protocol described previously (Yu et al., 2002). Briefly, 50 μL of the standard (gallic acid) solution or botanical WE/EE extract were mixed with 250 μL of FC reagent, followed by the addition of 3 mL of ultrapure water and 750 μL of a 20% Na2CO3 solution. The mixture was kept in the dark at ambient temperature for 2 h. The absorbance was measured at the wavelength of 765 nm. The results were expressed as milligram of gallic acid equivalents per gram of the dry botanical (mg GAE/g).
2.7. Radical scavenging capacity
Relative hydroxy (HO), DPPH and ABTS+ radical scavenging capacities of the botanical WE/EE were evaluated in accordance with established protocols previously published by our group (Moore et al., 2006; Cheng et al., 2006; Zhou et al., 2004; Zhang et al., 2012). Briefly, for the HO• scavenging capacity assay, 170 µL of 9.28 × 10−8 M fluorescein was mixed with 30 µL of a botanical extract or standard (Trolox), then 40 µL of 0.199 M H2O2 and 60 µL of 3.43 mM FeCl3 were added into the mixture. Fluorescence intensity of the mixture was recorded every five minutes for 8 h with an excitation wavelength of 485 nm and an emission wavelength of 535 nm. For the DPPH• scavenging capacity assay, 100 μL of a botanical extract or standard (Trolox) was mixed with 100 μL of freshly prepared 0.2 mM DPPH solution. The absorbance of the reaction mixture was determined every minute over a course of 90 min at 515 nm wavelength. For the ABTS•+ scavenging capacity assay, 160 μL a botanical extract or standard (Trolox) was mixed with 2 mL of freshly prepared ABTS•+ working solution to initiate the reaction. The absorbance of the mixture was recorded at 734 nm after 90 seconds. The scavenging capacities against HO•, DPPH• and ABTS•+ were reported in µmol of Trolox equivalents per gram of dry botanical (µmol TE/g).
2.8. Statistical analyses
The experiments were conducted in triplicate. All the results were reported as mean ± standard deviation (SD). One-way ANOVA was conducted to identify the differences between extraction solvents and extract concentrations (p < 0.05) using SPSS (Version 17.0, SPSS, Inc).
| 3. Results and discussion | ▴Top |
3.1. Chemical compositions in the water and ethanol extracts of clove (Syzygium aromaticum (L.) Merr. & L.), cinnamon (Cinnamomum verum) and honeysuckle (Lonicerae japonicae)
According to the high-resolution full MS scan, MS2 and the published literature, thirty-four compounds were tentatively identified in the water extract (WE) and ethanol extract (EE) of clove, with six compounds reported in the clove for the first time (Li et al., 2022). The six new compounds were myricetin-C-glucoside isomer I, myricetin-C-glucoside isomer II, rhamnocitrin 3-O-sulfate, rhamnazin 3-O-sulphate, rhamnocitrin 3-O-glucuronide, and rhamnocitrin 3-O-glucoside, respectively (Li et al., 2022). Using the same approaches, 27 and 23 compounds were tentatively identified in the cinnamon WE and EE, respectively (Xie et al., 2023). Seven compounds were reported in cinnamon extracts for the first time, including saccharumoside C, two emodin-glucuronide isomers, and two physcion-glucuronide isomers in the cinnamon water extract, as well as two type-A proanthocyanidin hexamers in both the water and ethanol extracts (Xie et al., 2023). In addition, a total of 36 compounds were tentatively identified in the honeysuckle extracts, with 27 in its water extract and 29 in its ethanol extract. Among them, 10 compounds including gardenoside, monotropein, oleoside, capparoside A, colossolactone E, salvigenin, afromosin, atractylon, zerumbone, and erucic acid were reported in the honeysuckle extracts for the first time (Gao et al., 2023). Sesamoside, loganic acid, secologanic acid, 4-caffeoylquinic acid and kaempferol were the five compounds with the higher ion intensity in the water extract, whereas luteolin, colossolactone E, salvigenin, atractylon and zerumbone had greater ion intensity in the ethanol extract. Capparoside A and 3-caffeoylquinic acid were the two major compounds in both honeysuckle water and ethanol extracts (Gao et al., 2023).
