| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 25, March 2024, pages 52-61
Fuji apple (Malus domestica Borkh. cv. Red Fuji) pomace extracts as a source of antimicrobial and antioxidant polyphenols
Jiankang Wang*, Zhengchun Liu, Lina Wei, Changyan Shao, Jing Wang, Yanan Zhu*, Yujiao Sun
School of Food Science and Engineering, Shaanxi University of Science & Technology, Xian 710021, China
*Corresponding author: Jiankang Wang and Yanan Zhu, School of Food Science and Engineering, Shaanxi University of Science & Technology, Xian 710021, China. E-mail: rjiankangwang@outlook.com (JW), yanan.zhu@sust.edu.cn (YZ)
DOI: 10.31665/JFB.2024.18371
Received: January 29, 2024
Revised received & accepted: February 27, 2024
| Abstract | Top |
This study aimed to assess the antibacterial and antioxidant properties of both crude polyphenol extract (CPE) and purified polyphenol extract (PPE) obtained from Fuji apple pomace. The antibacterial capacity of both extracts against Staphylococcus aureus was investigated by evaluating the inhibition zones, minimum bactericidal concentrations (MBC), growth curves and bacterial morphology of S. aureus treated with CPE or PPE, as well as the performance in simulated food systems. The antioxidant activity of CPE or PPE was assessed using the ABTS radical scavenging and ferric reducing antioxidant power (FRAP) assays. The results showed that PPE exhibited potent antibacterial activity against S. aureus, which was further confirmed by the bacterial morphology. It was revealed that PPE only exerted strong antibacterial effect in starch-based food system while CEP did not show such effect in all systems. The results of the ABTS and FRAP assays indicated that both PPE and CPE possess strong antioxidant activity, from which PPE showed much higher capacity than that of CPE. Therefore, PPE from Fuji apple pomace can be used as a novel antibacterial agent for food preservation and natural antioxidant for functional food and nutraceutical products.
Keywords: Fuji apple pomace; Crude polyphenol extract; Purified polyphenol extract; Antibacterial activity; Staphylococcus aureus; Antioxidant activity
| 1. Introduction | Top |
There has been a growing awareness about harmful effects of chemical preservatives in food, which has led to an increase in research on extracts from natural botanical sources, especially those possessing the antimicrobial and antioxidant properties (Shahidi and Santhiravel, 2022; Gupta and Yadav, 2021). Polyphenols have been known for their abundant sources and antibacterial effects, which primarily exert such effects by modifying the permeability of cell membrane, disrupting cell wall rigidity and integrity, and inhibiting the synthesis of biomolecules in bacterial cells (Bouarab Chibane et al., 2019; Fei et al., 2018). Moreover, their hydroxyl structure renders antioxidant properties, which was mainly achieved by scavenging radicals and chelating redox-active metals (Fraga, 2007). Apple is one of the most broadly distributed and consumed fruit all over the world (Gulzar, 2023). Apple polyphenols, which are secondary metabolites in apples, are a collective term for the polyphenolic compounds found in different parts of the fruit, primarily including phenolic acid derivatives and flavonoids. They have been reported to have various physiological functions, such as the prevention of cancer, diabetes, liver dysfunction and hypertension (Wang et al., 2023; Clemens and Shahidi, 2022). Apple pomace, a by-product from apple processing, is a rich source of high value-added bioactive compounds such as polyphenols. Millions of tons of apples are processed to produce apple cider, juices, or concentrates every year worldwide, which yield huge amounts of pomace (Fernandes et al., 2019), and apple pomace can be used for the extraction of polyphenols. Therefore, a complete understanding of functions of polyphenol extracts obtained from apple pomace can enhance the utilization of by-products from apple processing.
Staphylococcus aureus, a gram-positive bacterium, is among the prevalent foodborne pathogens. Chambers (2001) reported that only about 2030 % of the global population has never been a carrier of S. aureus, while about 20 % are persistent carriers. S. aureus is highly pathogenic, which can cause a spectrum of infections on the skin and soft tissues (Chmielowiec-Korzeniowska et al., 2020). The heat and salt resistance of S. aureus facilitate its growth and multiplication in food products (Yehia et al., 2020), and it affects human body mainly by secreting staphylococcal enterotoxins, causing pathological reactions such as vomiting, diarrhea and cramps in the host (Wattinger et al., 2012). The serious threat posed by S. aureus to human health necessitates the identification of effective and safe strategies for inhibiting its reproduction in food. The antibacterial effect of Golden Delicious apple pomace polyphenols on Escherichia coli was evaluated using the diameter of inhibition zone and the minimum inhibitory concentration as the indexes, confirming its robust antibacterial activity (Zhang et al., 2016). Thus, it is important to extend the evaluation of the inhibitory effect of apple polyphenol to other commonly found foodborne bacteria, such as S. aureus. Antioxidant capacity of polyphenols can be evaluated using a variety of methods, from which the most used ones are free radical scavenging and ferric reducing power (FRAP) assays (Bai et al., 2013). The antioxidant capacities measured by ABTS assay are strongly correlated with the phenolics and flavonoids from antioxidant-rich foods, and ABTS assay is superior to other free radical scavenging assays when used on a range of plant foods (Floegel et al., 2011). Therefore, ABTS radical scavenging assay is suitable for evaluating the antioxidant activity of polyphenol extracts from apple pomace. The FRAP assay has demonstrated features of high reproducibility and easy operation, and thus it is widely used to understand the antioxidant capacity of polyphenol extracts (Thaipong et al., 2006).
