| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 24, December 2023, pages 29-39
Potential role of nobiletin in Alzheimer’s disease
Huilin Fang, Lingling Zhang, Hui Zhao*
Tianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China
*Corresponding author: Hui Zhao, Tianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China. E-mail: zhaohui@tjcu.edu.cn
DOI: 10.31665/JFB.2023.18361
Received: December 14, 2023
Revised received & accepted: December 27, 2023
| Abstract | ▴Top |
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by amyloid plaques and neurofibrillary tangles. At present, there is no specific drugs for the prevention and treatment of AD, so it is urgent to find natural products contributing to alleviation of AD. Nobiletin is one of polymethoxyflavones (PMFs) extracted mainly from citrus peels. Nobiletin has multiple bioactivities, good bioavailability and low side effects. Studies have shown that nobiletin improves cognitive impairment and pathological features in animal models of AD and exerts neuroprotective effects. In addition, 5-demethylnobiletin (5-DN), the main derivative of nobiletin, achieves neuroprotection through anti-inflammatory, neurotrophic and cholinergic effects for the treatment of neurodegenerative diseases. Other PMFs (for example tangeretin) also have the potential to prevent neurodegenerative diseases due to their anti-inflammatory and neuroprotective effects similar to nobiletin. Taken together, PMFs, including nobiletin, have potential as therapeutic agents for AD.
Keywords: Nobiletin; PMF; Anti-neuroinflammatory; AD; AD animal model
| 1. Introduction | ▴Top |
In nature, polymethoxyflavones (PMFs) are found almost exclusively in citrus plants, especially in the peels of Citrus sinensis and Citrus reticulate (Xiao et al., 2009). While citrus peels are one of the main by-products of the fruit processing industry, extracting PMFs from waste citrus peels can increase the added value of citrus and reduce waste (Silva et al., 2018). Studies have shown that PMFs have a wide range of biological activities, including anti-cancer (Liu et al., 2020; Pereira et al., 2019; Silva et al., 2018), anti-inflammatory (Okuyama et al., 2015; Shin et al., 2012), antioxidant (Wang et al., 2018) and cardiovascular protection (Takada et al., 2013). Nobiletin is the most widely studied PMF because it is abundant in citrus plants and have significant pharmacological activity (Gao et al., 2018). It has various pharmacological activities such as anti-inflammatory (Li et al., 2007), anti-cancer (Sp et al., 2017), anti-bacterial (Yao et al., 2012), anti-obesity (Kanda et al., 2012), anti-allergy, anti-diabetic (Yuan et al., 2022) and anti-Alzheimer’s disease (AD) (Nakajima et al., 2015).
According to information provided by the Alzheimer’s Association (https://www.alz.org/alzheimers-dementia/treatments/medications-for-memory), the U.S. Food and Drug Administration (FDA) has approved medications that fall into two categories: medications that may slow the progression in people living with AD, and medications now available to treat AD. Aducanumab (Aduhelm™) is one of the medications that may slow the progression of the disease. Amyloid-related imaging abnormalities (ARIA), headache, and falls are the most prevalent side effects of this medication. An allergic reaction is another potentially dangerous adverse effect. Cholinesterase inhibitors, Glutamate regulators, and Orexin receptor antagonists are the three types of medications now available to treat symptoms. These medications are usually well tolerated, with the most common side effects being nausea, vomiting, lack of appetite, and increased bowel frequency. However, these drugs temporarily alleviate the symptoms of AD as well as intervene in the development of AD, but there are currently no drugs that completely cure and prevent AD.
In recent decades, a growing number of epidemiological and clinical studies have shown that the intake of natural products in the diet may have health benefits, including a reduced risk of neurodegenerative diseases. Current studies suggest that nobiletin one of PMFs, a secondary metabolite of citrus peel, achieves its neuroprotective effects through anti-inflammatory, antioxidant and modulatory effects on signaling pathways. In this review, we focus on the neuroprotective effects of nobiletin on AD from the conditions of AD formation and provide a basis for its development as an AD-related intervene candidate.
| 2. A brief introduction of AD | ▴Top |
AD is a complex multifactorial disease involving chronic neuroinflammation and neurodegeneration (Ozben and Ozben, 2019). The pathological hallmarks of Alzheimer’s disease include extracellular amyloid-β (Aβ) plaques, neurofibrillary tangles (NFT) containing hyper-phosphorylated tau-a microtubule protein, synaptic impairment, decreased mitochondrial membrane potential and mitochondrial malfunction, and cholinergic defects (Braidy et al., 2017). AD has been a huge influence not only on affected people, caregivers, but also on society in both developed and developing countries (Qiu et al., 2009). Memory loss, aphasia, expression disorders, personality and behavioral abnormalities, as well as diet problems and infections in advanced dementia, can occur in patients with AD (Ozben and Ozben, 2019).
Given the demand for ongoing care and rehabilitation, it will create a significant financial strain on the family and society. It was expected that even a one-year delay in the development and progression of clinical AD would greatly lessen the global burden of the disease (Qiu et al., 2009).
| 3. The pathogenesis of AD | ▴Top |
There are many pathways to the formation of AD that may not be explained by a single hypothesis pathway. The formation hypotheses include the amyloid cascade hypothesis, the tau hypothesis, the mitochondrial cascade hypothesis, the apolipoprotein cascade hypothesis, the lipid invasion model, and the neuroinflammation hypothesis. The treatment of AD has shifted from single amyloid therapy to multi-target therapy, and each of the two classical hypothesized mechanisms of AD formation will be explained below.
3.1. The amyloid cascade hypothesis
Amyloid precursor protein (APP) is a type I transmembrane protein. In the Central nervous system (CNS), APP has two hydrolytic pathways: a non-amyloidogenic pathway in which APP is cleaved by α-secretase to produce a soluble APP fragment (sAPPα) and a carboxy-terminal fragment containing 83 amino acids (C83), which is further cleaved by γ-secretase to produce the secretory fragment a 3 kDa product (p3) and the APP intracellular domain (AICD) (Soria Lopez et al., 2019; Yuksel and Tacal, 2019); The other is the amyloidogenic pathway, in which APP is cleaved by β-secretase to produce soluble APPβ (sAPPβ) peptide and C-terminal membrane-bound fragment C99, and release sAPPβ into the extracellular compartment, while γ-secretase continues to cleave the C99 fragment embedded in the plasma membrane to produce Aβ and AICD, in which Aβ is secreted into the extracellular compartment and AICD is released into the cytoplasm (Soria Lopez et al., 2019; Wilkins and Swerdlow, 2017). Studies have demonstrated that sAPPα has neuroprotective properties, encourages synaptogenesis and neurite outgrowth, and has the ability to improve learning and memory in APP-deficient circumstances (Yuksel and Tacal, 2019). sAPPβ is a ligand for Death Receptor 6 (DR6), which triggers caspase activation and mediates degeneration of neuronal cell bodies and axons by binding to DR6 (Nikolaev et al., 2009). Furthermore, though the sequences of AICD peptides produced via the non-amyloidogenic and amyloidogenic pathways are similar, the latter produces a more stable AICD with stronger transcriptional activity (Bukhari et al., 2017; Guo et al., 2021). The non-amyloidogenic AICD is rapidly degraded in the cytoplasm, while the amyloidogenic AICD enters the nucleus and binds to Tip60 to form an ATF complex with regulatory effects on gene expression (Bukhari et al., 2017; Guo et al., 2021). Aβ peptides secreted by γ-secretase treatment of C99 range in length from 37 to 42 amino acids, of which 40 and 42 are most likely to form toxic oligomers, and although the concentration of Aβ42 is only 10% of that of Aβ40, Aβ42 is a major component of amyloid plaques (Bitan et al., 2003). The balance between the production and clearance of these Aβ fragments in the AD brain is disrupted, resulting in the deposition of soluble Aβ oligomers and insoluble amyloid in the brain to form amyloid plaques (Guo et al., 2021). They interact with microglia, astrocytes, blood-brain barrier, glial lymph, meningeal lymph, and neurons to trigger a variety of deleterious cellular responses that ultimately lead to neuronal malfunction (Long and Holtzman, 2019).
