| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 24, December 2023, pages 13-28
Binding of carotenoids to proteins: a review
Fereidoon Shahidi*, Chandrika Sewwandi Dissanayaka
Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, A1C 5S7, Canada
*Corresponding author: Fereidoon Shahidi, Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, A1C 5S7, Canada. E-mail: fshahidi@mun.ca
DOI: 10.31665/JFB.2023.18360
Received: December 26, 2023
Revised received & accepted: December 30, 2023
| Abstract | ▴Top |
Carotenoids are lipophilic natural pigments distributed in plants, certain types of algae, fungi and animals. The extensive conjugated double bond system in carotenoids is responsible for their unique color, antioxidant capacity and provide health benefits. However, the hydrophobic nature of carotenoids impacts their color and bioactivity during the development of food products due to their low solubility in aqueous media. The complexation of these molecules with proteins has proven to be an efficient approach for enhancing carotenoid’s solubility and protection against oxidative degradation and hence improving their functional properties and biological activities. This review compiles the molecular interactions between carotenoids and proteins, their physiological relevance, potential applications and characterization of their binding affinities, stabilities, and activities in terms of in-silico analysis and beyond. Overall, the deep understanding and interpretation of binding at the molecular level provide fundamental aspects for the inclusion of carotenoid bioactive compounds in fortified foods and pharmaceuticals.
Keywords: Carotenoids; Carotenoproteins; Carotenoid-protein molecular interactions; Docking
| 1. Introduction | ▴Top |
Carotenoids are natural pigments found in plants, algae, fungi, cyanobacteria and animals and are responsible for the yellow, orange, and red colors in various fruits and vegetables, among others (Saini et al., 2015). In plants, they function as protectors against photooxidative stress, precursors of various hormones, serve as light-harvesting pigments and contribute to seed dispersal and pollination by attracting pollinators (Saini et al., 2015; Shete and Quadro, 2013). They are also one of the major classes of natural pigments in marine animals such as fish and invertebrates as well as certain birds. In living organisms, carotenoids are responsible for coloration of the body, tissues or biological fluids and function as a chromatic adaptation to the living environment (Shahidi and Brown, 1998).
Carotenoids play a significant role in the human body by acting as provitamin A. They are essential for eye health and are associated with reducing the risk of cancers, cardiovascular ailments, neurodegenerative diseases, obesity, high blood pressure, aging and enhancing the immune system as well as benefiting skin health. The antioxidant property of carotenoids is the primary therapeutic characteristic of these compounds, where the conjugated polyene chain is involved in scavenging free radicals (Lakey-Beitia et al., 2019; Rehman et al., 2020). Generally, an inadequate ingestion level of carotenoids leads to visual disability by causing signs of corneal ulceration, irreversible blindness, keratomalacia, xeropthalmia, corneal aberration and night blindness (Rehman et al., 2020; Sommer, 2008).