In this study, crude polyphenol extract (CPE) and purified polyphenol extract (PPE) derived from Fuji apple (Malus domestica Borkh. cv. Red Fuji) pomace were selected to evaluate their bactericidal and antioxidant activities in various systems, respectively. Antibacterial effects of these polyphenol extracts were assessed by investigating the inhibition zones, minimum bactericidal concentrations (MBC), growth curves and bacterial morphology of S. aureus treated with CPE or PPE, as well as the bacteriostatic activity of both two extracts in simulated food matrices. The antioxidant activities of CPE or PPE were assessed according to their ferric reduction capacity using the FRAP method.
| 2. Materials and methods | Top |
2.1. Reagents and materials
Crude polyphenol extract (CPE) and purified polyphenol extract (PPE) from Fuji apple (Malus domestica Borkh. cv. Red Fuji) pomace were prepared in our laboratory. Staphylococcus aureus 25923 were purchased from ATCC. Phosphate buffered saline (1, pH 7.4), acetic acid buffer (pH 3.6), 2,2-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS), 2,4,6-tris-(2-pyridyl)-s-triazine (TPTZ), ethanol (ACS grade), K2S2O8 (AR grade), NaOH (AR grade), NaCl (AR grade), HCl (AR grade), FeCl3 (AR grade), FeSO4 (AR grade), L-ascorbic acid (AR grade), and Tween-80 (CR grade) was purchased from Merck (Shanghai, China). Isoamyl acetate (AR grade) was purchased from FUCHEN (Tianjin, China). Other reagents were purchased from OXOID (UK) unless otherwise specified.
2.2. Antibacterial activity determination
2.2.1. Preparation of bacterial strains inoculum
S. aureus was cultured for 40 h in tryptone soya broth (TSB) at 37C accompanying a passage at the 24 h. The cultured bacteria were centrifuged at 1,700 r/min for 5 min and the supernatant was removed. The bacterial pellet was re-suspended in PBS buffer at an optical density (OD) at 600 nm of 1 followed by twice dilutions to get a final concentration of 6 log CFU/mL.
2.2.2. Inhibition zones measurement
The growth inhibition of S. aureus by CPE or PPE was determined using a modified agar diffusion method by measuring the diameters of inhibition zones (Singh et al., 2005). The assay involved coating 100 L of the bacterial suspension uniformly on the surface of Luria-Bertani (LB) agar plates followed by attaching four sterile filter papers with a diameter of 6 mm. Ten L of PBS buffer containing PPE (100 mg/mL) or CPE (100 mg/mL) was added onto each of three filter papers. In the negative control, the PBS buffer without PPE or CPE was added to the remaining filter paper. All tests were performed in triplicate. After incubating at 37C for 24 h, the inhibition zones were measured accurately using a vernier caliper. When diameters of inhibitory zones were greater than 7 mm, inhibitory effects were considered. The larger the diameter of the inhibitory zone, the stronger the inhibitory effect is. On the other hand, when the diameter was 7 mm or less, it was considered ineffective.
2.2.3. Minimum bactericidal concentration
The minimum bactericidal concentrations (MBC) of polyphenol extracts against S. aureus were determined following the method described by Obroh et al. (2021) with minor modifications. CPE and PPE were diluted using the two-fold serial dilution method (Chandrasekaran and Venkatesalu, 2004) to concentrations of 25, 12.50, 6.25, 3.13, and 1.56 mg/mL. Four hundred microliter of polyphenol solution was mixed with the same volume of TSB medium containing 100 L of bacterial suspension. Additionally, PBS buffer and penicillin-streptomycin mixture (1%) were used as negative and positive controls, respectively. The assay was conducted in triplicate. Following an incubation at 37C for 24 h, 200 L of the mixture was coated onto LB agar plate and further incubated at 37C for 24 h. The MBC was determined as the lowest concentration of the polyphenol extracts applied that did not permit the presence of any visible bacterial colonies on the agar plate after the incubation.
2.2.4. Growth curves
The growth curves of S. aureus under treatments of CPE or PPE with different concentrations were established following the method of Pagliarulo et al. (2016) with minor modifications. Bacterial suspensions were prepared as described in section 2.2.1. Subsequently, PBS buffer containing either CPE or PPE were added to the bacterial-containing TSB culture to achieve final polyphenol concentrations of 50, 25, 12.50, 6.25 and 3.13 mg/mL. The aforementioned polyphenol concentrations were selected based on the results of the MBC assay. Moreover, the CPE was tested using the same concentration as PPE, although its MBC was not obtained. PBS buffer was included as the negative control, and sterile TSB medium only containing equal concentration of CPE or PPE was used as the blank control. All samples were set in triplicate. Two-hundred microliter of tested samples were incubated in a 96-well plate covered by a film at 37C for 12h at OD600 nm, with 10-min intervals to determine growths of bacteria in a microplate reader. The absorbance value of bacteria in the results was calculated by the formula: OD600 = A A0, where OD600 was the ordinate in Figure 1, a was the absorbance value of S. aureus treated with CPE or PPE at 600 nm, and A0 was the absorbance value of blank control at 600 nm.