The amyloid-β oligomer (AβO) hypothesis is a variation of the amyloid cascade theory, and it proposes that AβO, the most toxic and pathogenic form of Aβ, which are produced when Aβ peptides are cleaved from the membrane and released extracellularly, are responsible for brain damage in AD (Cline et al., 2018; Tyler and Tyler, 2022). Studies have shown that AβO accumulates in human and animal model brain tissue in an AD-dependent manner and triggers tau pathology, synaptic degeneration, oxidative stress, cholinergic impairment, and selective neuronal cell death (Cline et al., 2018).
3.2. The Tau Hypothesis
The Tau hypothesis points to tau as the primary causative agent of AD, not Aβ (Tyler and Tyler, 2022). Tau is a microtubule-associated protein located primarily in axons that stabilizes microtubules and regulates axonal transport of organelles, including mitochondria (Medina and Avila, 2014; Spires-Jones and Hyman, 2014). The human tau protein gene is located on chromosome 17 (Serý et al., 2013). It has 15 exons, where exons 2, 3, and 10 alternately splice together to create six isoforms (Serý et al., 2013). Tau missplicing promotes the development of longer tau isoforms and may result in tau lesions (Bakota and Brandt, 2016). Because oligomeric or misfolded tau proteins can acquire toxic activity and kill neurons (Bakota and Brandt, 2016). In AD tau is hyperphosphorylated, has reduced affinity for microtubules and detaches from them, transferring to neurons to aggregate and accumulate in the somatodendritic compartment as paired helical and straight filaments (Medina and Avila, 2014; Serý et al., 2013; Spires-Jones and Hyman, 2014). Studies have shown that tau mediates Aβ toxicity production in AD and has a central role in the downstream cascade of Aβ-induced neurodegenerative triad (Bakota and Brandt, 2016; Medina and Avila, 2014).
| 4. Chemical aspects of nobiletin and its main derivative 5-demethylnobiletin | ▴Top |
4.1. Chemical aspects of nobiletin
Flavonoid is a benzopyran derivative containing a tricyclic skeleton (C6-C3-C6) and is colorless to yellow crystalline at room temperature and pressure, soluble in water and ethanol. Flavonoids have the chemical formula C15H10O2. The three rings (C6-C3-C6) are called the A-ring, C-ring and B-ring (Figure 1). Nobiletin substitutes -OH and -H on the A ring with -OCH3, and connects -OCH3 at the 2′,3′- positions on the B ring (Singh et al., 2014) (Figure 2).
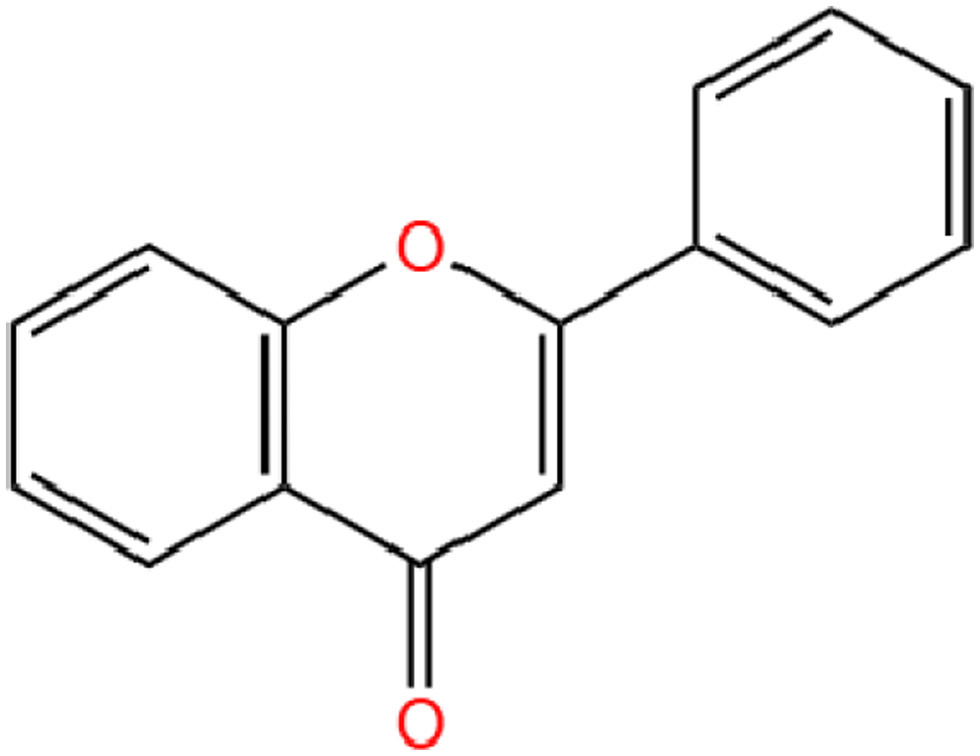 Click for large image | Figure 1. The chemical structure of Flavonoid basic skeleton. |
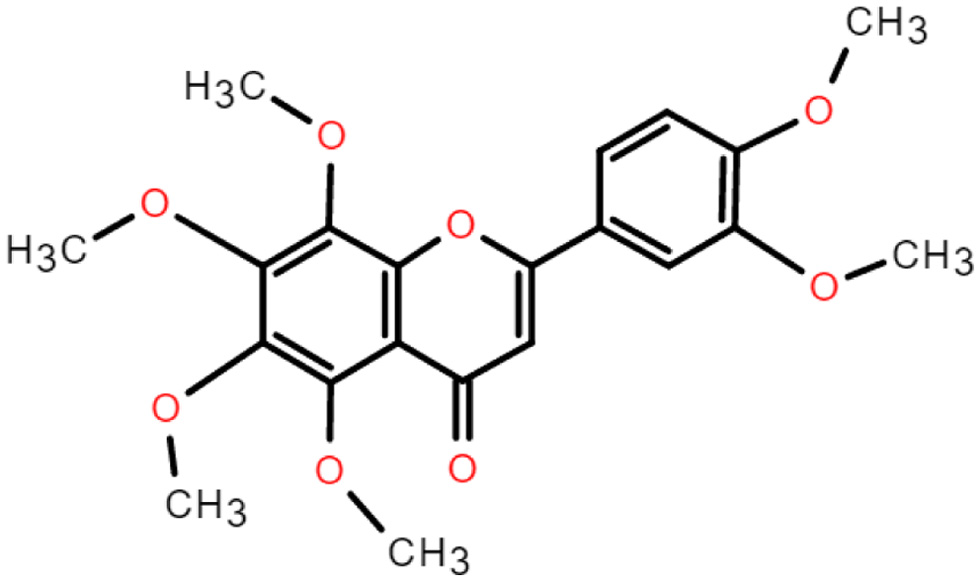 Click for large image | Figure 2. The chemical structure of nobiletin. |
Nobiletin is mainly derived from citrus plants, especially in the peel of Citrus reticulate and Citrus sinensis. The study showed that among the four extraction methods of ethanol, boiling, steaming and soaking, the highest concentration of nobiletin was obtained from ethanol, followed by soaking, boiling and steaming (Yang et al., 2020). The relative molecular mass of nobiletin was 402.39, containing six methoxy per molecule, with low polarity and high activity. Moreover, due to high methylation, polymethoxyflavones have better lipid solubility than other polyhydroxyflavonoids and flavonoid glycosides, and therefore have better bioavailability (Yang et al., 2020). Increasing evidence suggests that PMF metabolites have greater biological activity than the parent compounds (Zheng et al., 2015). 3′-demethylnobiletin (3′-DMN), 4′-demethylnobiletin (4′-DMN) and 3′,4′-demethylnobiletin (3′,4′-DMN) are the most common nobiletin phase I metabolites (Kesharwani et al., 2020). Li et al. showed that the major nobiletin metabolite in mouse urine was 4′-DMN, while 3′-DMN was a minor metabolite (Li et al., 2006).
4.2. Chemical aspects of 5-demethylnobiletin