Carotenoids are synthesized in plastids of all photosynthetic organisms and play a major role in photosynthesis, protect chlorophylls by absorbing light energy and aid in stabilization of cell membrane by scavenging oxygen radicals (Rehman et al., 2020; Rosas-Saavedra and Stange, 2016). The conjugated polyene system of carotenoids with the functional groups attached determines their color, configuration, reactivity and photochemical properties with their biological function. Most carotenoids absorb blue and violet light in the range between 400 and 500 nm and exhibit colors in the yellow, orange and red (Rodriguez-Concepcion et al., 2018). In addition to these colors, esterification of carotenoids with fatty acids during fruit ripening may affect the color intensity (Britton, 2008). Animals, including humans, do not synthesize carotenoids de novo and accumulate them from ingested foods. Marine animals contain various carotenoids which are taken from foods such as algae and other animals. They accumulate carotenoids in their integuments and also in the gonads. Integumentary carotenoids play an important role in photoprotection, camouflage, and signaling such as breeding color in marine animals (Maoka, 2011). In this framework, this review further describes their structure, classification, molecular interactions between carotenoids and proteins and their potential application in food, pharmaceuticals and cosmetics industries.
| 2. Structure of carotenoid pigments and classification | ▴Top |
Carotenoids belong to the isoprenoid family and their basic structure is made up of eight isoprene units, resulting in a C40 backbone known as phytoene. The majority of carotenoids exist in a more stable all trans-isomeric form compared to cis-isomeric conjugation in plants. Further modification through desaturation, cyclization and hydroxylation converts colorless phytoene into the colored carotenoids that are present in photosynthetic systems. Carotenoids can be classified into two groups on the basis of functional groups present and are named carotenes and xanthophylls. Carotenes are hydrocarbons and classified as α-carotene, β-carotene, ε-carotene, and lycopene (Figure 1). Xanthophylls are derivatives of carotenes that contain one or more oxygen as a functional group and are classified as hydroxylated carotenoids such as lutein, zeaxanthin and cryptoxanthin, ketocarotenoids such as astaxanthin, methoxylated carotenoids such as rhodovibrin, oxocarotenoids such as rhodoxanthin and canthaxanthin, depending on the oxygenated substituents attached on the terminal rings as shown in Figure 2 (Shahidi and Brown, 1998). In addition to this, fucoxanthin is considered as allenic carotenoid of the xanthophyll family which has an unusual allenic bond and some oxygenic functional groups such as epoxy, hydroxyl, carbonyl and carboxyl moieties in its molecule (Peng et al., 2011).
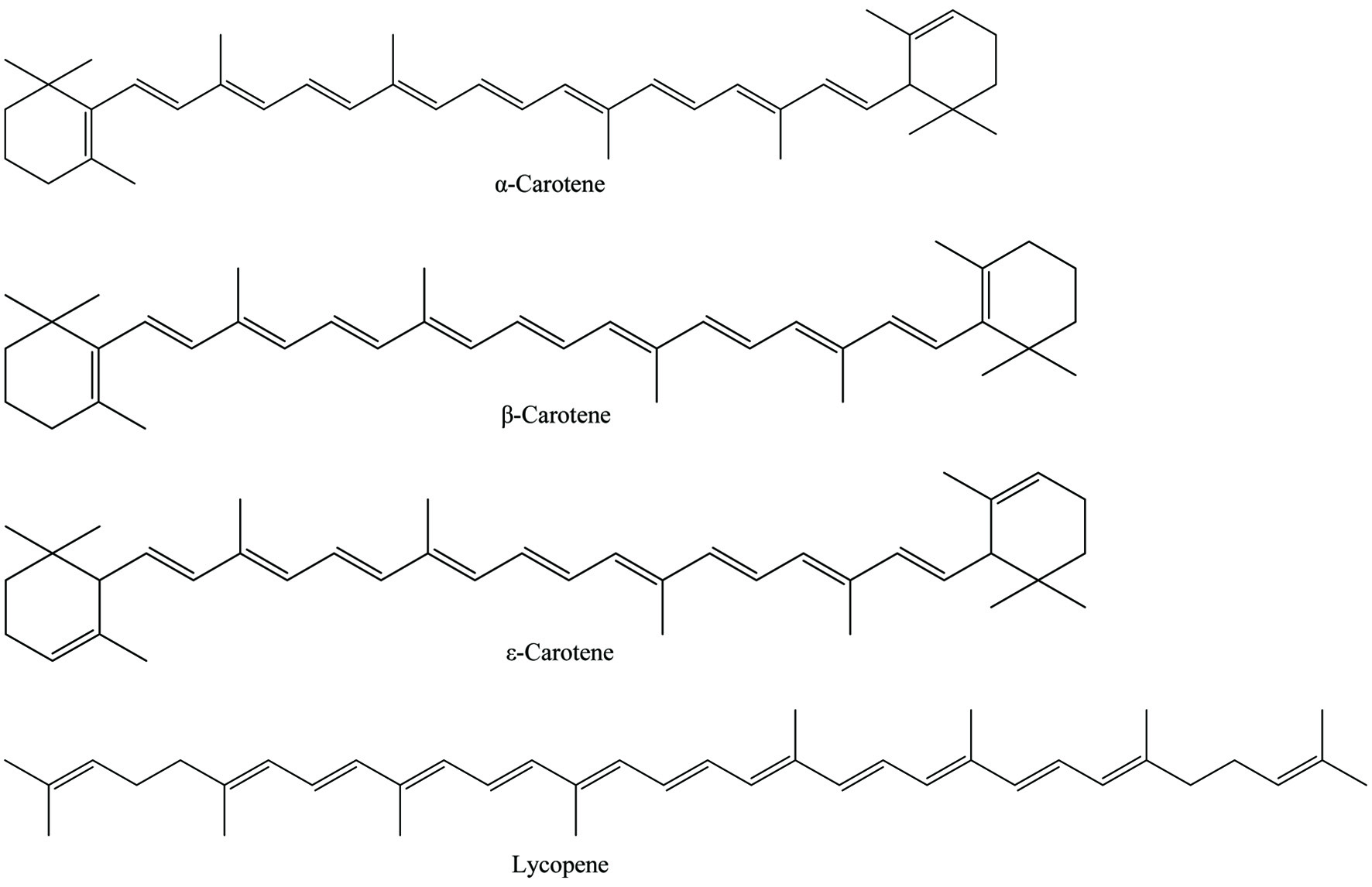 Click for large image | Figure 1. Chemical structures of carotenes. |
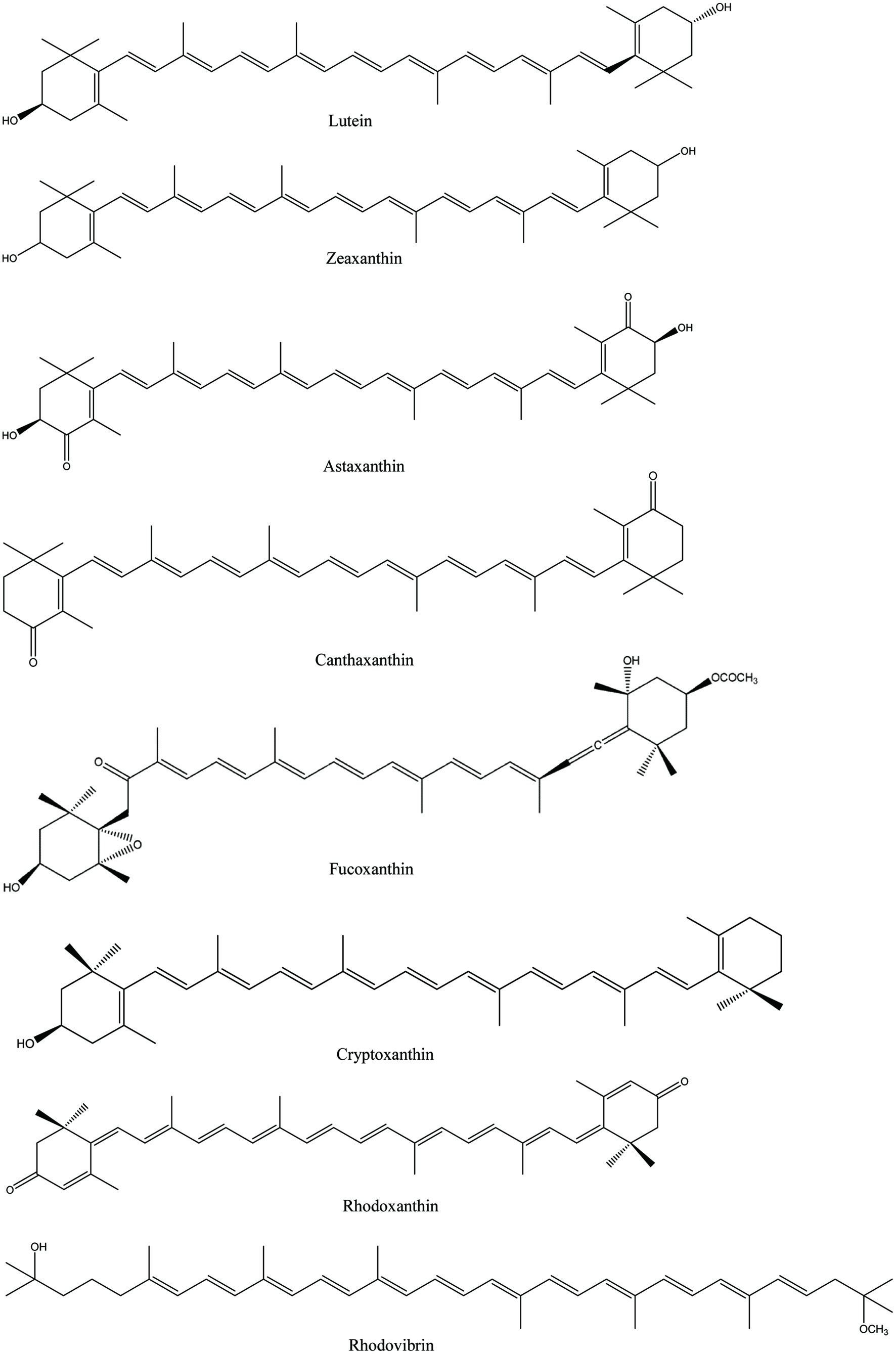 Click for large image | Figure 2. Chemical structures of xanthophylls. |
Furthermore, oxidative cleavage of carotenoids leads to the production of apocarotenoids which is catalyzed by carotenoid cleavage dioxygenases (CCDs) or non-enzymatic processes. CCD enzymes catalyze carotenoid cleavage at specific double bonds at one or both ends of the molecule, which may contribute to the diversity of apocarotenoids while non-enzymatic apocarotenoid formation can occur via singlet oxygen attack, mainly on β-carotene. Crocetin and bixin are considered as carboxycarotenoids of the apocarotenoids family (Figure 3). Apocarotenoids are more soluble and volatile than carotenoids and are important in carotenogenesis, plant development and function as signals in response to environmental stress (Auldridge et al., 2006; Hou et al., 2016; Rodriguez-Concepcion et al., 2018).
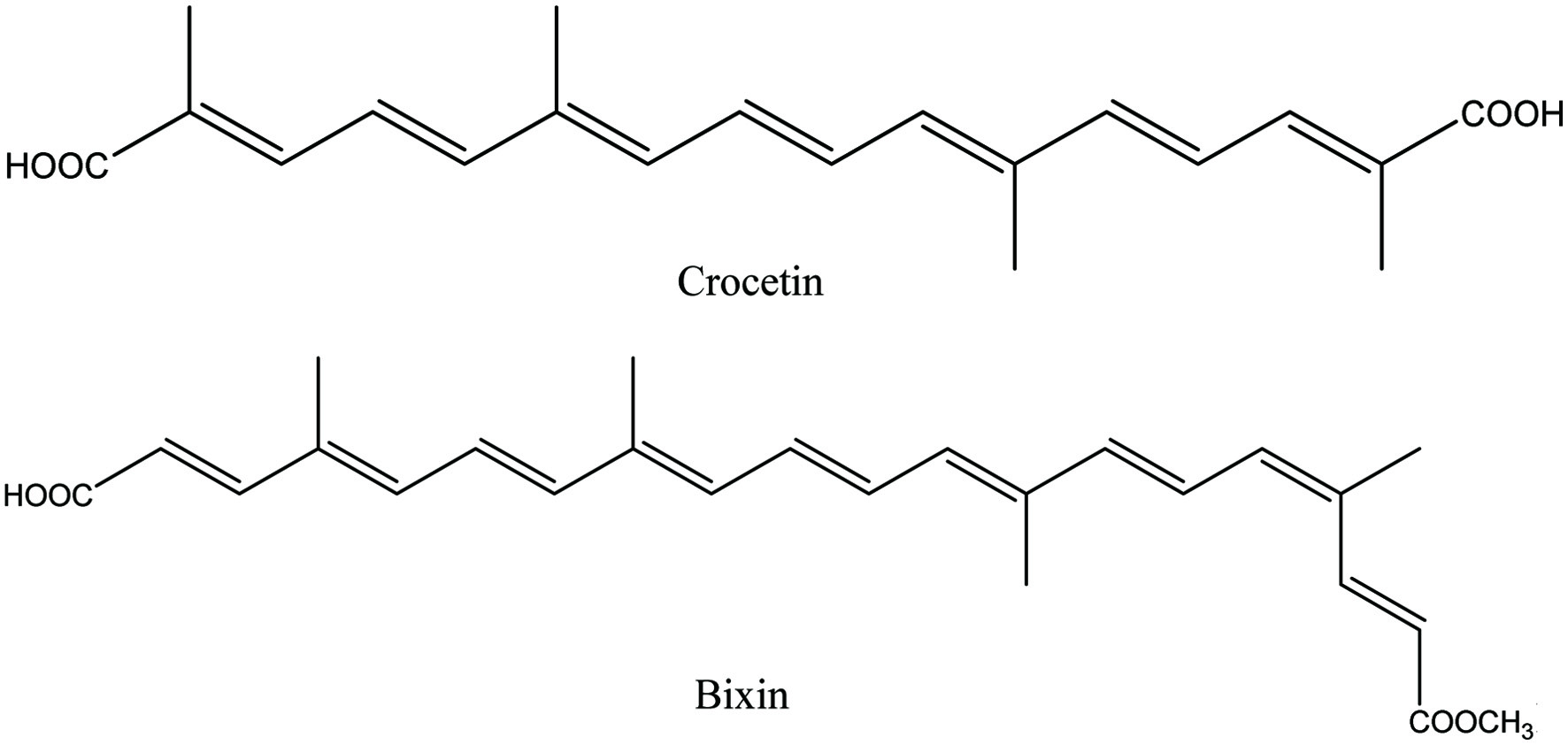 Click for large image | Figure 3. Chemical structures of apocarotenoids. |
Carotenes are fat-soluble pigments found in many dark green and yellow leafy vegetables. Both β-carotene and α-carotene may be present in yellow-orange vegetables and fruits such as carrots, butternut squash, sweet potatoes and lettuce. Lycopene is a powerful antioxidant among other carotenoids and lacks vitamin A activity. The red-colored fruits such as grapes, cranberries, watermelon and tomatoes are rich in lycopene (Khoo et al., 2011).