To further explain how compounds were identified, we provide an example of how an unknown compound in clove was identified. For the compound identified as compound 29 in the original manuscript (Li et al, 2022), the detected molecular ions in the negative ([M–H]−) mode was m/z 285.0383 (Figure 2). It was matched with elemental composition of C15H9O6 based on the elemental composition function of Thermo Xcalibur Qual Browser. The result indicated that the molecular formula of this compound was C15H10O6. The predominant fragment ions detected in the negative mode were at m/z 257.0437, 229.0488, 213.0540, and 185.0581, which account for [M–H–CO]–, [M–H–2CO]–, [M–H–2CO–O]– and [M–H–2CO–CO2]–. The fragment ion characterization is similar to that of kaempferol reported by March and Miao (2004). Therefore, compound 29 in clove was tentatively identified as kaempferol. Interestingly, kaempferol is found in both clove and honeysuckle (Li et al, 2022; Gao et al, 2023).
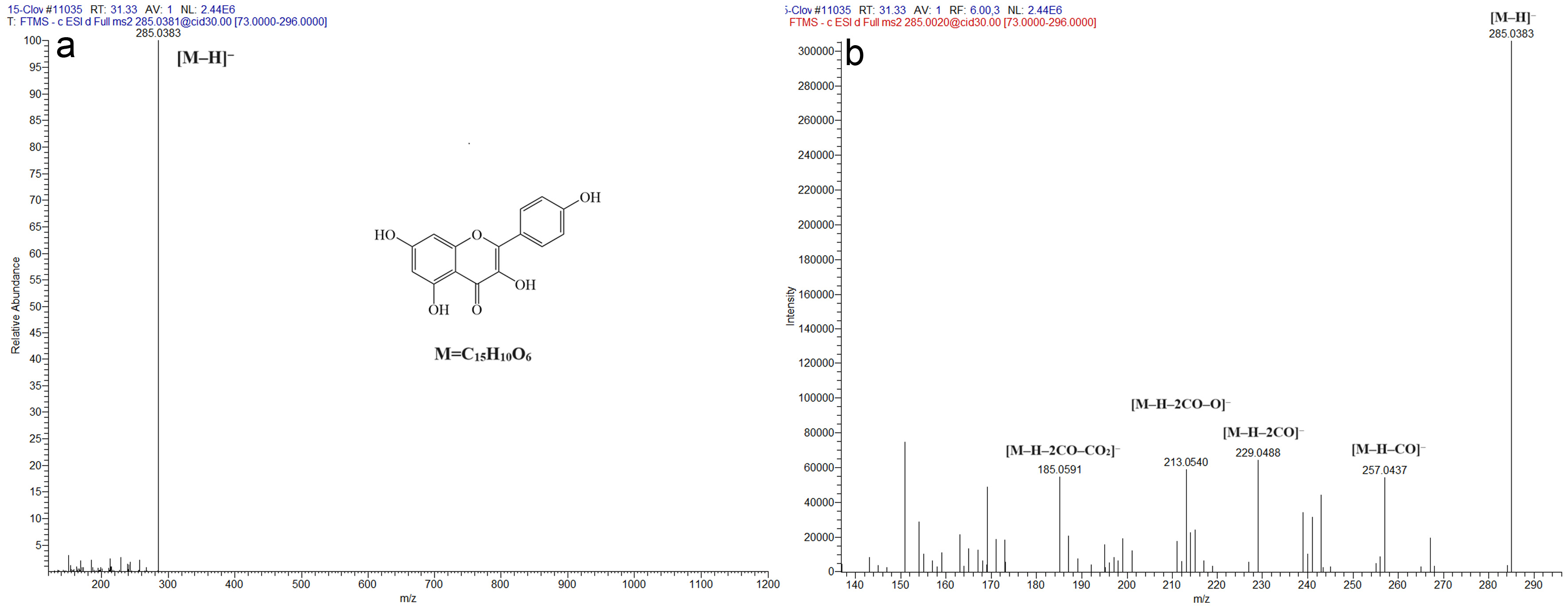 Click for large image | Figure 2. Identification of kaempferol (C15H10O6) in clove extract. (a) Full scan MS and (b) MS2 in negative ion mode. |
3.2. Inhibitory effects on SARS-CoV-2 spike protein binding to ACE2
The interaction between the SARS-CoV-2 spike protein (S-protein) and ACE2 on the surface of host cells initiates the virus entry into the host cells. The water extract (WE) and ethanol extract (EE) of clove (Syzygium aromaticum (L.) Merr. & L.), cinnamon (Cinnamomum verum) and honeysuckle (Lonicerae japonicae) dose-dependently suppressed the binding between the S-protein and ACE2 under the experimental conditions (Figure 3). The clove and cinnamon WEs at 33.3 mg dry botanical equivalents/mL concentration had almost 100% inhibition of the S-protein and ACE2 binding, whereas the cinnamon and honeysuckle EEs at the concentration of 3.3 mg dry botanical equivalents/mL had almost 100% inhibition of the S-protein and ACE2 binding (Figure 3). In