5-demethylnobiletin (5-DN) has a molecular weight of 388.37 g/mol and a molecular formula of C20H20O8 (Ding et al., 2022). 5-DN is obtained when the methoxy at the 5′ position of nobiletin is substituted with hydroxyl group (Figure 3). Studies have shown that the three main metabolites of 5-DN are 5,4′-didemethylnobiletin (M1), 5,3′,4′-tridemethylnobiletin (M2) and 5,3′-didemethylnobiletin (M3) (Song et al., 2020). The metabolites of 5DN (M1, M2, M3) inhibited human non-small cell lung cancer cells with stronger activity than the 5DN parent itself (Song et al., 2016). And the inhibitory effect of 3′-position (M3) and 4′-position (M1) substituents was higher than that of 3′- and 4′-position (M2). This suggests that the biological activity of PMFs depends on the structure of the substance, i.e. the position of the substituent. The current study shows that the pharmacological activity of metabolites is superior to their parent, such as 4′-DMN and nobiletin, 5,3′-DN and 5-DN, 5-tangeretin and tangeretin, etc. 5-DN is a flavonoid substance with in vivo Solubility and bioavailability are low (Chen et al., 2022). Studies have shown that the bioavailability of lipophilic bioactives can be improved by the action of delivery systems, for example, higher bioaccessibility can be achieved by using nanoemulsions containing triglycerides with different fatty acid chain lengths (Yao et al., 2020), and 5-DN bioaccessibility can be enhanced by using organogel-based emulsion (Zhang et al., 2021).
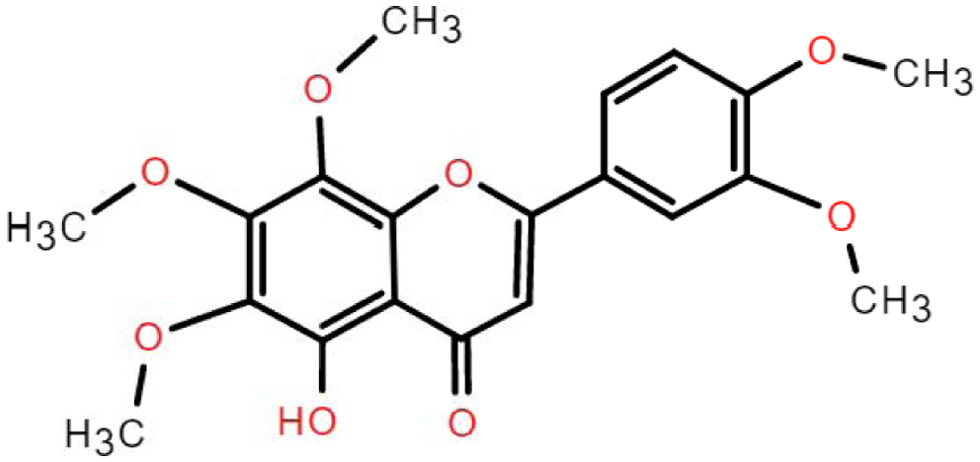 Click for large image | Figure 3. The chemical structure of 5-demethylnobiletin. |
| 5. Bioactive role of nobiletin and its main derivative 5-Demethylnobiletin on AD | ▴Top |
5.1. An overview of bioactivities of nobiletin
Nobiletin is a major component of PMFs extracted from the peel of citrus fruits regardless of reported methods, such as supercritical extraction, microwave assisted and Soxhlet extraction (Kesharwani et al., 2020). Likewise, its derivatives function akin to nobiletin although there are more or less bioactive capacity than nobiletin.
As the most prevalent compounds isolated from the peel of citrus fruits, Current research focused on nobiletin’s anti-inflammatory (Hagenlocher et al., 2019), anti-metastatic (Jiang et al., 2020), anti-allergic (Hagenlocher et al., 2017) and neuroprotective effects (Bi et al., 2016), as well as acting as an enhancer of existing drugs and reducing drug resistance (Moon et al., 2013; Moon et al., 2018). Yvonne et al., showed that nobiletin had an inhibitory effect on the expression of pro-inflammatory mediators and cytokines in mast cells (MC) stimulated with IgE/anti-IgE and LPS/sCD14 (Hagenlocher et al., 2017). This means that nobiletin has the potential to treat LPS or IgE-mediated diseases such as allergies. The study by Jeong et al. demonstrated that nobiletin treatment reduced the expression of neuroblastoma-derived MYC (MYCN) and multidrug resistance-associated protein 1 (MRP1), thereby sensitizing Adriamycin (ADR)-induced A549/ADR cytotoxicity. The above studies suggest that nobiletin can be used as an effective adjuvant to ADR chemotherapy in lung cancer (Moon et al., 2018). When the anticancer medicine 5-fluorouracil (5-FU) was employed at low doses, the combination of nobiletin and 5-FU reduced the viability of SNU-16 cells in a concentration-dependent way. In a p53-independent manner, nobiletin promoted apoptosis through caspase-3 activation and cell cycle arrest via a p21WAF1/CIP1and cyclin D1-mediated route. demonstrating that nobiletin and 5-FU inhibited the growth and induced apoptosis of SNU-16 human gastric cancer cells through different pathways, indicating that nobiletin and 5-FU have a synergistic antitumor effect (Moon et al., 2013). Nobiletin has a wide range of anti-dementia and neuroprotective effects, as shown in Figure 4.