Xanthophylls are more polar than carotenes found in the leaves of most plants, giving yellow to red colors. They are also considered as accessory pigments that include anthocyanins, carotenes, and phycobiliproteins (Khoo et al., 2011). Among xanthophylls, zeaxanthin acts as a key factor in vision protection, an anti-inflammatory agent, and also supports brain function (Demmig-Adams et al., 2020). Astaxanthin is found in various microorganisms and marine animals. Astaxanthin and its esters are used as food colorant in animal and fish feed and especially as a source of pigment in the feed for salmon, trout and shrimp (Lin et al., 2016). Lutein and zeaxanthin are the most common xanthophylls in green leafy vegetables, peas, asparagus, brussels sprouts, broccoli, lettuce, pistachios and egg yolks (Johnson, 2002). However, a large fraction of carotenoids is masked by the more dominant pigment chlorophyll in green plant tissues and appears only upon degradation of chlorophyll. This can be seen in the leaves of certain trees during the autumn in seasonally cold regions and the ripening of fruits. However, the color of these pigments in living organisms differ due to their chemical structural difference and interaction with other components, particularly proteins (Shahidi and Brown, 1998).
| 3. Carotenoproteins | ▴Top |
Carotenoproteins are complex compounds in which carotenoids are associated with proteins. They are found in combination with simple proteins, structural proteins, lipoproteins and glycoproteins in a particular ratio forming stable compounds (Britton and Helliwell, 2008). Since carotenoids are slightly soluble in aqueous media, their binding to hydrophobic sites of proteins facilitates their solubility in aqueous systems. Moreover, carotenoids in the free form are more susceptible to oxidative degradation due to their long conjugated double bond system. Thus association of carotenoids with proteins may help solubilizing them in fat-free media, stabilizing during processing and storage and change their color (Shahidi and Brown, 1998).
Carotenoproteins are widely distributed in ovaries, eggs, blood, cuticle and epidermis of marine invertebrates as shown in Table 1 (Shahidi and Brown, 1998). These complexes are mainly present in the carapace of crustaceans giving a grey, black, brown, green, purple or blue color appearance, but these colors change to yellow, orange or red upon cooking due to protein denaturation. In addition, carotenoid-protein complexes can be found in cyanobacteria, algae and plants which are responsible for color development, photooxidative protection and photosynthesis (Britton and Helliwell, 2008).
 Click to view | Table 1. Some examples of carotenoprotein complexes in animalsa |
Astaxanthin is one of the major carotenoids found in invertebrate animals such as shrimp, crab, crayfish and lobster as well as in some of fish and bird feathers which form complexes with proteins resulting in carotenoproteins. For instances, binding of astaxanthin to a carrier protein forms α-crustacyanin which is responsible for the blue coloration of lobster carapace (Dellisanti et al., 2003). Supporting this fact, Armenta-López et al. (2002) found a 265 kDa protein attached to astaxanthin in shrimp waste. Ovoverdin, astaxanthin-lipovitellin is responsible for the green color in lobster eggs and ovaries (Zagalsky, 1985). Moreover, red-orange color in salmonid fish is due to the binding of astaxanthin to their muscle protein. Furthermore, carotenoid-keratin binding in bird feathers, the purple color of asteriarubin carotenoprotein complex in starfish (Asterias rubens) and intensive blue color of linckiacyanin complex in blue sea star (Linckia laevigata) have been identified (Britton and Helliwell, 2008).
Salmonid fish species are unique among other types of fish with distinctive flesh coloration due to their ability to deposit carotenoids, particularly astaxanthin and its esters in their muscle tissues. It was found that the fish diet rich with astaxanthin was more efficiently utilized for pigmentation in Artic char (Salvelinus alpinus) than canthaxanthin and longer feeding period resulted a deeper color to the flesh. This pigmentation study also indicated possible improvement of fish flesh color by addition of carotenoids to fish diets (Shahidi et al., 1993). Furthermore, it was discovered that lipoproteins transport the ketocarotenoid astaxanthin and canthaxanthin in oncorhynchus species, including trout and salmon. For instance, it was assumed that astaxanthin present in chum salmon is transported to the skin by conjugating with high-density lipoprotein (HDL). Moreover, astaxanthin has shown a high affinity for very high-density lipoprotein (VHDL) than canthaxanthin while both have a similar affinity for binding with high-density lipoprotein (HDL) (Aas et al., 2000; Matthews et al., 2006).
Several drawbacks are associated with carotenoids’ addition in food formulations. The aggregation of carotenoids occurs during the development of formulated food products and storage due to their lipophilicity. Carotenoids can easily aggregate in an aqueous medium making them insoluble which may result in structural changes during food processing and influence their color, bioavailability, and bioactivity. The association of carotenoid molecules within aggregates is influenced by steric properties and attractive forces between neighboring molecules (Hempel et al., 2016). Thus, binding of carotenoids to food proteins appears to provide a promising strategy to overcome this aggregation (Mantovani et al., 2021).
The chemical structure of carotenoids, location in a cell and their interactions with other macronutrients, particularly proteins, result in their differential physiochemical characteristics. These factors can affect the absorption and metabolism of carotenoids and these account for synergistic or antagonistic effects on biological activities (During and Harrison, 2004). Therefore, understanding the interaction between carotenoids and proteins is crucial for the food, pharmaceutical and cosmetic industries which can be used to enhance the stability of food, design and manufacture of food additives and to develop protein-based delivery systems.
3.1. Molecular Interactions between carotenoids and proteins
Many of the carotenoids can be found in the exoskeleton, epidermis and reproductive organs of invertebrates and they are mainly stabilized by non-covalent bonding with the surrounding proteins (Heras et al., 2007). Formation of carotenoids-milk protein complexes is important for enhancing the carotenoid solubility in fat-free and low-fat media and protection against oxidative degradation. For instance, caseins and whey protein in milk have a great ability to bind with carotenoids at the sites of hydrophobic domains. These proteins interact with carotenoids mainly through hydrophobic interactions due to the long hydrocarbon chain of carotenoids (Mantovani et al., 2021). However, van der Waals forces and hydrogen bonding may occur between carotenoids and proteins depending on the chemical nature of these two components and the environmental conditions such as temperature, pH and ionic strength (Allahdad et al., 2018; Mantovani et al., 2021). Several studies have identified the interactions between proteins and these carotenoid bioactive compounds in terms of thermodynamic, spectroscopic and in-silico analysis (Allahdad et al., 2018; Hashemi et al., 2020a; Jafarisani et al., 2018; Mantovani et al., 2021; Zhang and Zhong, 2012b).
In the study of the interactions in casein-β-carotene complex, the thermodynamic parameters have indicated that κ-casein represented the highest binding affinity to β-carotene and van der Waals forces and hydrogen bonds were the main forces in the formation of the complex. Furthermore, it has been found that the interactions involving non-covalent bonds are highly susceptible to environmental conditions and hence factors such as temperature, pH and ionic strength affect the complexation of casein and β-carotene by forming different conformations of casein. The results have demonstrated that the binding is enthalpy-driven with 1:1 binding stoichiometry, favored at low temperatures, low ionic strengths and alkaline pH (Allahdad et al., 2018).
β-Casein is the most hydrophobic casein and has the largest regions with high hydrophobicity compared to κ-casein, αs1-casein and αs2-casein (McSweeney and Fox, 2013). Based on previous studies, β-casein has shown the weakest binding affinity for β-carotene. The self-association of hydrophobic tails in β-casein may block the C-terminal hydrophobic residues at the binding sites of the protein, resulting in a low affinity for β-carotene (Allahdad et al., 2018). Similar conformational changes occur in β-carotene-whey protein complexes that are bound with hydrophobic interactions. The binding affinity of whey protein to β-carotene was in an increasing order with β-lactoglobulin (β-LG) ≫ bovine serum albumin (BSA) > α-lactoalbumin (α-LA), indicating that β-LG may act as a suitable and protective carrier for β-carotene among other milk proteins (Allahdad et al., 2019).
According to thermodynamic investigations, the molecular interactions between β-carotene or astaxanthin with human serum albumin (HSA) and BSA have revealed that hydrophobic and electrostatic forces are involved in the formation of carotenoid-protein conjugates. Furthermore, binding of β-carotene or astaxanthin to HSA/BSA was synergistically driven by enthalpy and entropy. It has been suggested that these two carotenoids interact with both the C=O and C–N groups in the HSA/BSA protein and result in a change of secondary structure of the protein and hence decreasing the hydrophobicity of the microenvironment of Tyr and Trp residues (Li et al., 2015).