addition, the WE and EE of a same botanical sample may differ in their ability to inhibit S-protein and ACE2 binding. The WE of clove and cinnamon had greater inhibitory effect against S-protein binding to ACE2 than the WE of honeysuckle on a per same botanical weight concentration basis, but EE of cinnamon and honeysuckle has stronger inhibition than that of clove EE (Figure 3). The results suggested the potential of these three botanicals for reducing the risk of SARS-CoV-2 infection and COVID-19 disease.
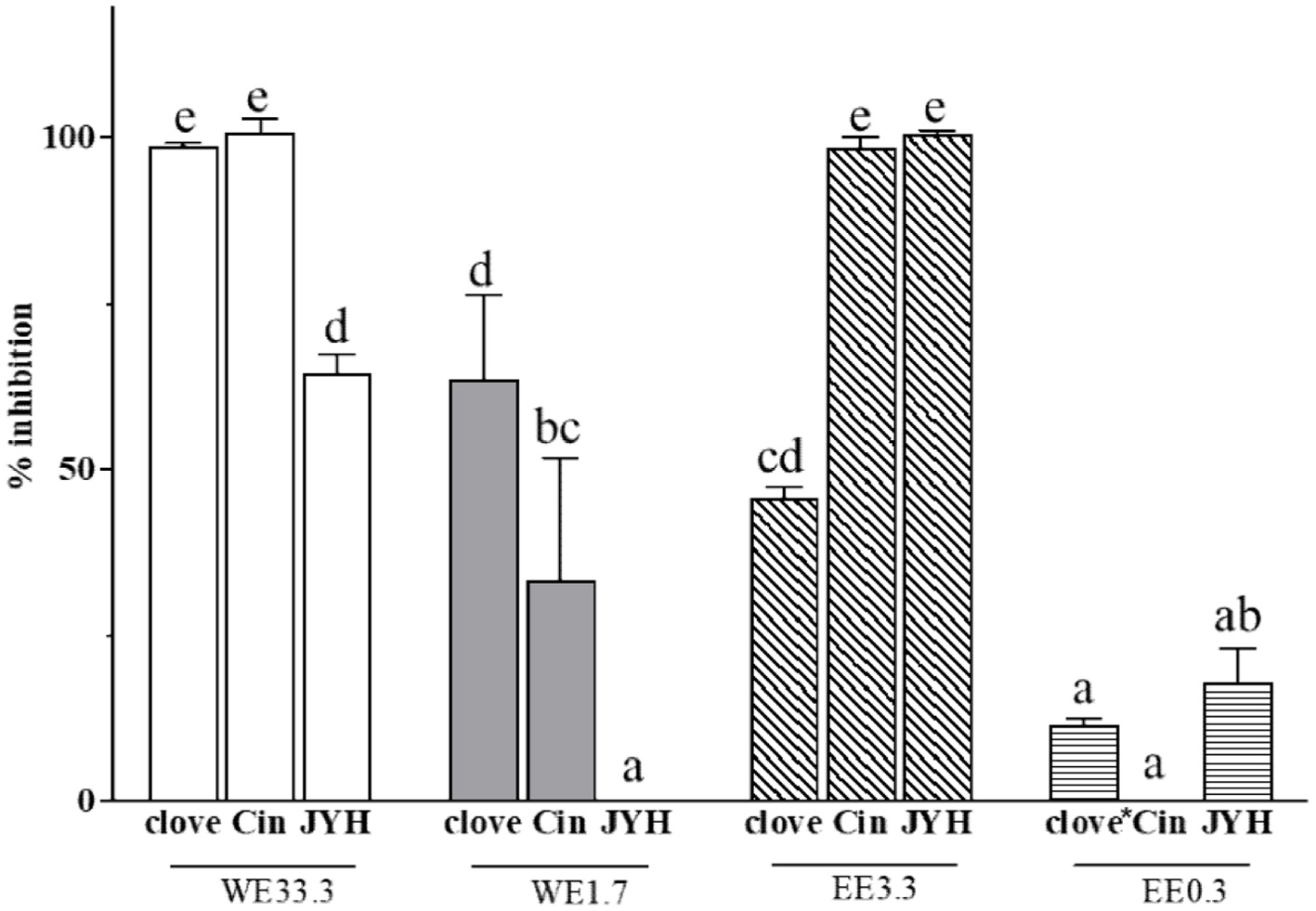 Click for large image | Figure 3. Inhibitory effects of clove, cinnamon (Cin) and honeysuckle (JYH) water (WE) and ethanol extracts (EE) at different concentrations on the binding interaction between SARS-CoV-2 spike protein and ACE2. WE33.3 and WE1.7 stand for the initial water extract concentrations of 33.3 and 1.7 mg dry botanical equivalents/mL in the testing mixture, respectively; whereas EE3.3 and EE0.3 stand for the initial ethanol extract concentrations of 3.3 and 0.3 mg dry botanical equivalents/mL in the testing mixture, respectively. Values are mean ± standard deviation (SD) of triplicate experiments. *clove was tested at 0.8 mg dry botanical equivalents/mL in the testing mixture. Different letters indicate significant difference (p < 0.05) by ANOVA. This figure was prepared using the data published previously (Li et al., 2022; Xie et al., 2023; Gao et al., 2023). |
Recently, the WE and EE of thyme (Thymus vulgaris), EE of parsley (Petroselinum crispum), WE of rosemary (Rosmarinus officinalis L.), and WE and EE of dill (Anethum graveolens L.) were found to dose-dependently suppress the binding between the S-protein and ACE2 (Yao et al., 2023a; Lee et al., 2023; Yao et al., 2023b; Choe et al., 2023). Rosemary WE at 33.3 and 1.7 mg botanical equivalents (BE)/mL concentrations had a 73 and 3% inhibition, respectively, while dill WE at 33.3 mg BE/mL had 66% inhibition (Yao et al., 2023b; Choe