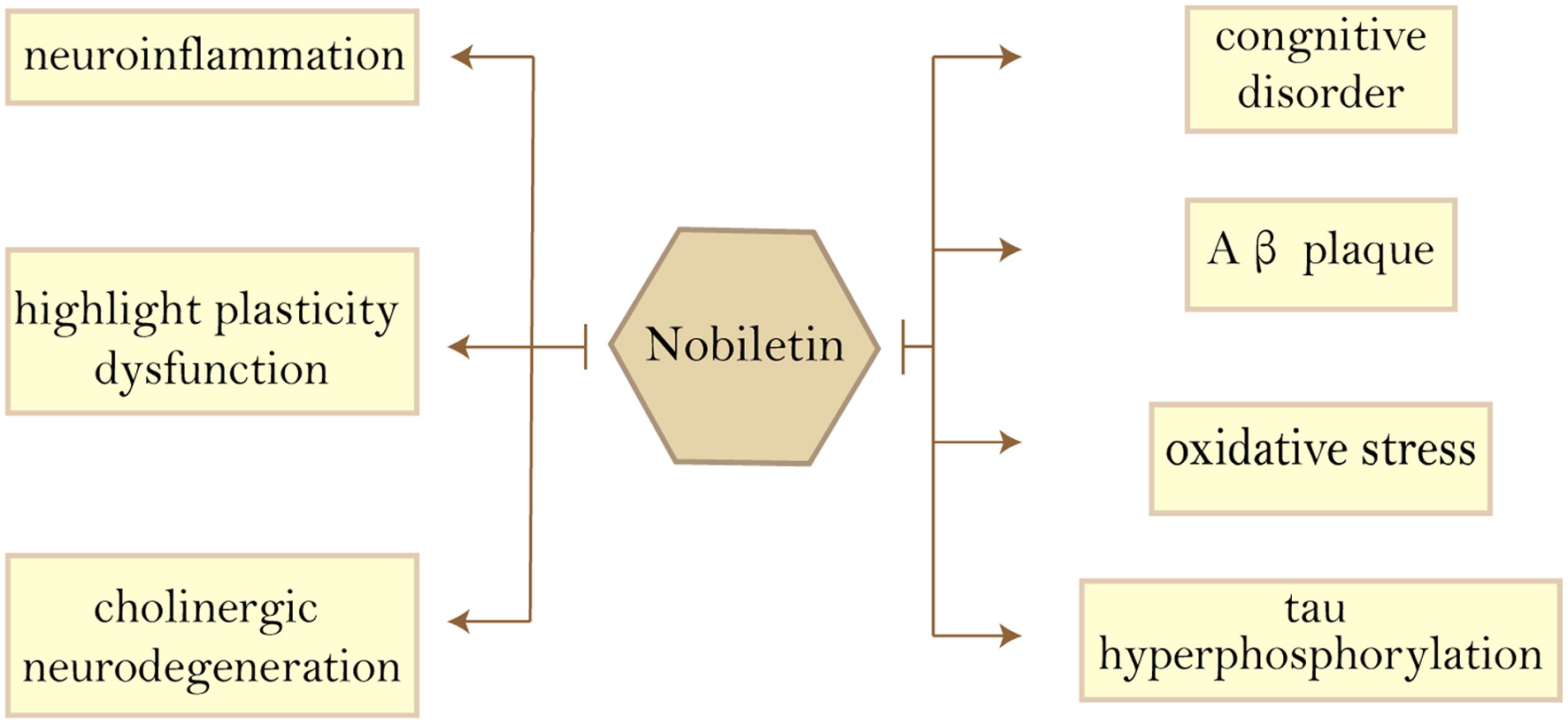 Click for large image | Figure 4. Beneficial effects of nobiletin on Alzheimer’s disease features. Abbreviations: Aβ, amyloid-β. |
5.2. An overview of bioactivities of nobiletin’s derivative 5-Demethylnobiletin
5-Demethylated polymethoxyflavones are a special subclass with abundant biological activity and more than 20 citrus PMFs have been isolated and identified (Song et al., 2017). Among citrus fruits 5-Demethylnobiletin (5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone, 5-DN) is one of the most abundant 5-demethylated PMFs, formed mainly through nobiletin autohydrolysis in the long-term storage of citrus peels, and is a demethylated product of nobiletin (Chen et al., 2022; Chiu et al., 2013; Ding et al., 2022). In addition, 5-DN has a variety of beneficial biological activities such as anti-inflammatory, anti-cancer, antioxidant, neuroprotective and anti-atherosclerotic (Chen et al., 2022; Song et al., 2020). Using 5-DN treatment in an 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced mouse lung tumor model, Song et al. found that 5-DN and its major metabolites (5,3′-DN and 5,4′-DN) inhibited lung tumor development (Song et al., 2017). Among them, 5-DN (20 μM) induced a significant increase in the number of G1/G0-phase cells in A549 cells and G2/M-phase cells in CL13 cells. It also enhanced the expression of cell cycle regulation-related proteins p21Cip1/Waf1 and p53 in A549 and CL13 cells. In addition, 5-DN (20 μM) also activated apoptosis-related proteins caspase3 and PARP in A549 and CL13 cells to trigger apoptosis. These results suggest that 5-DN can inhibit the development of lung tumors in mice by inducing cell cycle arrest and expression of apoptosis-related proteins in different lung cancer cells. Another study showed that 5-DN reduced CCL4-induced lipid peroxidation and improved cell viability by inhibiting the formation of lipid free radicals (Wang et al., 2018). Moreover, 5-DN inhibited lipid peroxidation more than nobiletin, suggesting that the demethylation of the 5′-position is more beneficial to improve lipid peroxidation damage.