β-LG is the major whey protein of cow’s milk which is a small protein with 162 amino acid residues (Kontopidis et al., 2004). The study of thermal stability of the complex formed between carotenoids from sea buckthorn (Hippophae rhamnoides L.) and bovine β-LG reported that slight levels of protein unfolding may favor its binding with carotenoids due to the exposure of hydrophobic sites in the protein structure, leading to a thermodynamically more stable assembly. Conversely, protein aggregation may diminish the binding affinity by blocking the binding sites. The carotenoid-bovine β-LG complex heated at temperatures higher than 80 °C showed a partial unfolding of the protein within the complex, followed by an increase of hydrophilicity in the vicinity of hydrophobic residues. Based on fluorescence spectroscopy and molecular dynamics simulations, it has been suggested that hydrophobic interactions stabilize the β-LG and β-carotene conjugate. Moreover, the binding of carotenoids to bovine β-LG upon thermal treatment led to a decrease in the polarity around the Tyr residues while increasing the hydrophobicity, whereas an increase in the polarity around the Trp residues decreased the hydrophobicity (Aprodu et al., 2017; Mantovani et al., 2021).
Binding of β-carotene to a globular protein such as BSA was found to protect β-carotene against oxidative degradation. Analysis of the complex showed that tryptophan residues bind β-carotene to BSA through noncovalent bonding, particularly via hydrophobic interactions. According to the circular dichroism data, binding of β-carotene to BSA affected the protein’s secondary structure by decreasing its α-helix part while increasing its random coil fraction. Interestingly, aggregated structure or the triplet-state of β-carotene remained unaffected by the presence of oxygen rather than its singlet state, resulting in near complete protection against photooxidation of β-carotene (Chang et al., 2016).
Lycopene extracted from tomato peels interacts with bovine β-LG through van der Waals forces and hydrogen bonding. According to the molecular modeling analysis, residues Ile29, Lys77 and His146 in the β-LG dimmer interface are responsible for the formation of β-LG–lycopene complex. The increase of protein concentration favors dimerization which may affect the access of the ligand (lycopene) to the binding site of β-LG, thus preventing complex formation. As indicated by the thermal effect, temperature increase from 25 to 90 °C affected the stability of the complex due to thermal denaturation of the protein (Gheonea et al., 2018).
The interaction of lutein with casein occurs at a single site of the protein, mainly driven by hydrophobic interactions. The investigation of the complex’s interaction at pH 7 has indicated that lutein molecules aggregate immediately before interacting with casein molecules present in the solution. This observation demonstrates that the majority of lutein-casein complexes are involved in forming casein aggregated-lutein conjugates. This might be the result of high aggregation constant between lutein molecules compared to that between casein and lutein (Mantovani et al., 2020).
The binding of lutein to BSA is entropically driven by the hydrophobic effect with 1:1 binding stoichiometry. Here, thermodynamic and fluorescence spectroscopic analyses have demonstrated that protein conformation or site conformation does not considerably affect the BSA-lutein interaction. However, denaturation of BSA may result in increased exposure and availability of the hydrophobic amino acids of the protein for interacting with the lutein molecule (Paiva et al., 2020). Similarly, thermodynamic analysis at pH 7.4 showed that the formation of the activated thermodynamically stable nanocomplexes between lutein and lysozyme are dominated by both hydrophobic and hydrophilic interactions (Rezende et al., 2020).
The interactions of bovine caseins (in cow) and caprine (goat) caseins with lutein have shown that the binding between lutein and milk protein involves tryptophan residues and some non-specific interactions. It was found that caprine caseins bind more lutein molecules with higher affinity than caseins in bovine, resulting in a significant increase in the chemical stability and solubility of lutein. Depending on the type of casein, the chemical stability of lutein can be enhanced in lutein-enriched emulsions and this will be useful in the development of low-fat dairy-like beverages (Mora-Gutierrez et al., 2018).
The study based on identifying the interaction of whey protein isolate (WPI), sodium caseinate (SC), with lutein has shown an improved aqueous solubility of lutein by forming a stable conjugate through hydrophobic interactions. According to the experimental results, a considerable effect on the protein’s secondary structure was not observed after interacting with lutein molecules. SC has shown a better capability in protecting lutein against oxidation and decomposition than WPI. In addition, the stability of lutein was directly proportional to protein concentration. Hence, milk proteins can be considered as effective carriers for lipophilic nutraceuticals and hence can be used to develop functional food materials (Yi et al., 2016).
Investigation of retinol and carotenoids binding to β-LG has shown that these two ligands bind at two different sites of β-LG. It was also identified that retinol binds to the interior cavity of β-LG in the presence of carotenoids. The results indicated a high binding affinity of β-carotene towards the hydrophobic pocket of β-Lg protein, creating hydrophobic interactions between them. The two β-ionone rings joined by isoprenoid chains present in carotenoids, and one β-ionone ring in retinol cause different levels of hydrophobicity and render non-competitive binding to the protein (Mensi et al., 2013).
Bixin is a major coloring compound present in annatto seeds which is used in colored cheese manufacturing with liquid whey proteins. In the study of molecular binding between bixin and three major whey proteins, namely β-LG, α-LA, and BSA, it was revealed that binding at pH 7.4 is a spontaneous process mainly driven by hydrophobic interactions. The binding was entropy-driven at a lower temperature and a higher ionic strength while the complexation transits to an enthalpy-driven process at higher temperatures. According to the secondary structural changes of whey proteins, BSA and α-LA showed a decreased content of α-helical structure and an increased content of β-sheet structure. A higher percentage of unordered structure was observed in β-LG at a high concentration of bixin. Furthermore, an enhancement of the binding affinity of whey proteins for bixin occurred after thermal denaturation due to the high level of unordered structure of proteins. Therefore, manipulated solvent conditions and temperatures that affect their binding could be used to minimize the residual annatto content in whey protein ingredients while maintaining the proteins’ functionality (Zhang and Zhong, 2012a, 2012b).
The way that carotenoids and proteins interact with each other depends on several internal and external factors which could affect their properties and functionalities. Thus, a deep understanding of the molecular interaction between protein and carotenoids will provide a fundamental framework for designing efficient protein-based carotenoid delivery systems.
| 4. Physiological relevance | ▴Top |
4.1. Digestion of the food matrix to absorb carotenoids
In the human body, carotenoids obtained from dietary sources are transported to tissues through systemic circulation (Figure 4). Digestion of carotenoids initiates in the oral cavity where they are released from the food matrix upon grinding into smaller pieces. β-Carotene is one of the most abundant carotenoids found in the human diet and the most potent vitamin A precursor of all provitamin A carotenoids (During and Harrison, 2004). β-Carotene absorption from oily solutions, aqueous dispersions, mild heating such as steaming, pureed or finely chopped vegetables is considerably higher than whole or sliced raw, uncooked vegetables (Erdman et al., 1993). For example, ingestion of tomato paste prepared by heating yields higher total and all-trans-lycopene concentrations in human blood plasma than ingestion of fresh tomatoes (Gärtner et al., 1997). Therefore, food processing and cooking cause mechanical breakdown of the food tissue and facilitate releasing the carotenoids and improving their bioaccessibility and bioavailability (Rao and Rao, 2007).