et al., 2023). In addition, the WE and EE of Huangqin (Scutellaria baicalensis Georgi.), a Chinese medicinal herb, showed dose-dependent inhibition on S-protein binding to ACE2 under the experimental conditions (Gao et al., 2024). The Huangqin WE at the concentration of 33.3 mg BE/mL resulted in a 76.9% inhibition 66% inhibition, and its EE had a 34.7% inhibition under the same experimental conditions (Gao et al., 2024). The observations from these studies warrant further investigations of these botanicals in their potential use reducing the risk of COVID-19 using cell and in vivo models.
| 3.3. ACE2 inhibition | ▴Top |
Inhibition of ACE2 activity may reduce the ability of SARS-CoV-2 to enter cells. Both water (WE) and ethanol (EE) extracts of clove were able to dose-dependently inhibit ACE2 activity under the experimental conditions, suggesting their potential in reducing the ACE2 availability (Figure 4). The clove WE at 5.0 and 0.5 mg BE/mL inhibited 100 and 82% of ACE2 activities, respectively (Li et al., 2022). The clove EE at 5.0 mg BE/mL concentration resulted in 92% ACE2 activity inhibition and 0.5 mg BE/mL did not have any inhibition (Figure 4) (Li et al., 2022), suggesting the clove WE had a stronger ACE2 inhibition at a same botanical weight concentration basis. Both WE and EE of cinnamon and honeysuckle were also able to dose-dependently inhibit ACE2 activity (Figure 4). Under the same experimental conditions, WE of clove showed a significantly greater ACE2 inhibitory effect as compared to honeysuckle WE (Figure 4) (Gao et al., 2023; Xie et al., 2023). WE of cinnamon had no difference in its ACE2 inhibitory effect to cinnamon EE, but WE of honeysuckle had a stronger ACE2 inhibition than its EE (Figure 4). Taken together, clove, cinnamon and honeysuckle may have potential in reducing the ACE2 availability, and consequently be used in developing nutraceuticals and functional foods for reducing the risk of SARS-CoV-2 infection.