5.3. Nobiletin and its derivatives act on AD
It was shown that 5-DN not only inhibited CCL4-induced fibrotic liver and inflammatory response in liver tissue (Chang et al., 2021), but also inhibited colitis-driven colon carcinogenesis in mice (Song et al., 2020). The above studies demonstrate that 5-DN has anti-inflammatory effects. Its anti-inflammatory activity is mainly manifested in reducing the production of inflammatory cytokines such as IL-1β, IL-6 and TNF-α and the expression of acute inflammatory biomarkers such as Cox-2 and iNOS (Chen et al., 2022; Ding et al., 2022; Wu et al., 2016). It has been reported that 5-DN not only inhibits the production of pro-inflammatory factors but also regulates aberrant inflammatory pathways, and it exerts anti-inflammatory effects mainly by regulating the JAK2/STAT3, ROS–AKT/mTOR, MAPK signaling pathways (Ding et al., 2022). Excessive inflammation has been mentioned above as a possible cause of AD formation, and the anti-inflammatory effect of 5-DN may be one of the reasons for its ability to protect the nerves and treat AD.
The main features of AD pathology are amyloid plaque deposition, tau hyperphosphorylation, synaptic damage, cholinergic neurodegeneration, cognitive impairment, oxidative stress, and neuroinflammation; therefore, there are many cellular and animal models of AD in basic research. The following will summarize the effects of nobiletin (Figure 5) and 5-DN in the treatment of AD from different models.
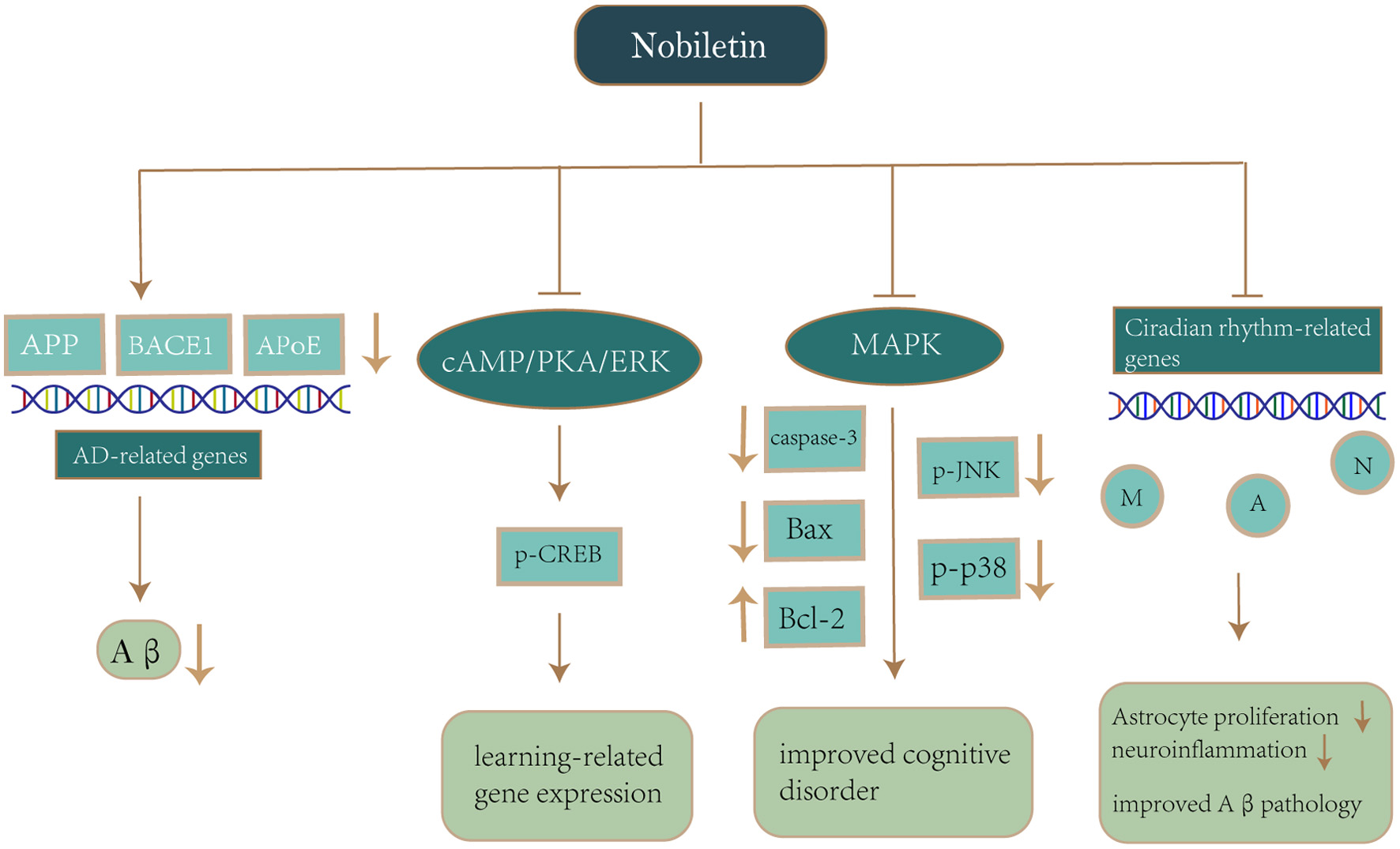 Click for large image | Figure 5. Mechanism of nobiletin on neuroprotection in Alzheimer’s disease. Notes: Nobiletin reduces APP production and Aβ deposition by downregulating AD-related genes (APP, BACE1, APoE). Nobiletin also activates the cAMP/PKA/ERK signaling cascade, which stimulates CREB phosphorylation and enhances the expression of learning-related genes. Nobiletin inhibits p-JNK and p-p38 production via the MAPK pathway, while suppressing caspase-3 and Bax activation as well as upregulation of Bcl-2 for the purpose of avoiding oxidative stress and improving cognitive impairment. Nobiletin reprograms the different circadian rhythms of gene expression in neurons, astrocytes and microglia, which in turn alleviates astrocyte hyperplasia and neuroinflammation, thereby improving Aβ pathology and recognition impairment. Abbreviations: APP, amyloid-β precursor protein; Aβ, amyloid-β; BACE1, β-Site amyloid precursor protein cleaving enzyme 1; APoE, Apolipoprotein E; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; ERK, extracellular signal-regulated kinases; CREB, cyclic-AMP-responsive-element-binding protein; p-JNK, Phosphorylated c-Jun aminoterminal kinase; MAPK, mitogen-activated protein kinases; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; M, Microglia; A, Astrocyte; N, neuronal cell. |
5.3.1. Effect of nobiletin and its derivatives on Aβ deposition and APP synthesis
Amyloid plaques are one of the pathological features of AD. Extracellular amyloid- β protein (Aβ) deposition and aggregation cause them to form. Aβ is formed from amyloid- β precursor protein (APP) by hydrolytic enzymatic cleavage by β-secretase and γ-secretase (Zhang and Song, 2013). The use of an Aβ-injected mouse model is economical and not time-consuming. Moreover, compared to non-pathogenic induced transgenic mice, a pathogen-induced AD model, the Aβ-injected mouse model, can exhibit not only AD-like behavioral abnormalities, but also Aβ pathology (Kim et al., 2016). Matsuzaki et al. used an eight-arm radial maze to examine the effects of nobiletin on learning cognitive impairment in rats, using reference memory errors (RMEs) and working memory errors (WMEs) as an index. nobiletin (10 - 50 mg/kg) was administered daily to rats injected with Aβ1–40 for 7 days before and after surgery. The results showed that injection of Aβ1–40 into the ventricles resulted in impaired reference memory and working memory in rats, while nobiletin administration significantly reduced RMEs and WMEs in Aβ1–40-injected rats, i.e., the compound restored the memory impairment caused by Aβ1–40. Indicates that nobiletin has a protective effect against Aβ1–40-induced impairment in learning cognitive abilities (Matsuzaki et al., 2006) (Table 1).