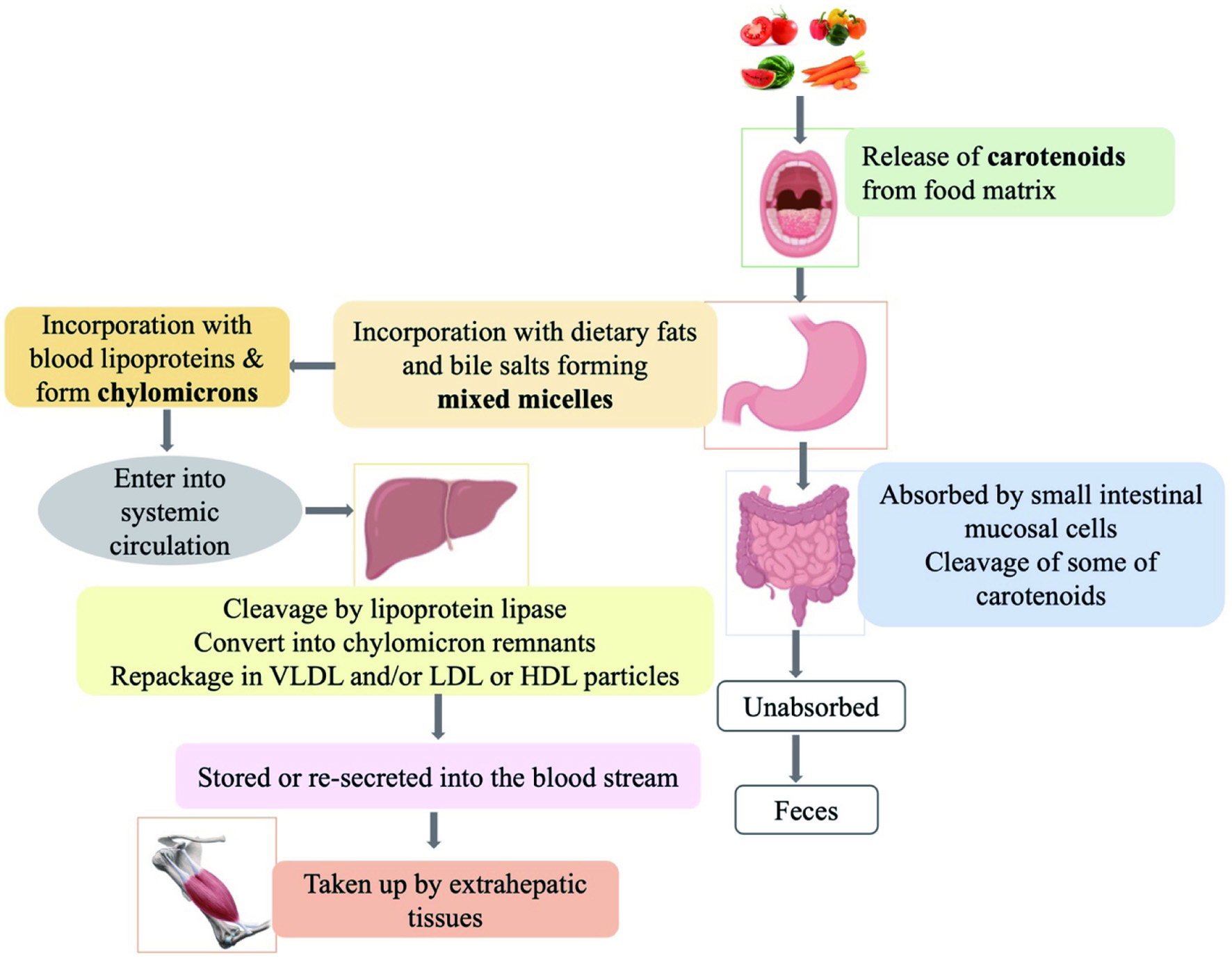 Click for large image | Figure 4. Carotenoids’ absorption and metabolism inside the body |
4.2. Formation of chylomicrons
Most carotenoids are absorbed into the gastrointestinal mucosal cells in the gastrointestinal tract and are not able to dissolve in aqueous solutions due to their lipophilicity. Therefore, carotenoids are incorporated into the mixed micelles in the stomach that are formed by dietary fats/oils and bile acids which are then absorbed by the small intestinal epithelium (enterocytes) through passive diffusion (Rao and Rao, 2007; Yonekura and Nagao, 2007). The amount of carotenoids incorporated into the micelles depends on the hydrophobicity of the carotenoid compound, constituent lipid composition and saturation (Rodriguez-Concepcion et al., 2018). Carotenes and unesterified xanthophylls are absorbed by intestinal mucosal cells and appear unchanged in the systemic circulation while esterified xanthophylls are cleaved prior to their uptake. In the circulation, the absorbed carotenoids are incorporated with blood lipoprotein known as chylomicrons and are then targeted to a variety of tissues such as macula, liver, lung, adipose, brain, prostate, serum, kidney, breast milk, and skin by acting as cytosolic carrier vesicles (Bhosale and Bernstein, 2007; Deming and Erdman, 1999). In chylomicron particles, the more polar carotenoids such as xanthophylls may remain at the surface while less polar carotenoids such as carotenes locate in the core of chylomicrons, on the basis of their structural properties. The ketocarotenoids such as astaxanthin and canthaxanthin interact with proteins by the formation of Schiff bases between their keto groups and specific lysine residues of the proteins while carotenes form hydrophobic interactions with amphipathic areas of the proteins or with the lipid components of lipoproteins (During and Harrison, 2004).
4.3. Intestinal uptake and intramucosal transport of carotenoids
The cleavage of β-carotene occurs mainly in the intestine and liver, but to a greater extent in the liver than in the intestine (During and Harrison, 2004). For instance, intestinal mucosal cells are involved in the metabolism of provitamin A carotenoids such as β-carotene by oxidative cleavage of β-carotene to retinal before binding with lipoprotein (Rao and Rao, 2007). The central cleavage is catalyzed by a cytosolic enzyme, β-carotene 15,15′-dioxygenase which cleaves β-carotene and generates two retinal molecules utilizing molecular oxygen (During and Harrison, 2004). In addition, the eccentric cleavage of β-carotene at non-central double bonds is catalyzed enzymatically or non-enzymatically to produce β-apocarotenals and β-apocarotenones with different chain length (Eroglu et al., 2012).
Humans absorb some β-carotene in the intact form and can also selectively convert it to vitamin A in other tissues. Carotenoids in the enterocyte will be metabolized and absorbed or some of them may be lost in the lumen of the gastrointestinal tract. When chylomicrons in the bloodstream reach the liver, they rapidly degrade by lipoprotein lipase associated with tissue endothelium and converted to chylomicron remnants. During this process, most of the chylomicron remnants deliver carotenoids to the liver, where they are repackaged into very low-density lipoprotein (VLDL) particles and are stored or re-secreted into the blood stream while some of carotenoids may be taken up by extrahepatic tissues (Deming and Erdman, 1999). Here, the non-polar carotenoids such as β-carotene and lycopene are associated primarily with VLDL and low-density lipoprotein (LDL), while more polar xanthophylls such as lutein and zeaxanthin associate with high-density lipoprotein (HDL) (During and Harrison, 2004).
Since carotenoids are lipophilic molecules, they are well represented within the stratum corneum (the outermost layer of the epidermis) of the skin. They are highly reproducible and act as antioxidants by protecting epidermal polyunsaturated fatty acids from oxygen radical peroxidation and provide protection from UV radiation (Hata et al., 2000). In addition, the polar xanthophylls such as lutein and zeaxanthin accumulate in the macular pigment of the human eye and are associated with decreased risk of blindness due to age-related macular degeneration (AMD) (Mozaffarieh et al., 2003). Cooking or processing can improve the solubilization of carotenoids in lipids and absorption in the body indicating that the structure of food matrix and the physical state of the carotenoid in the food affect their release and solubilization (Britton, 2020).
| 5. Potential applications | ▴Top |
5.1. Protein-based nanocarriers for encapsulation of carotenoids
The interactions between carotenoids and proteins greatly affect the light-absorption properties and hence the color and photochemistry of carotenoids. It also allows the hydrophobic carotenoids to be transported through the blood plasma while acting as non-lipid carriers for delivering and exerting their biological functions (Allahdad et al., 2018; Britton and Helliwell, 2008).
In recent years, nanoencapsulation has attracted much attention in nutraceutical delivery systems due to efficient controlled release of encapsulated ingredients, greater stability and effective delivery. This technique has a vast range of applications for protection of carotenoids. This is because the carotenoids are chemically unstable compounds and are easily oxidized with poor bioavailability, low solubility, quick release and low resistance to processing stresses. Protein-based nanoemulsions and nanoparticle platforms use natural proteins as templates or in combination with polysaccharides to encapsulate carotenoids. Therefore, nanoencapsulated carotenoids play an important role in improving carotenoids’ physiochemical properties and hence enhancing their health promoting effects (Fan et al., 2017; Rehman et al., 2020).
5.2. Milk proteins as nanocarrier systems for encapsulation of carotenoids
Milk proteins and milk protein aggregates are considered as natural delivery systems for hydrophobic bioactive compounds. The presence of hydrophobic regions within the protein allows their binding with small hydrophobic molecules via hydrophobic interactions, hydrogen bonding, and van der Waals forces. Caseins and whey proteins are the two major protein fractions in milk. Caseins are naturally found in milk as a supramolecular aggregated structure known as casein micelles. Whey proteins comprising mainly β-LG, α-LA, BSA, immunoglobulins as well as other minor proteins including lactoferrin, glycoproteins, lactoperoxidase and transferrin. Milk proteins are attributed with excellent surface activity and self-assemble properties, gelation, interaction with other polymers, high nutritional value, abundant availability and good sensory properties which make them highly suitable as transport vehicles for delivering various bioactives (Gangurde et al., 2011; Kimpel and Schmitt, 2015; Livney, 2010; Mantovani et al., 2021). Several studies have reported the application of milk proteins as nanocarrier systems by encapsulating carotenoid compounds.
β-Carotene loaded nanoemulsions which were encapsulated with native whey protein isolate (WPI) and WPI-dextran (DT) with 5, 20 and 70 kDa conjugates have been prepared for improving the physicochemical stability and in-vitro bioaccessibility of β-carotene. The storage of the prepared nanoemulsions for 30 days at 25 and 50 ˚C has shown that β-carotene retention was highest at both temperatures when conjugated with WPI-DT (5 kDa) than WPI due to the relatively higher DPPH scavenging ability. However, β-carotene -loaded nanoemulsions stabilized with native WPI resulted in an aggregation or flocculation under mild acidic pH of 4-5, especially when pH was close to the isoelectric point of 5.0 while no appreciable creaming or flocculation was observed after glycosylation with DT. The incorporation of glycosylated 70 kDa DT to the nanoemulsions demonstrated a considerable inhibition on the extent of lipolysis and release of β-carotene, suggesting that high molecular weight DT can be used to regulate nutraceuticals’ bioavailability (Fan et al., 2017). In addition, β-carotene was incorporated into whey protein capsules (WPC) through electrospraying using glycerol as a vehicle. The results demonstrated that β-carotene could be stabilized as functional additives in this manner, and the process of electrospraying did not require the use of high temperatures to generate whey protein capsules (López-Rubio and Lagaron, 2012).