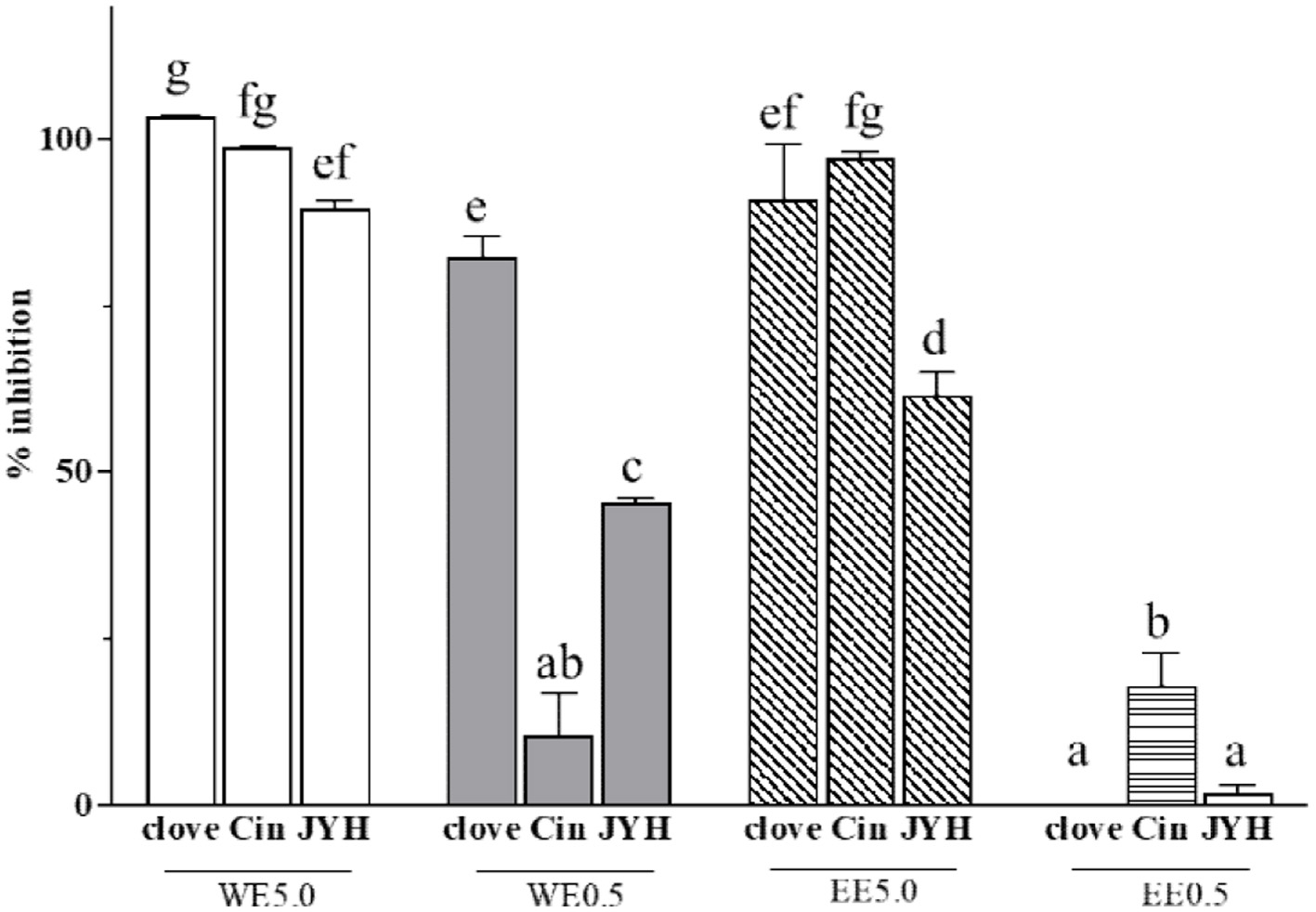 Click for large image | Figure 4. Inhibitory effects of clove, cinnamon (Cin) and honeysuckle (JYH) water (WE) and ethanol extracts (EE) at different concentrations on ACE2 activity. WE5.0 and WE0.5 stand for the water extract concentrations of 5.0 and 0.5 mg dry botanical equivalents/mL in the testing mixture, respectively; and EE5.0 and EE0.5 stand for the ethanol extract concentrations of 5.0 and 0.5 mg dry botanical equivalents/mL in the testing mixture, respectively. Values are mean ± standard deviation (SD) of triplicate experiments. Different letters indicate significant difference (p < 0.05) by ANOVA. This figure was prepared using the data published previously (Li et al., 2022; Xie et al., 2023; Gao et al., 2023). |
The WE and EE of thyme (Thymus vulgaris), EE of parsley (Petroselinum crispum), WE of rosemary (Rosmarinus officinalis L.), and WE and EE of dill (Anethum graveolens L.) were also found to dose-dependently inhibit ACE2 activity (Yao et al., 2023a; Lee et al., 2023; Yao et al., 2023b; Choe et al., 2023). The WE and EE of Huangqin (Scutellaria baicalensis Georgi.) was also able to dose-dependently inhibit ACE2 activity (Gao et al., 2024). These observations support the use of edible botanicals in prevention of COVID-19.
3.4. Total phenolic content (TPC) and free radical scavenging activities