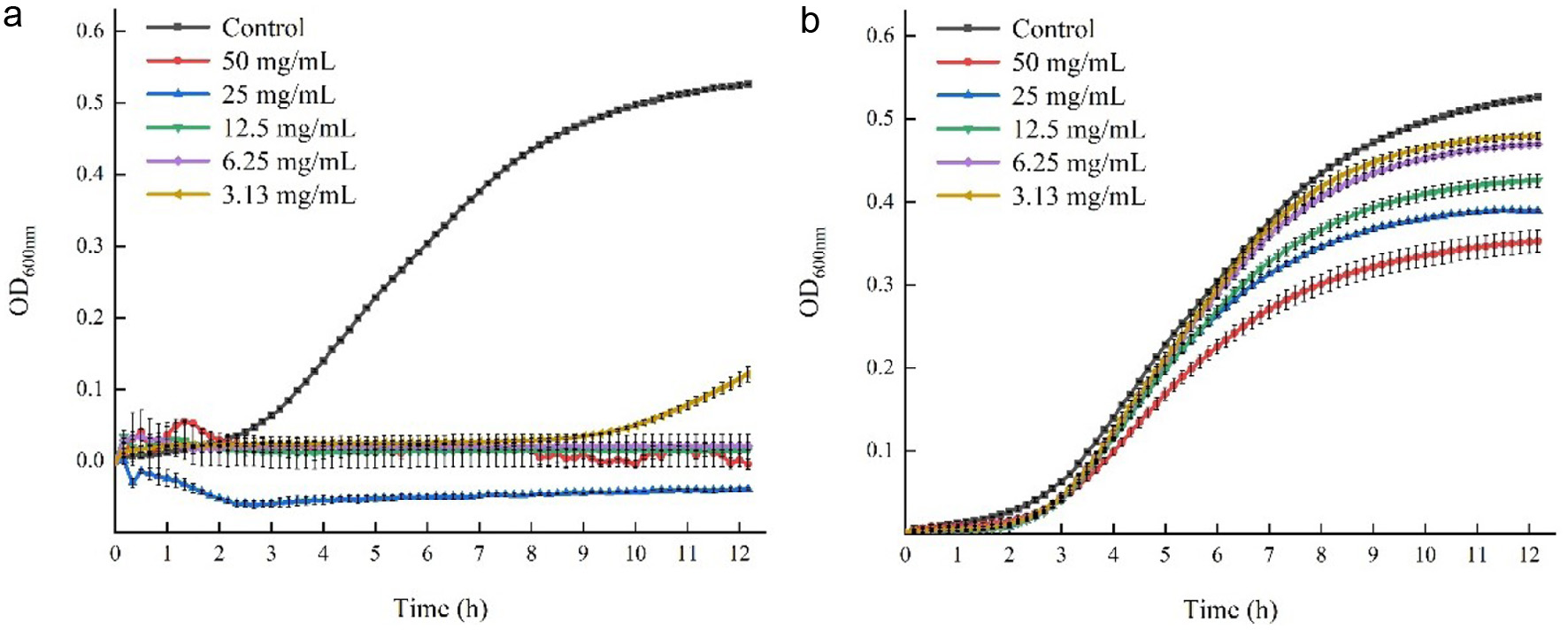 Click for large image |
Figure 1. The Effect of PPE and CPE on the growth curves of S. aureus during 12 h of incubation. (a) S. aureus was treated with PPE; (b) S. aureus was treated with CPE. |
2.2.5. Observation by scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was employed to examine the morphological changes of S. aureus cells treated with CPE or PPE with minor modifications (El-Maati et al., 2016). The cultured bacteria were diluted to an OD (600 nm) of 0.6 before use in subsequent assay. CPE or PPE was added to the bacterial suspension to achieve final concentrations of 0, 12.50 and 25 mg/mL. The cells were centrifuged at 8,000 r/min for 10 min at room temperature after an incubation at 37C for 4 h. After twice re-suspensions in PBS buffer and centrifugations followed by a 10 min static standing for intermissions, the cells were fixed with 2.5% glutaraldehyde-PBS (v/v) solution and incubated at 4C for 12 h. The cells were fixed with 2.5% glutaraldehyde-PBS (v/v) solution and allowed to stand at 4C for 12 h. After washes with PBS buffer and sterile water, respectively, the cells were dehydrated using ethanol solutions with graded concentrations (30%, 50%, 70%, 80%, 90%, 100%) and left undisturbed for 10 min before centrifugation. Subsequently, the cells were resuspended in isoamyl acetate and were set to stand for 30 min before being centrifuged at 8,000 r/min for 10 min. The samples were finally dried at 40C for 2 h, freeze-dried for 2 h to dryness, then loaded onto a carrier stage and sprayed with platinum before being visualized using a SEM.
2.2.6. Evaluation of the bacteriostatic effects of CPE and PPE in simulated food systems
The bacteriostatic activities of polyphenol extracts were further analyzed in simulated food systems containing starch, lipid or protein, respectively. The samples were prepared using the method described by Li et al. (2022) with minor modifications as a lower concentration of bacterial suspension was used.
2.2.6.1. Simulated starch-based food system
Different weights of corn starch (CS) were dissolved in 5 mL of 0.5% NaCl (w/v) solution and stirred evenly, and their concentrations of 0%, 1%, 2%, 3%, 4% and 5% (w/v) were obtained. The pH of the solutions was adjusted to 8.0 with 0.1 mol/L NaOH, and then the solutions were boiled for 2 min until the simulated system was formed with a high clarity. The test solution was prepared by adding CPE or PPE into the above simulated starch-based food matrices until the final concentration of 50 mg/mL (2 MBC) was achieved. The solutions were left undisturbed for 60 min following mixing, and then they were centrifuged at 5,000 r/min for 2 min, which was followed by the collection of the supernatant.
Four sterilized Oxford cups (stainless cylinders with an outside diameter of 8.0 mm, inner diameter of 6.0 mm, and height of 10.0 mm) were placed on each LB agar plate previously coated by 0.1 mL bacterial suspension followed by filling each cup with 200 L of test solution. Additionally, the PBS buffer was used instead of the test solution as a control in one of the Oxford cups.
2.2.6.2. Simulated lipid-based food system
Different volumes of canola oil (CO) were pipetted to a 50-mL volumetric flask with the addition of 1% (v/v) Tween-80 and diluted to volume with sterile water, which was shaken vigorously for preliminary mixing. The above solution was transferred to the beaker, they were then emulsified at 10,000 r/min three times with 1min intervals and 30 seconds each time to form the lipid-based food system, and the final concentrations of the canola oil were 0%, 1%, 2%, 3%, 4% and 5% (v/v). Further steps are described in Section 2.2.6.1.
2.2.6.3. Simulated protein-based food system
Different weights of soybean protein isolate (SPI) were added into 5mL of sterile water, and then the solutions were stirred magnetically at 500 r/min for 1 h at room temperature to form the protein-based food system, and the final concentrations of SPI were 0%, 1%, 2%, 3%, 4% and 5% (w/v). Further steps are described in Section 2.2.6.1.