It was found that hydrophobic interactions are involved in encapsulation of β-carotene in casein-graft-dextran nanoparticles where the simultaneous nanoparticle formation and encapsulation were induced by hydrophobic interaction. During the process, solubility of the hydrophobic complex of casein and β-carotene decreases whereas the solubility of dextran increases gradually, forming particles with casein and β-carotene core and dextran shell. These encapsulated β-carotene can be released by pepsin or trypsin hydrolysis indicating that these types of particles are able to deliver unstable and hydrophobic nutrients and drugs (Pan et al., 2007).
Moreover, the influence of the aggregate structure of casein on the encapsulation efficiency of β-carotene has been studied. It was found that the encapsulation efficiency of aggregated casein was higher than that of the re-assembled casein micelles. The aggregated casein prepared at pH 6.0 showed the highest encapsulation efficiency and enhancement of action against time while dried re-assembled casein micelles did not improve the encapsulation efficiency over time. Basically, the deformation of aggregated casein clusters is linked to the entrapment of β-carotene and stabilization with casein via hydrophobic interactions. The increased encapsulation efficiency could be due to the relocation of free β-carotene to the inner region of the aggregated casein cluster which may increase the surface hydrophobicity in casein under low pH conditions, thus showing a significant improvement of the encapsulation properties of β-carotene (Jarunglumlert et al., 2015).
The food processing treatments such as sterilization, pasteurization, high hydrostatic pressure (HPP) and baking used in the food industry cause degradation of thermolabile carotenoids. Casein micelles are used to develop protective nanostructures against the degradation of carotenoids during these processes. Using casein micelles to encapsulate, stabilize and protect β-carotene have proven to lower degradation of β-carotene under high-pressure processing. Moreover, subsequent release of the entrapped β-carotene in nanomicelles occurrs during digestion, thus ensuring the release of the compound after intake (Sáiz-Abajo et al., 2013). As the hydrophobic β-carotene binds to the hydrophobic domain of casein and retained inside the hydrophobic environment of the micelle in an aqueous environment, it results in increased intra- and intermolecular hydrophobic interactions upon thermal treatment in order to protect β-carotene from degradation. (Raikos, 2010; Sáiz-Abajo et al., 2013). Norbixin, a derivative of bixin, is widely used as a natural coloring agent in food, cosmetics and pharmaceuticals. However, its precipitation under acidic conditions has limited its use in acidic food products. Interestingly, the conjugation of norbixin with WPI prevents its precipitation under both neutral (pH 7) and acidic (pH 3) conditions while improving its color stability. Results indicated the potential application of norbixin-WPI as a stable colorant in aqueous foods under acidic pH such as dairy drinks, cheese and juices (Møller et al., 2020).
5.3. Other protein-based nanocarriers for encapsulation of carotenoids
In addition to milk proteins, several investigations have reported the use of other animal and plant proteins for the delivery of lipophilic bioactive compounds. The structural variability of protein molecules in different sources is helpful for the manufacture of complex nanocarriers offering multiple benefits. Intake of lutein (Lut) provides retinal health benefits, particularly for the treatment of early age-related macular degeneration (AMD), thus attention has been paid to discover potential lutein-protein nanocarriers. The nanocomplexes between Lut and lysozyme (Lys) were developed in order to transport lutein and to improve its chemical stability. According to the thermodynamic analysis, the formation of the activated complex by association of the free Lut and Lys molecules was fast and the complex so formed was stable. Moreover, no temperature effect was observed on the stability of the complex. Therefore, the positive outcomes obtained through this study provided the opportunity to produce a thermodynamically favorable Lut-Lys complex for use as a potential lutein delivery systems and in pharmacokinetic-pharmacodynamics prediction models (Rezende et al., 2020).
The hydrophobic surface and aggregated structure of soy protein isolate (SPI) can be considered as a promising agent as effective nanocarriers for lipophilic compounds. The complexation between SPI and β-carotene demonstrated that SPI significantly improves water dispersibility and stability against heating and β-carotene-to-protein ratio determines the freeze-drying of β-carotene. The interactions between SPI with β-carotene mainly occurred by intermolecular hydrophobic interactions built on the surface of the protein. However, the complexation resulted in interparticle aggregation in a concentration-dependent manner without affecting the structure of protein nanoparticles. Interestingly, the thermal degradation of β-carotene was remarkably inhibited by combining with SPI. The significant enhancement of the stability and biaccessibility of β-carotene upon conjugation with SPI nanoparticles provided the insight of converting lipophilic β-carotene to water-soluble agents that could be used in the formulations of functional foods (Deng et al., 2016).
Previous studies have revealed that barley endosperm protein, glutelin, possesses excellent oil-binding property and emulsifying stability, whereas barley endosperm protein, hordein, exhibits good foaming capacity (Wang et al., 2010). Barley protein-based emulsion microparticles for the oral administration of β-carotene was designed by Wang et al. (2011). The in-vitro studies indicated that β-carotene with whey protein microparticles, delivered to the simulated human intestinal tract, were degraded by pancreatic enzymes and steadily released the entrapped β-carotene. The uniquely structured protein matrix microparticles did not show any aggregation during storage or in harsh human gastric conditions and demonstrated that barley protein may be used to develop targeted delivery systems for lipophilic bioactive compounds (Wang et al., 2011).
Similarly, Yang et al. (2014) developed barley protein-based nanoparticles comprising of β-carotene and indicated that they were relatively safe, stable and had a controlled discharge of lipophilic bioactives. In a simulated intestinal environment, it was identified that bulk protein nanoparticles were degraded in the simulated gastric tract conditions and even smaller nanoparticles were released which protected the lipophilic compounds by a layer of barley protein. Unique colloidal structure of the designed nanoparticles, low cytotoxicity and their stability supported the fact that they have strong potential to be used as delivery systems for food bioactive compounds, pharmaceutical and cosmetic applications (Yang et al., 2014).
5.4. Protein-based nanoemulsions
Oil-in-water emulsions have proven to be effective systems for encapsulation and delivery of lipophilic bioactive ingredients and to generate mixed micelles that enhance their solubilization in gastrointestinal fluids. Protein-type emulsifiers or combination with additional antioxidants such as protein-polyphenol conjugates have provided novel approach to improve the stability of β-carotene emulsions by protecting it from degradation (Fu et al., 2019; Mao et al., 2018). Encapsulated β-carotene in pickering emulsions stabilized by wheat gluten nanoparticles (WGN) or wheat gluten nanoparticle-xanthan gum (WGN-XG) complexes have been designed to improve its stability and biaccessibility. It was noticed that 94.3 and 70.1% of the carotenoids incorporated with wheat gluten complexes was retained after one-month of storage at 25 and 37 °C, respectively; thus, wheat gluten complexes are effective in protecting β-carotene from chemical degradation during storage. Based on in-vitro digestion studies β-carotene in the WGN-XG emulsions showed a higher bioaccessibility than in the WGN ones. Further analysis indicated that relatively stable β-carotene-loaded pickering emulsions consisting of protein nanoparticles and xanthan gum could be generated by electrostatic interactions. These results may facilitate the designing of an effective method for nutraceutical delivery based on a pickering emulsion system (Fu et al., 2019). Similarly,formulating oil-in-water (o/w) emulsions by using a carotenoid (astaxanthin/ β-carotene)–BSA complex as surface active substance showed that the carotenoid (astaxanthin/ β-carotene) was first bound to BSA and then acted as an emulsion in o/w system (Wackerbarth et al., 2009).
Lutein loaded into whey protein isolate (WPI) or polymerized whey protein isolate (PWP) nanoemulsion was prepared with high intensity ultrasound and its stability under different storage conditions evaluated. Accordingly, the lutein content of the system was reduced by only 4% after four weeks of storage at 4˚C indicating that WPI-based nanoemulsion possessed a high physiochemical stability at 4˚C. This effective encapsulation method could provide a promising strategy to be used as a good carrier system with high potential stability for hydrophobic bioactive molecules such as carotenoids (Zhao et al., 2018).
Moreover, the stability of β-carotene in protein-oil-in-water delivery systems has been studied by incorporating whey protein isolate (WPI) and sodium caseinate (NaCas) in sunflower oil (SO) or hydrogenated palm kernel oil (HPKO) dispersions. The results showed high β-carotene stability in HPKO dispersions than in SO dispersions whereas NaCas significantly reduced the degradation of β-carotene by providing better barrier properties than WPI. It was suggested that the partially solid property and lacking a reaction medium in HPKO effectively minimizes the β-carotene degradation compared to the liquid property in SO lipid carrier. Specifically, an improved stability was observed with protein concentrations in the range of 0.2-0.4%. Therefore, the lipid carrier type and amino acid composition of the protein could be considered as two key factors that regulate the stability of labile lipophilic bioactive molecules in food model systems (Cornacchia and Roos, 2011).