Phenolics are known antioxidant and anti-inflammatory components in edible botanicals, and may contribute to their overall ability in reducing the risk of SARS-CoV-2 infection and COVID-19 disease. WEs and EEs of clove, cinnamon and honeysuckle contained significant levels of phenolic components measured as TPC (Figure 5a). The greatest TPC of 180.0 mg GAE/g was observed in the clove EE, and the lowest TPC of 5.3 mg GAE/g was detected in the EE of honeysuckle. GAE stands for gallic acid equivalents, and gallic acid was used as a phenolic standard for TPC values in all three studies (Li et al., 2022; Xie et al., 2023; Gao et al., 2023). These TPC values of herbs and spieces are much greater than that observed in grains and pulses, for example 487.9−927.8 μg GAE/g (0.5−0.9 mg GAE/g) for three winter wheat grains (Yu et al., 2002), 2.2−2.9 mg GAE/g in the 7 wheat bran samples (Zhou et al., 2004), and that of 0.8−2.2 mg GAE/g for 13 soybean samples (Zhang et al., 2012). The TPC values for clove, cinnamon and honeysuckle are aligned with values observed in other herbs and spices, although differences in solvent and extraction method can hinder direct comparisons across publications. (Ulewicz-Magulska and Wesolowski, 2019). The WEs of clove and honeysuckle had greater TPC values than their counterpart EEs, whereas the EE of cinnamon had a higher TPC value than its WE, suggesting the phenolic compounds in these botanicals differ in their structures and polarity. Furthermore, the EE of a selected botanical with a high TPC value does not warrant its WE to have a high TPC value (Figure 5a).