2.3. Antioxidant potential determination
2.3.1. ABTS radical scavenging assay
The ABTS radical scavenging capacity of CPE or PPE was determined according to the method by Nguyen et al. (2020) with minor modifications. The ABTS stock solution (7 mmol/L) was evenly mixed with K2S2O8 (7.35 mmol/L) solution at the ratio of 1:1 (v/v), and kept in the dark at room temperature for 12 h to became ABTS radical solution. Further, the absorbance of the ABTS radical solution at 734 nm was adjusted to 0.7 0.02 by dilution with ethanol, and the ABTS working solution was obtained. An aliquot of 200 L of CPE or PPE with different concentrations (12.50, 25, 50, 100, 200 g/mL) was added to 1 mL of ABTS working solution, and they were placed under the condition of avoiding lights before the measurement of their absorbance. Additionally, distilled water and L-ascorbic were used as negative and positive controls, respectively. Distilled water mixed with CPE or PPE solution was used as a blank control. The absorbance of all samples was measured at 734 nm after incubating at 30C for 6 min, and the results were calculated by the following formula:
2.3.2. Ferric reducing antioxidant power (FRAP) assay
The ferric reducing antioxidant power (FRAP) was used to determine the antioxidant capacity of CPE or PPE (Stojiljkovi et al., 2016). An aliquot of 2.5 mL of 0.01 mol/L TPTZ (0.04 mol/L HCl) solution and 2.5 mL of 0.02 mol/L FeCl3 were added to 25 mL of 0.3 mol/L acetic acid buffer (pH = 3.6) to create the FRAP reagent. Then, 120 L of FeS04 solutions with various concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mmol/L) were added to 3.6 mL of FRAP reagent to prepare standard curve samples. After standing at 37C for 10 min, the absorbance of all the samples were measured at 593 nm with a microplate reader, and a standard curve was created based on the absorbance. An aliquot of 120 L of CPE or PPE with different concentrations (200, 400, 600, 800, 1000 g/mL) was added to 3.6 mL of FRAP reagent, which was placed under the condition of avoiding lights before the measurement of their absorbance. Additionally, the samples with L-ascorbic acid instead of CPE or PPE were used as a control. The absorbance of samples was measured at 593 nm after incubating at 37C for 10 min. The results were expressed as mmol/g FeS04 equivalent.
2.4. Statistical analysis
Each assay was carried out in triplicate, and the results were recorded as means standard deviation (SD). The data were analyzed using SPSS 26.0.
| 3. Results and discussion | Top |
3.1. Antibacterial activity
3.1.1. Inhibition zone measurement
The agar diffusion assay was based on the formation of inhibition zones, due to the diffusion of the antimicrobial agent in the solid agar culture medium inhibiting the growth of microorganisms (Galvo et al., 2016). Zhu et al. (2019) reported that the inhibitory zone of purified polyphenol extract constituents from Sanguisorba offcinalis L. against S. aureus was twice that of crude polyphenol extract. In this study, the results of the antibacterial activities of PPE and CPE against S. aureus based on the diameter of inhibition zones are shown in Figure 2 and Table 1. It was observed that colonies formed on LB agar showed a transparent halo around the filter paper with the addition of PPE compared to the control group, while the filter paper treated CPE did not exhibit this effect. The diameter of the inhibition zone recorded in Table 1 further reflects the antibacterial capacities of PPE and CPE. PPE exhibited robust bacteriostatic activity with a diameter up to 11.15 0.39 mm, while CPE had no inhibitory effect against S. aureus as a limited small zone diameter (7 mm) was formed. The results could be attributed to the higher content of polyphenols of PPE, which mainly include epicatechin acid, ferulic acid and chlorogenic acid, the key polyphenol species in Fuji apple pomace (Jakobek et al., 2020; Liu et al., 2021), which was confirmed to have strong antibacterial activity against gram-positive bacteria (Martinengo et al., 2021; Motallebi et al., 2020). It was reported that the purification process effectively increased the total phenolic content by 3.35 folds, from 13.83 to 46.45 mg of gallic acid equivalents per g of CPE/PPE (Mohammadi et al., 2024).
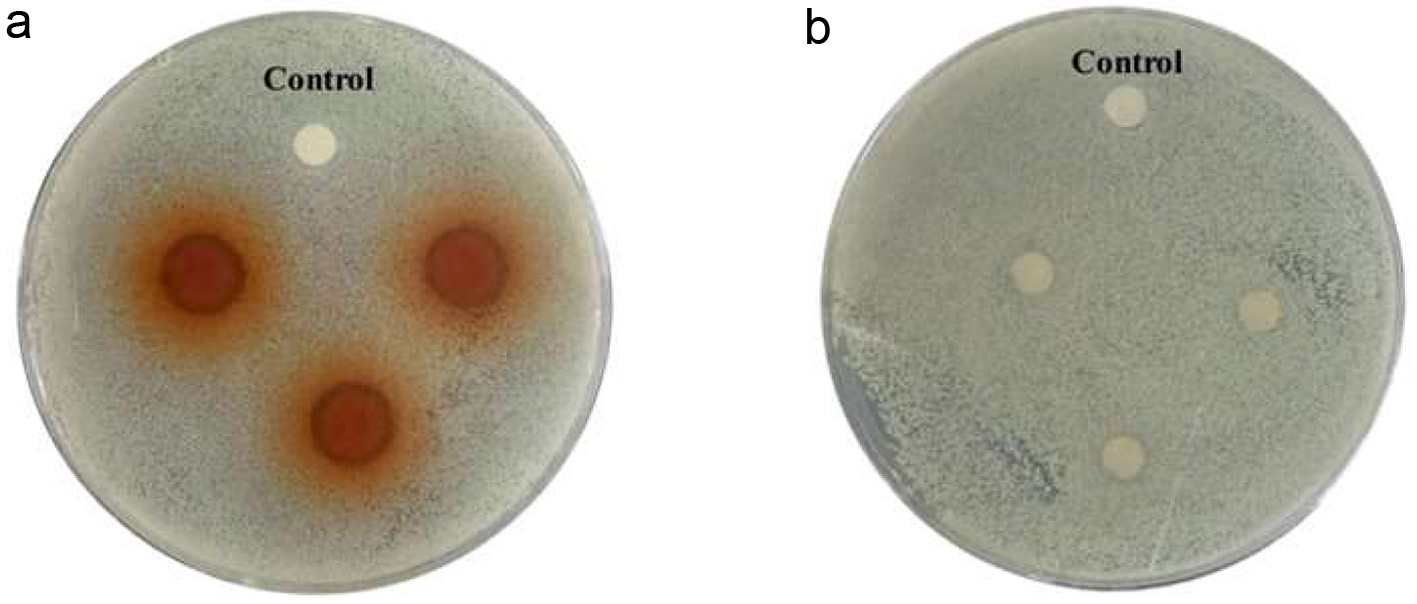 Click for large image |
Figure 2. Halos (clear zones) of inhibition of growth of S. aureus formed around colonies of PPE and CPE on LB agar. (a) circle filter papers treated with PPE; (b) circle filter papers treated with CPE; Control: the control group. |
 Click to view |
Table 1. The diameters of Inhibition zone of PPE and CPE against S. aureus on LB agar |
3.1.2. Minimum bactericidal concentration