 Click to view | Table 1. Effect of nobiletin on AD animal models |
In neuronal cell lines APP acts as an upstream positive regulator of Ras-dependent signaling pathways, while Ras-ERK signaling induces hyperphosphorylation of APP and tau in AD brain tissue, and inhibition of Ras-MAPK activation prevents tau and APP hyperphosphorylation and neuronal cell cycle entry (Kirouac et al., 2017). This suggests that APP activates the Ras-ERK signaling axis and glycogen synthase kinase 3 (GSK-3), and is therefore critical in promoting neurodegeneration, so disrupting these signaling pathways may hinder the development of AD (Kirouac et al., 2017).
In cultured rat hippocampal neurons and PC12 cells, nobiletin stimulates cyclic-AMP-responsive-element-binding protein (CREB) phosphorylation by activating the cAMP/protein kinase A/ERK signaling cascade and increases cAMP-mediated dependent transcription in the presence of forskolin (Nagase et al., 2005; Nakajima et al., 2007). cAMP is involved in a number of crucial and critical physiological activities, including normal metabolism, gene transcription, cell growth, and cell cycle completion (Sharma and Singh, 2020). The transcriptional regulator cAMP response element binding protein (CREB) identifies CRE sequences and promotes gene transcription, which is critical for learning and memory (Moosavi et al., 2016). Matsuzaki et al. demonstrated that in rat hippocampal neurons, nobiletin reversed the Aβ-induced reduction in CREB phosphorylation (Matsuzaki et al., 2006). Phosphorylated CREB inhibits apoptosis, enhances survival-promoting pathways, maintains neuro homeostasis, and decreases pathological markers of Alzheimer’s disease (Sharma and Singh, 2020). In the cell culture system, nobiletin, a neurotrophin, increases CRE-dependent transcription by activating the PKA/ERK/CREB signaling pathway, resulting in increased expression of learning and memory-related genes (Kimura et al., 2015; Nagase et al., 2005). Furthermore, 5-DN induced neurite growth in PC12 cells through activation of MAPK/ERK- and PKA-dependent CREB signaling pathways (Chiu et al., 2013). This suggests that 5-DN has the ability to promote neuronal differentiation and the formation of mature synapses, supporting the neurotrophic effects of the nervous system.
In APP-SL 7-5 and 7-9 Tg mice Aβ is produced by overexpression of the human APP695 gene, which contains the double Swedish/ Lsondon mutation. Compared to the non-transgenic group, the levels of Aβ40 and Aβ42 were up to 4,000-fold and 9,000-fold in the 7-5 older group, while only 28-fold and 30-fold in the younger group. It indicates an age-dependent increase in Aβ deposition levels in APP-SL Tg mice. And the level of 7-5 increased Aβ in transgenic group mice was higher than that in 7-9. Therefore, we chose APP-SL 7-5 Tg mice in the older group as the AD mouse model (Shin et al., 2007). Nine-month-old APP-SL 7-5 Tg mice were selected and administered nobiletin (10 mg/kg i.p.) daily for 4 months. Enzyme-linked immunosorbent assay (ELISA) showed that Aβ1– 40 and Aβ1– 42 dissolved in guanine were significantly reduced in the brain of mice after 4 months of continuous administration. The results of immunohistochemistry and anti-Aβ antibody assays were consistent with ELISA, and it was clear that nobiletin reduced Aβ burden and plaques in the hippocampus of APP-SL 7-5 Tg mice. Horizontal and vertical activity was reduced in APP-SL 7-5 Tg mice, but horizontal activity in the nobiletin-treated group tended to be normal. Thus, nobiletin may inhibit the inflammatory response induced by extensive amyloid deposition in the brain of APP-SL 7-5 Tg mice (Onozuka et al., 2008).
The 3xTg-AD mice contain three human mutant genes APP, tau and PS1 and can exhibit AD-related pathological features such as accumulation of plaques and tangles, as well as age-dependent cognitive decline and neuroinflammation (Belfiore et al., 2019). Therefore, this model can represent the FAD model (Chen et al., 2013). Nakajima et al. showed that male 3XTg-AD mice aged 6–7 months were used and started behavioral experiments at the age of 9–10 months (Nakajima et al., 2015). Meanwhile, 3XTg AD mice were treated with nobiletin (10 or 30 mg/kg, i.p.) daily for three months until the end of the behavioral experiment. The results showed that NOB had a protective effect on impaired short-term and recognition memory in 3XTg-AD mice, and had no effect on their motor activity. This suggests that NOB ameliorates cognitive impairment in 3XTg-AD mice by reducing soluble Aβ1–40 levels in their brains
In AD model mice, Kim et al. studied the influence of nobiletin as a clock modulator on circadian rhythm (Kim et al., 2021). Circadian rhythms are biological changes that occur every 24 hours during the day and night (Fifel and Videnovic, 2020). The circadian system is essential for maintaining synchrony between internal physiology, behavior, and environmental stimuli (Leng et al., 2019). Patients with neurodegenerative illnesses may experience deterioration in their health and quality of life if this function is affected. The circadian network’s core or master clock is found in two clusters of neurons called the suprachiasmatic nucleus (SCN) in the anterior hypothalamus of mammals (Leng et al., 2019). In the SCN of AD patients, considerable neuronal loss was seen, as well as the buildup of amyloid plaques and neurofibrillogenic tangles. Furthermore, a damaged SCN clock appears early in Alzheimer’s disease and worsens as the disease progresses (Fifel and Videnovic, 2020). This shows that SCN central clock malfunction could be a factor in altered circadian rhythms and disease progression in Alzheimer’s patients. nobiletin showed a potential to restore sleep bout duration in female APP/PS1 mice to some level, as well as alleviate excessive oxygen consumption and CO2 overproduction in mice with a certain glucose tolerance (Kim et al., 2021). Furthermore, nobiletin reversed the APP/PS1 mice’s poor adaptive thermogenesis caused by the chilly environment, indicating that nobiletin protects against impaired thermoregulation in the AD mouse model (Kim et al., 2021). On the other hand, nobiletin changed the expression of cortical clock-controlled genes and activated various clock-controlled metabolic genes involved in insulin signaling and mitochondrial function, as well as attenuating Aβ plaque deposition in APP/PS1 mice by attenuating the expression of AD-related genes APP, Bace1 and Apolipoprotein E (ApoE) (Kim et al., 2021). This suggests that nobiletin inhibits the synthesis of APP proteins and the deposition of Aβ in a circadian time-dependent manner.