The formation of astaxanthin nanodispersions via an emulsification-evaporation process was carried out by Anarjan et al. (2014). In this work, gelatin and its combinations with sucrose oleate as a low-molecular-weight emulsifier, sodium caseinate as a protein and gum Arabic as a polysaccharide were used as stabilizer systems. The addition of sodium caseinate to gelatin caused a significant increase in the chemical stability of astaxanthin nanodispersions. This demonstrated that suitable combination of stabilizers can enhance desired dispersion properties compared to the stabilizer alone due to the formation of intermolecular complexes at interfaces and thereby increasing the surface activity (Anarjan et al., 2012). Based on the chemical structure, cysteinyl residues, disulfide bonds and thiol functional groups on sodium caseinate structure prevented lipid oxidation by scavenging free radicals and resulted in only minor degradation of astaxanthin. Furthermore, the mixture of gelatin with gum Arabic that was used to produce astaxanthin nanodispersions rendered optimal physical stability whereas gelatin and its combinations with sucrose oleate generated nanodispersions with smallest particle size. It was identified that the combination of surface-active compounds affords higher emulsifying and stabilizing functionality compared to individual components in the preparation of astaxanthin nanodispersions (Anarjan et al., 2014).
These studies demonstrated the potential application of carotenoid-protein conjugates in nanoemulsion-based delivery systems, food emulsions and pharmaceutical encapsulations. However, the physicochemical and functional properties, stabilities and bioactivities are governed by the encapsulant material used for the encapsulation of carotenoids. Thus, the choice of an appropriate encapsulant or emulsion is one of the most important steps in successful nanoencapsulation of carotenoids.
| 6. Methods of analysis | ▴Top |
In-vitro techniques such as fluorescence quenching, isothermal titration calorimetry (ITC), circular dichroism (CD), dynamic light scattering (DLS), nuclear magnetic resonance (NMR), electrospray ionization-mass spectrometry (ESI-MS), X-ray diffraction and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and in-silico analysis such as molecular docking and molecular dynamics simulations, quantitative structure-activity relationship (QSAR), quantitative structure-property relationship (QSPR) analysis are the major techniques used for characterization of the binding between receptors and ligands (Bandyopadhyay et al., 2012; Buitimea-Cantúa et al., 2018; Mantovani et al., 2021; Vidal-Limon et al., 2022). Among these techniques, virtual screening in-silico approaches have gained much attention over the past decades as a fundamental step in elucidating the structure−activity relationships between bioactive molecules and their potential targets. Molecular docking is one of the most used, efficient and feasible tools to study the biomolecular interactions, the behavior of small molecules in the binding site of target proteins and the stability of conjugation, which adds a new level of understanding and interpretation of experimental data (Vidal-Limon et al., 2022).
6.1. Molecular docking/dynamics analysis for studying carotenoid-protein interactions
Molecular docking is a structure-based drug design method that analyses the interactions and predicts binding modes and affinity between receptors and ligands at the molecular level (Fan et al., 2019; Vidal-Limon et al., 2022). This provides a fundamental understanding of the behavior of small molecules in the binding site of target proteins as well as elucidating their biological and physiological properties (Meng et al., 2012). Several studies have used molecular docking analysis to examine target structures for possible binding and to predict active sites, functionalities of proteins and their interacting compounds.
Lycopene binding to bovine β-LG was investigated at the molecular level using molecular docking. It was observed that lycopene binds on the top of the calyx of the protein at 25 °C to form a stable complex, whereas lower affinity between lycopene and β-LG occurs at 90 °C due to conformational changes upon thermal treatment. However, conjugation of lycopene to the heat treated β-LG caused no alteration of the protein conformation. The hydrophobic patches of the folded protein exposed to the solvent was decreased at high temperatures resulting in a lower solvation energy (Gheonea et al., 2018).
The molecular docking and molecular dynamics simulation analyses for studying the effect of thermal treatment on the complex formed by β-LG and β-carotene from sea buckthorn revealed that high temperature causes changes in the β-carotene binding site, therefore leading to a more thermodynamically stable assembly. Heating the β-LG- β-carotene complex led to changes in the hydrogen bonding pattern and consequently in the secondary structure motifs. The thermal treatment up to 90 °C resulted in favorable molecular rearrangements due to significant changes in β- and γ-turn motifs and formation of new non-interacting native-like α-helical motifs, altering the conformation of the helices. Here, Ile2, Val3, Thr4, Thr6, Lys8, Ile78 and Glu89 amino acids from the β-LG binding surface are involved in forming hydrophobic interactions with β-carotene molecule at 25 °C while more hydrophobic interactions occur at 90 °C. Specifically, the Lys8 residue gets buried within the protein core and does not interact with the ligand molecule at high temperatures. It was also identified that Glu89 and Lys91 were not involved in the interaction with β-carotene at 90 °C due to the side chain reorientation. The results of the molecular dynamics simulations as well as free energy of assembly dissociation and the solvation energy of β-LG folding indicated that the complex between the β-LG and β-carotene molecules leads to a more thermodynamically-stable assembly by forming noncovalent hydrophobic interactions (Aprodu et al., 2017). Similarly, combination of in-silico analysis with thermodynamic parameters revealed that the conjugation between α-LA and β-carotene is a spontaneous process driven by enthalpy, was dominated mainly by the van der Waals and hydrophobic interactions. A partial folding of the α-LA structure with 5% reduction of hydrogen bonds of the protein structure was observed at 90 °C, but with no major changes associated with the secondary structure of the protein. Thus, a good thermodynamic stability of the milk protein-β-carotene complex at high temperatures demonstrates its applicability in formulating new dairy products and for enhancing the nutritional value of the sea buckthorn end products as pharmaceutical and bioactive compounds (Dumitraşcu et al., 2016).
The interactions between saffron carotenoids (crocin/Cro and crocetin/Crt) with human serum albumin (HSA) showed a reduction of α-helix content of HSA due to its unfolding upon binding with saffron carotenoids as well as by affecting the hydrophobic environment of Trp241. Binding of Cro to HSA forms hydrogen bond with Arg117 and Tyr138 while Crt is in hydrogen bond with Tyr138 of the protein. Moreover, docking analysis showed the formation of hydrogen bonds between the polar groups of the ligands, the glycosyl in Cro and carboxyl in Crt, with the hydroxyl group of Tyr138 and the amine group of Arg118 of the HSA. According to the binding energies, Cro shows relatively higher binding affinity for HSA than Crt, indicating that Cro has more hydrophilic character than Crt. Therefore, intensive docking analysis of HSA-Cro/Crt conjugation revealed that the presence of hydrophobic amino acids around the carotenoid backbone forms hydrophobic interactions while polar groups of the ligand form hydrogen bond with the amino acids of the protein resulting in a stable complex (Jafarisani et al., 2018).
In the same manner, in-silico analysis of saffron carotenoids, crocetin (Crt) and crocin (Cro) with catalase (CAT) indicated the formation of stable complex through hydrophobic interactions and hydrogen bonding. Crt with a hydrophobic backbone interacts with a hydrophobic bottle neck-like structure in the entrance of CAT active site and forms a hydrogen bond with Glu461 in the hydrophobic cavity while Cro binds far away from the CAT active site. Depending on the hydrophobic nature of ligands, these docking results also indicated different binding sites for Crt and Cro on CAT enzyme (Hashemi et al., 2020a).
The complexes comprising fucoxanthin and whey proteins, including BSA, β-LG, and α-LA, were constructed and investigated using molecular docking. The results indicated that conjugation of fucoxanthin with whey protein occurs through van der Waals forces, hydrogen bonds and hydrophobic interactions. Thus, the binding affinity of each protein for fucoxanthin in decreasing order was with BSA, α-LA and then β -LG. Detailed analysis of BSA-fucoxanthin complex indicated that fucoxanthin binds to the hydrophobic pocket of the protein containing Trp213, Leu237, Val240, Leu259, Ala260, and Ala290, while the oxygen atom of the epoxy group in fucoxanthin forms two hydrogen bonds with the hydrogen atoms of Tyr149 and Arg256 residues. In β-LG- fucoxanthin complex, the ligand binds at the top of calyx through van der Waals forces, hydrogen bonds with Asp28 residue and hydrophobic forces with Leu31, Pro38, Ile71, Ile72, Ile84 residues. However, fucoxanthin spans α-lobe and β-lobe subunits of the α-LA protein and mainly binds via hydrophobic interactions at the residues of Phe31, Tyr36, Ile41, Val42, and Trp118. Furthermore, loosening and unfolding of the whey protein structure occurs upon binding with fucoxanthin through noncovalent interactions as the main driving force (Zhu et al., 2017).
The interaction mechanism between oleic acid-bovine serum albumin (OA-BSA) and astaxanthin has been studied to encapsulate astaxanthin using fatty acid-protein complexes as nanovehicles. According to the computational docking analysis, astaxanthin is bound to the junction area of subdomains while more tended to ?ibuprofen for site II. In the astaxanthin –BSA complex, amino acid residues in the 209–480 region of the sequence was involved in astaxanthin binding and formed two hydrogen bonds between oxygen atoms of hexatomic ring in astaxanthin and the hydrogen atoms of Lys294 and Arg217 residues of the protein. However, astaxanthin binds to the subdomain IIA of the BSA protein in the OA-BSA complex and amino acid residues located in the 100–207 region of the sequence. Combining multispectral analysis and molecular docking indicated an enhancement of the binding ability of astaxanthin to the subdomain IIA of BSA incorporated with OA as a protein ligand. Therefore, fatty acid-protein complexes such as OA-BSA can be used as efficient delivery carriers of fat-soluble carotenoids (Liu et al., 2020).