It had been reported that some of the bactericidal agents can act as both bacteriostatic and bactericidal depending on their concentration (Chikezie, 2017). The lowest concentrations of PPE or CPE required to kill bacteria were identified as the minimum bactericidal concentrations (MBCs). Table 2 showed the MBC of PPE and CPE against S. aureus, which provided the basis for the selection of concentration of PPE or CPE in the subsequent bacteriostatic assay. PPE exhibited a strong bactericidal effect even at relatively low concentrations with an MBC of 25 mg/mL. The MBC of PPE was lower than the reported MBC of polyphenol extract from pomegranate against S. aureus (Lima et al., 2019), which could be explained by variations in their phenol content, strains sensitivity as well as antibacterial procedures employed in the test (Gullon et al., 2016). On the contrary, no apparent bactericidal activity was observed for CPE within the tested concentration range (1.5650 mg/mL). Additional assays with high concentrations of CPE (100400 mg/mL) were carried out (data not shown) to identify its MBC, and even though the number of bacteria decreased with increasing concentrations, colonies still did not completely disappear from the growing medium, and thus the MBC of CPE was not identified. As no specific MBC value was identified for CPE, the three subsequent antibacterial assays including growth curves, observation by scanning electron microscopy as well as antibacterial effect in simulated food systems were used the same concentration as PPE where the concentration selection was based on MBC.
 Click to view |
Table 2. The minimum bactericidal concentrations (mg/mL) of PPE and CPE against S. aureus |
3.1.3. Growth curves
The growth curves of S. aureus with the treatment of PPE (3.1350 mg/mL) or CPE (3.1350 mg/mL) over 12 h of incubation period were plotted to monitor the impact of these polyphenol extracts on the growth of S. aureus, which was depicted in Figure 1. The growth and life cycle of bacteria are divided into four periods: adaptation period, logarithmic period, stationary period, and decline period, and these stages are determined based on the number of surviving organisms at various intervals (Chesney, 1916). In this study, the growth curve of S. aureus in the control group showed the first three stages aforementioned, which indicated a normal growth of S. aureus cells. However, when PPE with concentrations from 6.25 to 50 mg/mL were added, the growth of S. aureus was completely inhibited, but when the concentration of PPE decreased to 3.13 mg/mL, the growth of S. aureus was inhibited at the initial stage. The growth curve showed different fluctuations when the concentration of PPE increased to 25 or 50 mg/mL. Higher concentrations of PPE may react with casein in tryptone soya broth (TSB), thereby impacting the absorbance (El-Messery et al., 2020). However, the trend of growth curves of S. aureus treated with CPE showed limited changes compared to the control group, the growth rate of the S. aureus decreased slightly, but the effect on the growth curve were stronger when higher concentrations of CPE were applied. Thus, both PPE and CPE demonstrated inhibitory effect on the growth of S. aureus, with PPE being more potent, which could be due to the higher polyphenol content of PPE and lower interference from the impurities, and the inhibitory effect improved as the concentrations of PPE or CPE increased.
3.1.4. Effect on the morphology of S. aureus
The morphological damage of S. aureus was observed by scanning electron microscope (SEM) in order to further reveal the antibacterial activity of PPE or CPE against S. aureus and its mechanism. The results are shown using photomicrographs at 30.0 K and 50.0 K magnification in Figure 3. It can be clearly seen that the majority cells of S. aureus in the control group were in uniform, full, and spherical shape with relatively smooth surfaces, and they displayed normal cell morphology without extracellular spillage or apparent damage. In spite of this, there were still a few cells with a small degree of invagination, which could be due to the long drying time of cells before being loaded onto a carrier stage. In contrast to the control group, the surface of S. aureus cells treated with PPE or CPE showed wrinkles and cracks with some particulate matters, and these particulate matters might consist of cellular debris or leakage of cellular contents (Shen et al., 2015). These observations were similar to the morphological changes of S. aureus after being treated with punicalagin, a phenolic compound present in leaves of pomegranate peel, which was reported by Xu et al. (2017). In summary, PPE and CPE exhibited strong antibacterial activity against S. aureus at different degrees. The antibacterial activity of polyphenol extracts derived from Fuji apple pomace was primarily due to their ability to damage the cell membrane and cell wall of S. aureus. This may be because polyphenols can alter the fatty acid composition in the cell membrane, and inhibit the synthesis of ergosterol, thereby changing the cell membranes permeability (Di Pasqua et al., 2006). Additionally, polyphenols can bind with the bivalent cations of the bacterial outer membrane (Nohynek et al., 2006). The destruction of the bacterial cell membrane and cell wall can lead to the leakage of intracellular components, which affected its normal growth and could even cause cell death (Zhong et al., 2023).