In different AD models, nobiletin exhibited significant inhibition of APP synthesis and Aβ deposition and reversed Aβ-induced learning and cognitive impairment.
5.3.2. Effect of nobiletin and its derivatives on tau hyperphosphorylation
Neuroinflammatory amyloid plaques and intracellular neurofibrillary tangles (NFT) are used in the definition of AD in neuropathology when present together (Bakota and Brandt, 2016). Among them, NFT is generated by massive aggregation and accumulation of hyperphosphorylated tau. Tau is a microtubule-associated protein, which is mainly expressed in the cell bodies and axons of neurons cells in the central and peripheral nervous system of vertebrates, and plays a central role in mediating the downstream cascade of Aβ-induced neurodegenerative triad during the progression of AD (Bakota and Brandt, 2016; Boutajangout and Wisniewski, 2014). Under pathological conditions, tau undergoes oligomerization or misfolding to form insoluble aggregates that acquire toxic activity and kill neurons (Bakota and Brandt, 2016; Dai et al., 2018). Therefore, the study of tau contributes to the improvement of neurodegenerative changes downstream of Aβ action.
The senescence-accelerated mouse prone 8 (SAMP8) exhibits behavioral and histopathological features of AD, showing age-related impairments in spatial learning and memory as well as oxidative stress, tau hyperphosphorylation, and abnormal Aβ expression and deposition (Cheng et al., 2014; Liu et al., 2020). Therefore, SAMP8 mice can be used for the study of AD etiology and provide a way to find new targets for AD treatment. Nobiletin (10–50 mg/kg, i.p.) was administered to 4–6 months old SAMP8 mice daily for one month until all behavioral experiments were completed (Nakajima et al., 2013). On the basis of the behavioral experiments, SAMP8 mice fed nobiletin significantly reversed recognition memory impairment and context-dependent fear memory deficits in SAMP8 mice compared to SAMP8 mice fed standard food (Nakajima et al., 2013).
5.3.3. Effects of nobiletin and its derivatives on cholinergic neurodegeneration
Acetylcholine (ACh), cholinergic receptors (AChRs), choline acetyltransferase (ChAT), and acetylcholinesterase (AChE) are all part of the cholinergic system (Halder and Lal, 2021). It is crucial for memory, learning, and other elements of cognition. As a result, ongoing research into normal cognition and age-related cognitive deficits, such as Alzheimer’s disease, is focused on the cholinergic system. A progressive loss of limbic and neocortical cholinergic innervation is central to the cholinergic hypothesis of Alzheimer’s disease. The main cause of forebrain cholinergic neuronal dysfunction and death is neurogenic fiber degeneration in the basal forebrain, which leads to extensive presynaptic cholinergic denervation. Furthermore, cholinesterase inhibitors are available for the treatment of Alzheimer’s disease, demonstrating that the cholinergic system is a key therapeutic target for the disease (Hampel et al., 2018). Shalini Trivedi et al. investigated the effect of 5-DN on cholinergic function using Caenorhabditis elegans as a model organism (Trivedi et al., 2017). The results showed that 5-DN enhances cholinergic neurotransmission by increasing synaptic Ach levels and nicotinic Ach receptor activity.
Destruction of the bilateral olfactory bulbs will lead to chronic changes in brain state and complex changes in behavioral, neurochemical, neuroendocrine and neuroimmunological parameters (Zueger et al., 2005). Studies have shown that five weeks after olfactory bulb removal, olfactory bulbectomized (OBX) mice develop AD neurodegeneration, such as loss of spatial memory, elevated Aβ levels in the brain, energy deficits, cognitive deficits, and decreased cholinergic activity in the hippocampus (Avetisyan et al., 2016; Moriguchi et al., 2021). These phenomena are similar to those occurring in vivo in familial AD models and sporadic AD patients (Avetisyan et al., 2016). Therefore, OBX mice can be used as an effective AD model for the study of AD pathogenesis and the development of related drugs. Nagase et al. examined the effect of 11-day nobiletin treatment in OBX mice with cholinergic neurodegeneration using passive avoidance test and Y-maze test (Nagase et al., 2005). Nakajima et al. based on adult male ddY mice, which could be treated in a certain way to obtain OBX mice, and control mice were treated in the same way but without removal of the olfactory bulb. Three days after surgery, OBX mice were injected intraperitoneally with nobiletin (50 mg/kg per) for 11 days. After 11 days of administration, nobiletin caused a decrease in acetylcholinesterase staining density and ChAT expression in the hippocampus of OBX-induced mice. These results suggest that nobiletin has a protective effect on OBX-induced cholinergic neurodegeneration, while improving impaired memory in OBX mice (Nakajima et al., 2007).
5.3.4. Effects of nobiletin and its derivatives on cognitive impairment
Previous studies have shown that the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway is linked to cell proliferation, differentiation, migration, senescence, and apoptosis (Sun et al., 2015). Nakajima et al. showed that nobiletin restored N-methyl-D-aspartate (NMDA)-stimulated ERK phosphorylation impairment in rat hippocampal neurons induced by the NMDA receptor antagonist dizocilpine maleate (MK-801) in a concentration-dependent manner (Nakajima et al., 2007). In other words, activating ERK in hippocampus neurons, nobiletin may help to alleviate the cognitive deficits and learning problems associated with NMDA receptor hypofunction.
5.3.5. Effect of nobiletin and its derivatives on oxidative stress
Hyun et al. treated HT22 cells, a murine hippocampal neurons model by hydrogen peroxide-induced, with C. unshiu immature peel (CUIP) and nobiletin, showed that CUIP and nobiletin could affect the MAPKs pathway and protect HT22 cells from death (Cho et al., 2015) (Table 2). Specifically, they inhibited the production of p-N-terminal kinase (p-JNK) and p-p38 in HT22 cells through the major component nobiletin, while inhibiting caspase-3 and pro-apoptotic protein Bax activation and upregulating B-cell lymphoma-2 (Bcl-2) to prevent H2O2-induced HT22 cell death (Cho et al., 2015). A common trigger for death signaling in neurons is dysregulation of intracellular oxidative stress, which mediates neuronal dysfunction and death in Alzheimer’s disease (Wu et al., 2018). Therefore, inhibition of oxidative stress may be a therapeutic route for AD. Furthermore, nobiletin (10–50 mg/kg, i.p.) administration reversed the decrease in the glutathione (GSH)/glutathione disulfide (GSSG) ratio in the cerebral cortex and hippocampus, significantly increased glutathione peroxidase and manganese superoxide dismutase activities, and decreased protein carbonyl levels in the brains of SAMP8 mice (Nakajima et al., 2013). Nobiletin also significantly reduced ROS levels in the hippocampus of 3XTg-AD mice and wild-type mice (Nakajima et al., 2015). The above studies demonstrate that nobiletin could be a promising therapeutic approach to prevent or treat AD by attenuating oxidative stress.