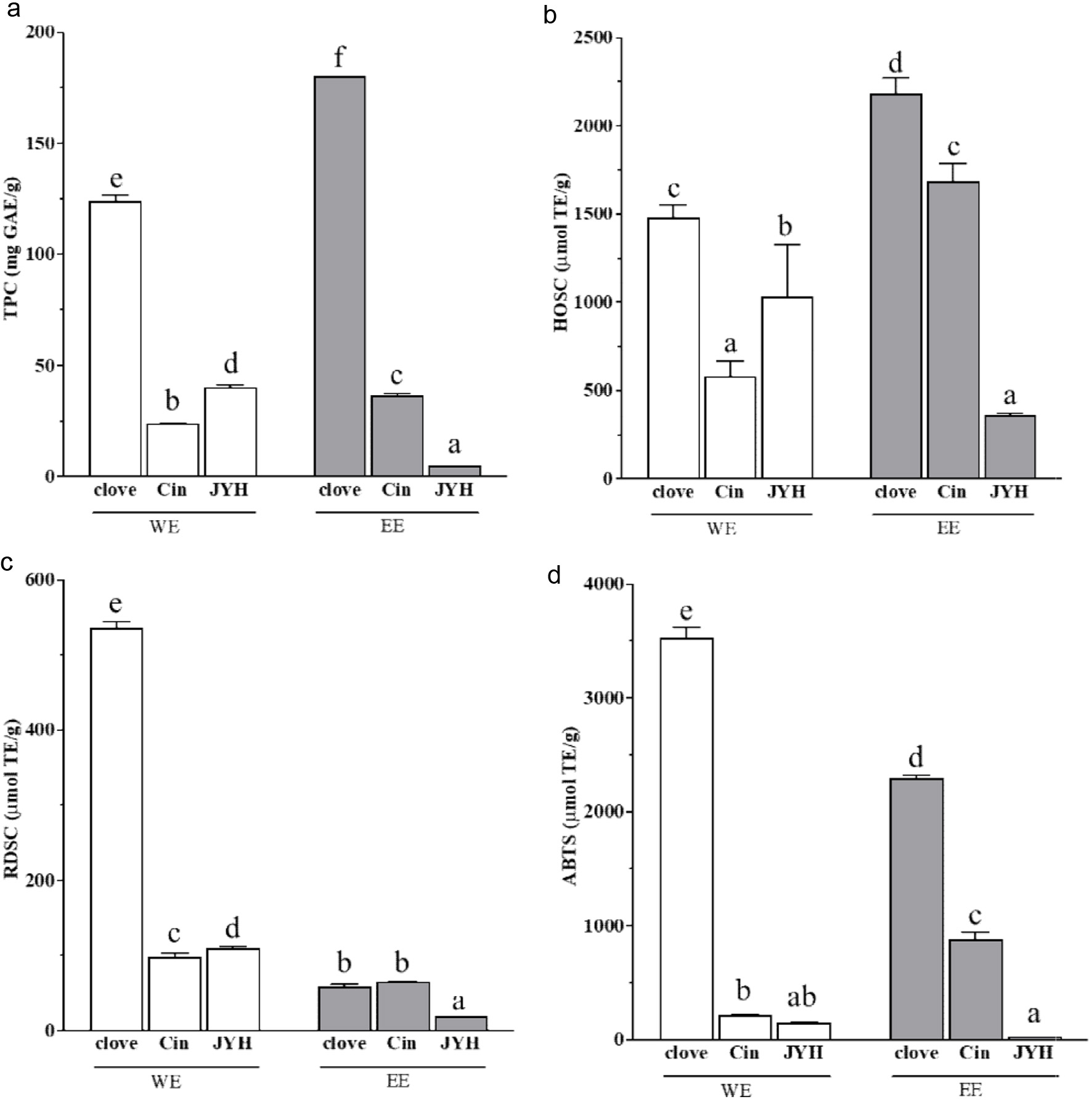 Click for large image | Figure 5. (a) Total phenolic content and relative free radical scavenging activities of clove, cinnamon (Cin) and honeysuckle (JYH) water (WE) and ethanol extracts (EE) against (b) HO•, (c) DPPH• and (d) ABTS•+. Values are reported as mean ± SD of experiments performed in triplicate on a per dry botanical weight basis. Different letters indicate significant difference (p < 0.05) by ANOVA. This figure was prepared using the data published previously (Li et al., 2022; Xie et al., 2023; Gao et al., 2023). |
Hydroxyl radicals (HO•) are generated under the general physiological conditions, and they are the most reactive radicals in the human body. Significant relative HO• scavenging activities (HOSC) were observed in the WEs and EEs of clove, cinnamon and honeysuckle under the same experimental conditions (Figure 5b). Clove extract had highest level of HOSC among the 3 tested spices, regardless of the extraction solvent. The greatest HOSC of 2,181 µmol TE/g, was detected in the clove EE, which is significantly higher than that of about 1,500 µmol TE/g for the clove WE. The HOSC order for the EEs was clove > cinnamon > honeysuckle on a per botanical weight basis. This order is different to the HOSC order for their WEs as clove > cinnamon > honeysuckle (Figure 5b). The HOSC values ranged from 2,181 µmol TE/g for clove EE to 365 µmol TE/g for honeysuckle EE. The HOSC for the three spice extracts was positively correlated with their TPC values (Figure 5a and b).