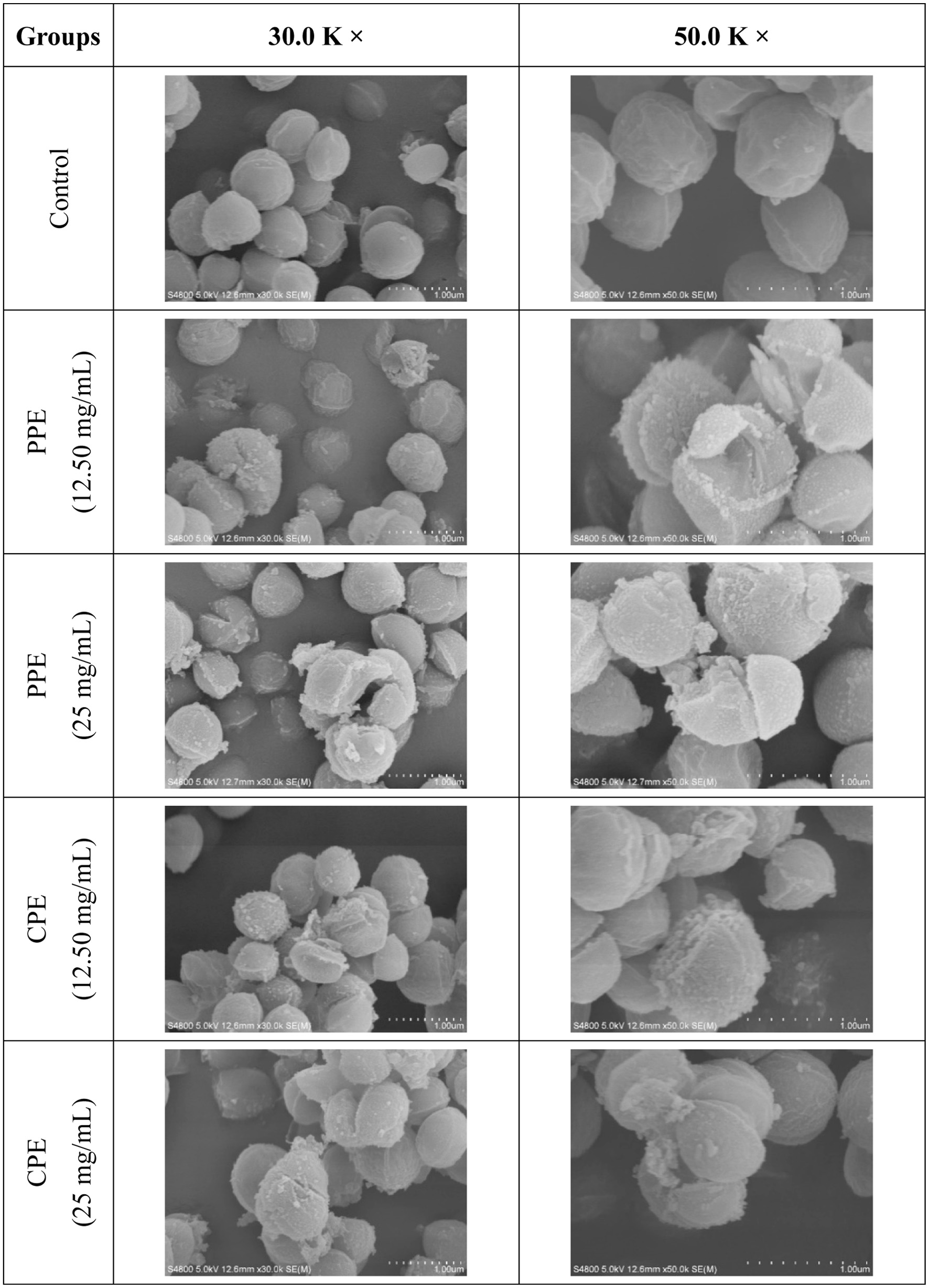 Click for large image |
Figure 3. Scanning electron microscope (SEM) images at 30.0 K and 50.0 K magnification showing morphological changes of S. aureus treated with PPE or CPE. |
3.1.5. Evaluation of the bacteriostatic effects of PPE and CPE in simulated food systems
Natural preservatives antimicrobial efficacy would be hampered by the intricate, multi-scale structure of food, which may consist of starch, lipid or protein (Wang et al., 2023). Thus, corn starch (CS), canola oil (CO) and soy protein isolate (SPI) were used to form simulated starch-based, lipid-based and protein-based food systems with their varying concentrations in order to evaluate the antibacterial activity of PPE or CPE in food matrices with different macronutrients. It was observed that PPE has strong antibacterial activity in simulated starch-based food matrices against S. aureus, which is supported by the presence of inhibition zones, but there was no significant difference (p > 0.05) among the effects of all concentrations employed. However, the antibacterial activity was not demonstrated in the simulated lipid-based and protein-based food matrices (data not shown), which were proved by the absence of the inhibition zone. Limited miscibility of PPE in lipid-based food systems may lead to its limited contact time with bacteria present in the emulsion (Pastene et al., 2009), and thus improving the solubility of polyphenol extracts can be one of the means to improve its antibacterial activity in the lipid-based food systems. On the other hand, polyphenols from the PPE could interact with proteins or lipids, which is leading to the formation of complexes that restrict their antimicrobial activity (Mandalari et al., 2016). CPE did not show any antibacterial activity in the three simulated food matrices, which could be due to its lower content of polyphenols as well as the interference from its impurities (Table 3).