 Click to view | Table 2. Effect of nobiletin on cell models |
5.3.6. Effects of nobiletin and its derivatives on neuroinflammation
In the central nervous system, there are two types of cells: neurons and glial cells. In the central nervous system, there are two types of cells: neurons and glial cells. And glial cells include microglia, oligodendrocytes and astrocytes, which have the role of regulating neurons (Kwon and Koh, 2020). Astrocytes are central nervous system (CNS) secretory cells that secrete neurotransmitters, growth factors, inflammatory mediators, and toxic proteins. Their primary role is to restore water and ion homeostasis, as well as to keep the blood-brain barrier (BBB) intact (Rostami et al., 2021). However, the activation of astrocytes is heterogeneous and traditionally divided into A1-phenotype astrocytes, which are neurotoxic, and A2-phenotype astrocytes, which are neuroprotective (Kwon and Koh, 2020). If large amounts of pathogenic proteins such as amyloid-β, tau, and α-synuclein accumulate in the extracellular space, then these stimuli drive astrocytes to transform into a pro-inflammatory phenotype and thus neurotoxic (Kwon and Koh, 2020). Neuroinflammation is actually a neuroprotective mechanism, but due to a combination of endogenous and exogenous environmental factors, persistent inflammatory stimuli induce a chronic inflammatory response in astrocytes, leading to the development of neurodegenerative diseases such as AD (Kwon and Koh, 2020). Recent studies have shown that nobiletin acts as a biological clock regulator to improve Aβ pathology and recognition deficits by reprogramming different circadian rhythms of gene expression in neurons, astrocytes and microglia, which in turn alleviates astrocyte proliferation and neuroinflammation (Wirianto et al., 2022).
| 6. Other PMFs function with anti-AD effects | ▴Top |
Citrus PMF is a safe natural product, with few side effects. No information on PMF toxicity has been reported in current studies, and long-term use of reticulated citrus extracts containing high concentrations of PMF has no negative effects on animals or humans. In addition to nobiletin, other PMFs such as tangeretin and HMF may have neurotrophic and preventive potential against neurodegenerative diseases due to their anti-inflammatory and antioxidant effects.
6.1. Tangeretin
Tangeretin [5, 6, 7, 8-tetramethoxy-2-(4-methoxyphenyl)-4H1-benzopyran-4-one] is a naturally occurring O-polymethoxylated flavonoid found in citrus fruits (Yumnam et al., 2018). Tangeretin and Nobiletin both belong to the polyphenolic flavonoid class of plant secondary metabolites.
β-Site amyloid precursor protein cleaving enzyme 1 (BACE1) is one of the major therapeutic targets in AD and belongs to a class of type I membrane-bound aspartyl proteases responsible for APP cleavage and production of Aβ plaques (An et al., 2017; Das and Yan, 2019; Sayad et al., 2022). According to studies, BACE1 excision not only reduces Aβ production but also improves cognitive and behavioral impairments (Das and Yan, 2019). Excision of the BACE1 gene in a transgenic mouse model of AD rescued memory deficits, while BACE1 inhibitors reduced amyloid accumulation in the brain of animal models, improving memory impairment (Evin and Hince, 2013). The aforementioned studies show that BACE1 inhibitors have the potential to treat Alzheimer’s disease. Tangeretin has the largest inhibitory impact on BACE1 among PMFs, and because tangeretin is a natural BACE1 inhibitor with a relatively modest inhibitory effect, the adverse effects of excessive BACE1 inhibition may be reduced (Youn et al., 2017). Bao et al. recently discovered that tangeretin suppressed BACE1 activity in the cortex of APP/PS1 mice, lowering Aβ plaque formation (Bao et al., 2022). This suggests that tangeretin can attenuate synaptic damage, reduce Aβ accumulation and improve cognitive impairment in mice.
Recent studies have shown that tangeretin can inhibit LPS-induced expression of inflammatory cytokines in primary rat microglia and BV-2 microglia (Shu et al., 2014). It also exerts anti-neuroinflammatory effects by reducing the inhibition of interleukin 1 beta (IL-1β), interleukin 6 (IL-6), transforming growth factor beta 1 (TGF-β1) and vascular endothelial growth factor (VEGF) expression through PI3K/Akt pathway (Guo et al., 2017; Lv et al., 2021). In addition, tangeretin can also exert neuroprotective effects by reducing the expression of insulin receptor (InsR) and Insulin-like growth factor-1 (IGF-1) receptor in the Mammalian/mechanistic target of rapamycin (mTOR) signaling pathway (Qin and Jiang, 2020).
6.2. HMF
HMF is a type of PMF with significantly higher permeability in brain tissue than nobiletin and tangeretin (Okuyama et al., 2017). in other words, HMF has the ability to act directly in the brain. Recent studies have shown that HMF can inhibit LPS-induced NO release and reduce the expression of pro-inflammatory factors IL-1β, IL-6 and TNFα (Wang et al., 2019). Subcutaneous injection of HMF inhibited LPS-induced activation of hippocampal microglia and the expression of pro-inflammatory factors in the hippocampus (Okuyama et al., 2015). The above results demonstrate that HMF has anti-inflammatory effects and can reduce neuroinflammation in the brain.
Brain-derived neurotrophic factor (BDNF) is the representative and most abundant neurotrophic factor in the brain and has an important role in neurogenesis and neuroplasticity (Sawamoto et al., 2016). Satoshi Okuyama et al. showed that HMF prevents neuronal cell death in the CA1 cell layer and stimulates autophosphorylation of Ca(2+)/calmodulin-dependent protein kinase II (CaMK-II) by inducing BDNF production, thereby inhibiting the pro-inflammatory phenotype of microglia activation (Okuyama et al., 2014). In addition, HMF also rescued the corticosterone-induced decrease in hippocampal BDNF, p-ERK1/2, and p-CaMK II (Sawamoto et al., 2016).
Taken together, it is clear that HMF has the potential to prevent and protect against neurodegenerative diseases due to its anti-inflammatory and neuroprotective effect.
| 7. Conclusion | ▴Top |
It is widely accepted that dietary habits have a powerful influence on AD risks. Here, we analyzed the mechanisms of impact on AD regarding citrus PMF nobiletin and its derivative 5-DN, together with its PMFs analogues. On the basis of experimental and sporadic clinical trial reports, nobiletin, as well as its PMF analogues and derivative 5-DN, potentially exerts neuroprotective effects through multiple molecular pathways. Overall, citrus PMFs might be good candidates for the prevention and treatment of AD although more profound mechanism investigation and clinical trials remain request.
| References | ▴Top |