Two or more radical systems are needed to draw a general conclusion of the radical scavenging properties for a selected botanical sample, since the test system may alter the test outcomes. The relative DPPH• scavenging capacities (RDSC) were evaluated for the WEs and EEs of clove, cinnamon and honeysuckle. All the WEs and EEs of clove, cinnamon and honeysuckle were able to significantly scavenge DPPH• under the same testing conditions (Figure 5c). The greatest RDSC of 536.91 µmol TE/g was observed in the clove WE, and the lowest RDSC at 18.7 µmol TE/g was detected in the honeysuckle EE (Figure 5c). The WE of clove, cinnamon and honeysuckle had stronger RDSC than their corresponding counterpart EE on a per botanical weight concentration basis, under the same testing conditions.
In addition, ABTS•+ scavenging capacities were determined for the WEs and EEs of clove, cinnamon and honeysuckle under the same experimental conditions. Similar as RDSC and HOSC, clove WE had the greatest ABTS•+ scavenging capacity among the 3 WEs, and clove EE had the strongest ABTS•+ scavenging capacity among the EEs of clove, cinnamon and honeysuckle (Figure 5d). This observation agreed well with TPC, HOSC and RDSC determinations. The strongest ABTS•+ scavenging capacity of 3,525.1 µmol TE/g was detected in the clove WE. The EE of honeysuckle had the lowest ABTS•+ scavenging capacity at 24.8 µmol TE/g (Figure 5d). Taken together, the TPC and radical scavenging capacities of water and ethanol extracts for clove, cinnamon and honeysuckle suggested their potential in reducing oxidative stress.
It needs to be pointed out that the WE and EE of thyme (Thymus vulgaris), EE of parsley (Petroselinum crispum), WE of rosemary (Rosmarinus officinalis L.), and WE and EE of dill (Anethum graveolens L.) also had significant TPC and scavenging capacity against HO•, DPPH•, and ABTS•+ (Yao et al., 2023a; Lee et al., 2023; Yao et al., 2023b; Choe et al., 2023). In addition, the WE and EE of Huangqin (Scutellaria baicalensis Georgi.) showed significant TPC and scavenging capacity against HO•, DPPH• and ABTS•+ (Gao et al., 2024). Taken together, these research results suggested the potential use of edible botanicals in prevention of COVID-19. These results also warrant additional research to evaluate their effectiveness in cell and animal models, and potential toxicity and safety.
| 4. Conclusion | ▴Top |
Plant extracts are, and have been, an important pillar in the study of nutraceuticals. In this study, both water and ethanol plant extracts were analyzed in the context of reducing the risk of SARS-CoV-2 infection and its complications. Clove, cinnamon and honeysuckle extracts showed significant capabilities of inhibiting not only the binding of the SARS-CoV-2 spike protein to ACE2, but also to ACE2 activity itself in laboratory conditions. Current evidence does not directly point to specific molecular components responsible for this activity, but molecular docking studies have identified a variety of polyphenols in these and other botanicals which might bind to ACE2 or SARS-CoV-2 spike protein and thus provide a possible mechanism of action. (Wang et al, 2022; Prasanth et al, 2021). In addition, the plant extracts showed exceptional free radical scavenging capabilities, which could theoretically help mitigate the effects of cytokine release syndrome in response to SARS-CoV-2 infection. In conjuncture with the UHPLC-MS/MS data, it is possible that the data here can be used to find compounds of interest, that may be responsible for the anti-viral effects of the extracts and subject to further study. As this data only applies to in vitro testing, extrapolation to the human level is not yet possible. However, results are promising to suggest the merit of further investigations using in vitro human cells, in vivo animal models, or even clinical testing. Botanicals are widely accessible to large populations and contribute other aesthetic food properties and health benefits, and future work may elucidate whether they also belong in the anti-viral toolkit.
| References | ▴Top |