 Click to view |
Table 3. Bacteriostatic effects of PPE and CPE against S. aureus in simulated starch-based food matrices |
3.2. Antioxidant activity
3.2.1. ABTS radical scavenging capacity
The ABTS radical scavenging assay is one of the commonly used methods to evaluate the antioxidant capacity of polyphenol extracts (Floegel et al., 2011). The principle of this method is that K2S2O8 solution reacts with ABTS solution to oxidize ABTS into blue-green ABTS radicals, and after the addition of antioxidant substances such as L-ascorbic and polyphenols, ABTS radicals will be reduced into ABTS, and the color of the solution will change. Therefore, the ABTS radical scavenging capacity of L-ascorbic acid, polyphenols and other substances can be evaluated by detecting the absorbance of the sample at 734 nm. The ABTS radical scavenging ability of PPE and CPE were shown in Figure 4. The results indicated that the ABTS radical scavenging capacity of PPE increased when concentrations increased, and was significantly stronger (p < 0.05) than that of CPE with all concentrations tested. When the concentration is 12.5100 g/mL, the ABTS radical scavenging capacity of tested materials was as follows: L-ascorbic acid > PPE > CPE. However, when the concentration increased to 200 g/mL, the ABTS radical scavenging rates of both PPE and L-ascorbic acid were about 99.99%, which were five times of that of CPE, from which demonstrated the strong antioxidant activity of PPE. In conclusion, both PPE and CPE showed strong ABTS radical scavenging capacity, from which PPE being more potent, which could be due to its higher content of polyphenols, mainly including chlorogenic acid, epicatechin and catechin (Qing et al., 2023; Serra et al., 2021).
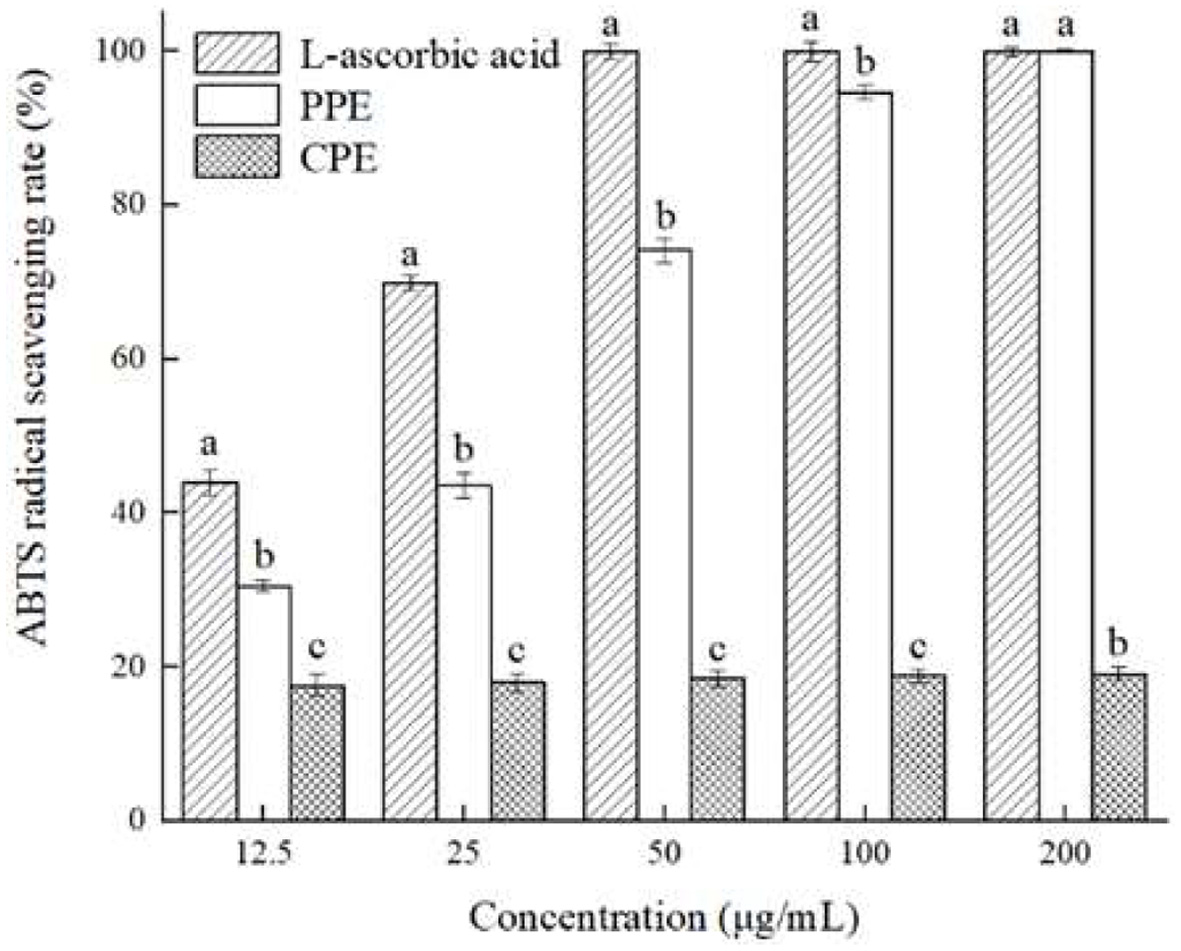 Click for large image |
Figure 4. Antioxidant activity of PPE and CPE in ABTS radical scavenging test as compared with L-ascorbic acid. Different letters marked in the same concentration indicate significant differences (p < 0.05) between any two groups. |
3.2.2. Ferric reducing antioxidant power (FRAP)
The ferric reducing antioxidant power (FRAP) assay demonstrated features of high reproducibility, easy operation, and thus it is widely used to evaluate the antioxidant capacity of polyphenol extracts (Thaipong et al., 2006). The principle of this assay is to determine the reduction of Fe3+ to Fe2+ by measuring the absorbance of the Perls Prussian blue complex (El-Maati et al., 2016), and a higher absorbance indicates a higher Fe3+ reducing capacity. Figure 5 illustrated the ferric reducing antioxidant power of PPE and CPE. The results indicated that PPE had a potent reducing capacity for Fe3+, and its capacity increased when concentrations increased. However, CPE exhibited limited Fe3+ reducing activity at the concentration range selected. Both PPE and CPE showed significantly lower (p < 0.05) reducing power with all concentrations tested compared to that of L-ascorbic acid; meanwhile, PPE exhibited a significantly higher (p < 0.05) reducing power than that of CPE with all concentrations tested. In summary, the reducing capacity of tested materials to Fe3+ in this assay was as follows: L-ascorbic acid > PPE > CPE, and it demonstrated that PPE had stronger antioxidant activity than that of CPE, which could be due to its higher content of polyphenols (Guo et al., 2018).
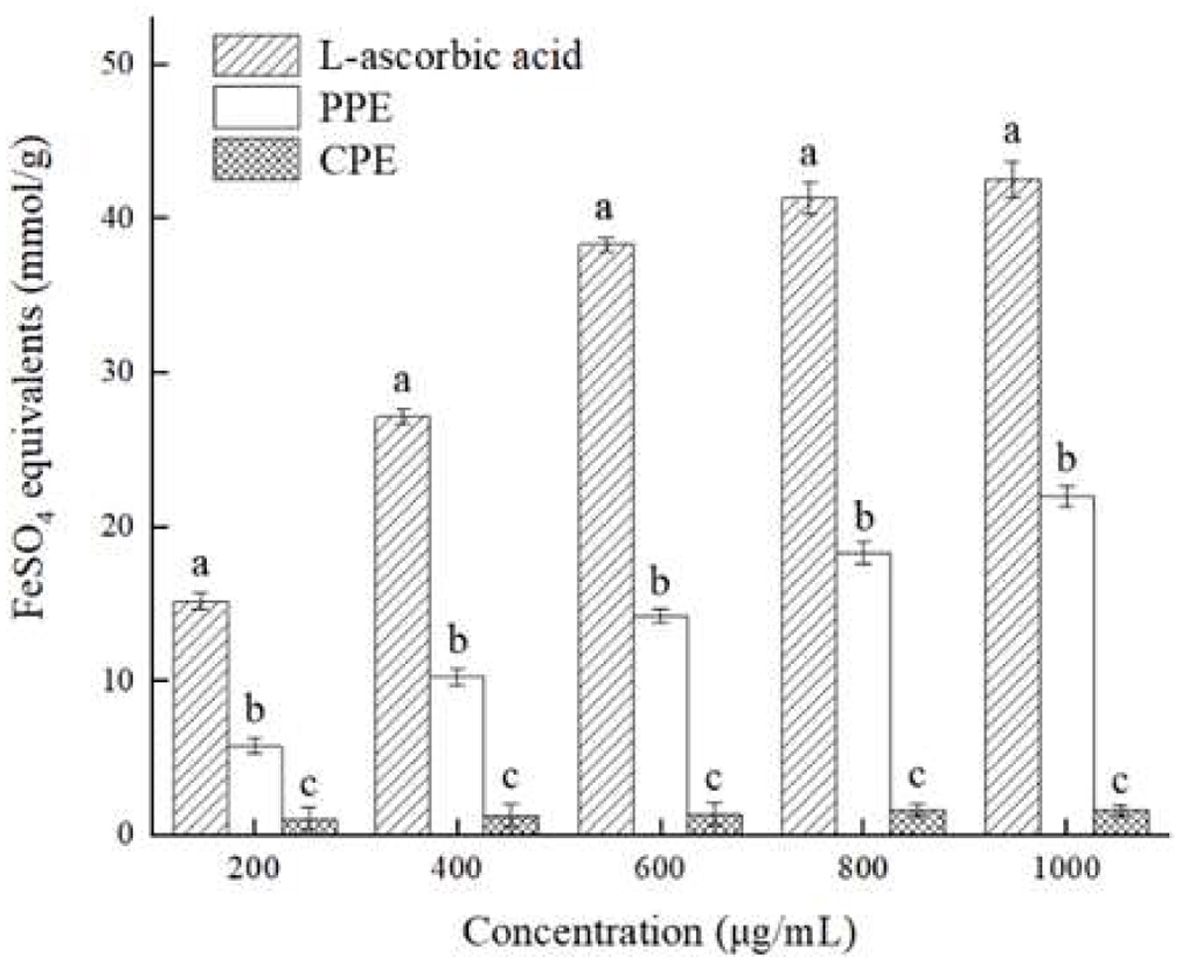 Click for large image |
Figure 5. Antioxidant activity of PPE and CPE in FRAP test as compared with L-ascorbic acid. Different letters marked in the same concentration indicate significant differences (p < 0.05) between any two groups. |
| 4. Conclusion | Top |
Purified polyphenol extract (PPE) from Fuji apple pomace showed strong antibacterial activities in various systems tested, mainly by damaging the cell wall and membrane of bacteria, while it also demonstrated potent antioxidant activity in ABTS radical scavenging and FRAP assays due to its strong quenching capacity of ABTS radicals and reducing effect of Fe3+ to Fe2+. The crude polyphenol extract exhibited limited antimicrobial and antioxidant properties in tested systems comparing to that of PPE, which may be due to its lower polyphenol content and the interference from the impurities. Therefore, polyphenol extract, especially its purified forms from Fuji apple pomace, can be used as a novel antibacterial agent for food preservation as well as natural antioxidant for functional food and nutraceutical products.
Acknowledgments
This work was supported by the National Foreign Expert Project Funded by the Ministry of Science and Technology of the Peoples Republic of China (G2022041012L), the Shaanxi Province Key Research and Development Program (Project No. 2017TSCXL-NY-02-03), the Department of Science and Technology of Shaanxi Province, P. R. China (No. 2023-YBNY-108), and Xian Science and Technology Bureau (22NYYF048).
Conflict of interest
The authors declare that there is no conflict of interest.
| References | Top |