6.2. Carotenoids-protein conjugates as potential therapeutic targets
The effects of saffron carotenoids crocetin (Crt) and crocin (Cro) on superoxide dismutase (SOD) was analyzed by Hashemi et al. (2020b). The docking analysis performed using AutoDock Vina (version 1.1.2) showed a strong binding affinity of Cro/Crt to SOD and induced slight conformational changes. Cro binds at the entrance of the active site channel of the protein via hydrogen bonds and van der Waals interactions while decreasing the α-helical content thereby inducing conformational changes and limiting substrate accessibility. Moreover, Crt was stabilized through a hydrogen bonding with Asn65 and van der Waals interactions with 8 amino acids residues mainly around the copper and zinc atoms in the active site. These data predicted that Cro inhibits SOD activity by scavenging superoxide radical, while Crt inhibits SOD by affecting the copper-binding site which can be used as therapeutic target for cancer treatment.
The anti-amyloidogenic activity of three carotenoids, cryptocapsin, cryptocapsin-5,6-epoxide and zeaxanthin, was studied at the molecular level to discover their ability to inhibit amyloid beta (Aβ) aggregation. According to the molecular modeling analysis, cryptocapsin showed the highest bioactivity by exhibiting a high binding affinity for Aβ peptide, while cryptocapsin-5,6-epoxide and zeaxanthin exhibited similar activity on anti-aggregation effect. The keto group of cryptocapsin forms hydrogen bonds with the Aβ peptide at Glu22 and Asp23 and cryptocapsin-5,6-epoxide interacts with amyloidic regions of Aβ forming hydrogen bonds at Leu17, Phe19 of amyloidic region 1 and Gly33 of amyloidic region. Zeaxanthin has one hydroxyl group at each β-ionone ring and these hydroxyl groups interact with Val39 and Ile41 while others interact with Gly25. This study performed by molecular modeling has provided novel perspectives on the anti-amyloidogenic potential of these bioactive compounds as therapeutics for Alzheimer’s disease (Lakey-Beitia et al., 2017).
The tomato lycopene and lycophyll was subjected to molecular modeling in order to investigate their predicted binding interactions with human 5-lipoxygenase enzyme (5-LOX). Blind docking of the lycopene and lycophyll resulted in the most favorable bindings at the cleavage site or the putative allosteric site of the enzyme suggesting the potential direct competitive 5-LOX inhibitory effect of these compounds. Particularly, all-trans geometric forms of lycopene have shown a proper binding with the cleft at the interface of the N-terminal and C-terminal domains of the protein. The long molecular length of lycopene and its derivatives restricts their binding at the catalytic site of the enzyme. The results obtained through in-silico analysis can be utilized in direct experimental studies to modulate the 5-LOX pathway in prostate cancer using tomato carotenoids (Hazai et al., 2006).
Human calcium/calmodulin-dependent protein kinase IV (CAMKIV) is a multifunctional protein kinase and a factor in the development of neurodegenerative diseases, cerebral hypoxia and cancer (Cunningham et al., 2019; Naz et al., 2017). Therefore, bioactive compounds that can inhibit the CAMKIV enzyme activity are a potential target in the pharmaceutical industry. Docking analysis on conjugation between CAMKIV and β-carotene using Autodock (version 4.2) indicated a strong binding affinity of β-carotene towards CAMKIV forming a stable complex by noncovalent bonding including several van der Waals interactions in the vicinity of the active site of the protein. Interestingly, it has been identified that the pigment molecule enters deeper into the active site cavity of CAMKIV which may decrease the substrate accessibility thereby inhibiting enzyme’s activity (Naz et al., 2017).
Human serum albumin (HSA) is the most abundant protein in blood plasma which functions as a transporter and free-radical scavenger. However, albumin incurs some conformational changes in diabetes mellitus (DM) conditions which affect its scavenging activity (Roche et al., 2008; Wibowo et al., 2019). The strong bioactivities of astaxanthin such as antioxidant, anti-diabetic, anti-inflammatory and anti-cancer activities has made it a potent target for this phenomenon. Wibowo et al. (2019) expected to improve the function of albumin in DM conditions by its conjugation with astaxanthin, thereby preventing conformational changes in albumin. Molecular docking and dynamics results showed that astaxanthin binds with HSA via hydrogen bonds and alkyl bonds forming a stable complex. Moreover, binding of astaxanthin to HSA indicated an enhancement of the scavenging activity while binding of astaxanthin to glycated albumin (gHSA) showed an improvement of the stability of the protein. Thus, the improvement of protein structure from gHSA to normal HSA condition upon binding with astaxanthin has demonstrated the suitability of astaxanthin as a novel therapeutic agent for DM.
Binding of dietary carotenoids with brain-derived neurotrophic factor (BDNF) protein have been studied via molecular docking analysis for anti-depressive effects. A total of 13, 10, and 14 hydrophobic interactions were found for α-carotene, β-cryptoxanthin, and lycopene in the docked complex, respectively. Thr82, Thr83, Gln84, Arg104, and Asp106 were found to be the common amino acids that form these interactions. The most stable binding occurred between α-carotene and BDNF and all ligands bound in a similar manner with almost similar binding affinity at the site distant from the N-terminal region involved in tropomyosin receptor kinase B (TrkB) binding site of BDNF and indicating an allosteric effect of these carotenoids on BDNF protein. Therefore, identifying potential anti-depression properties of dietary carotenoids and knowing the possible mechanisms through docking analysis is of great importance in the pharmaceutical industry (Park et al., 2022).
More recently, tumor necrosis factor-alpha (TNF-α) inhibiting capacity of natural carotenoids was investigated as a drug target against cytokine in COVID-19 patients. The in-silico approaches based on molecular docking, and molecular dynamics (MD) simulations showed a favorable binding of apocarotenoids, namely sorgomol, strigol and orobanchol to TNF-α protein. Sorgomol exhibited a considerably higher affinity for the protein, among others, where it binds to the active site of the protein and disrupts TNF-α-Tumor necrosis factor receptor 1 (TNFR1) interaction. Thus, sorgomol apocarotenoid can be considered as a competitive inhibitor that can interrupt the binding of TNFR1 receptor to TNF-α protein, thereby preventing the activation of the inflammatory cascade (Taghipour et al., 2022). Marine carotenoids fucoxanthin and siphonaxanthin have been shown to possess powerful antioxidant activity and several potential bioactivities including anticancer, anti-inflammatory, and anti-obesity effects (Pangestuti and Kim, 2015; Yim et al., 2021). The ability of binding of these two compounds with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike-protein was predicted using AutoDock Tools. In molecular docking fucoxanthin and siphonaxanthin displayed stable conjugation with a low binding energy. However, SX fixed exactly into the angiotensin-converting enzyme 2 (ACE2) binding region of SARS-CoV-2, suggesting the polar nature of siphonaxanthi compared to fucoxanthin facilitates siphonaxanthin’s affinity for the protein. Thus, the inhibitory activity of polar xanthophylls on SARS-CoV-2 spike-protein provides useful information in the application of these marine carotenoids as anti-viral agents for possible treatment and prevention of COVID-19 (Yim et al., 2021).
β-Carotene and astaxanthin extracted from Spondias mombin plant leaves against 3-hydroxy-3-methylglutaryl coenzyme A /HMG-CoA reductase (HMGCR) inhibition activity was studied in-silico. The molecules docked using Autodock 4.0 demonstrated a better binding affinity for HMG-CoA reductase when compared with mevastatin which is a known HMG-CoA reductase inhibitor. The extensive hydrophobic interactions displayed by carotenoids within the orthosteric site of HMGCR may be responsible for a stable conjugation of mevastatin as it forms only one hydrogen bond and one π–π stacking interaction within the orthosteric site. The results suggest the potential inhibitory effect of β-carotene and astaxanthin on HMG-CoA reductase enzyme in preventing cholesterol biogenesis (Metibemu et al., 2021).
Thus, fully elucidating the carotenoid-protein interactions and mechanisms based on docking analysis may be helpful in better development of novel products that can be used in the food and pharmaceutical industries.
| 7. Conclusion | ▴Top |
Carotenoids are the most widely distributed natural pigments with distinctive colors varying from red to orange to yellow in plants and animals. The hydrophobic nature of carotenoids makes them less soluble in the aqueous environment, more susceptible to photodegradation and poorly absorbed from unprocessed food. Conjugation of carotenoids with proteins via noncovalent bonding provides a protective effect against their oxidative and thermal degradation under intensive processing and storage conditions. Carotenoids and proteins associate mainly through hydrophobic interactions while van der Waals forces and hydrogen bonding may occur depending on the type and chemical structure of components involved and environmental conditions such as temperature, pH and ionic strength. In several studies, carotenoid-protein conjugates/ carotenoprotein complexes generated by encapsulating carotenoids with protein-based nanoparticle platforms have been identified as an effective method of targeted nutraceutical delivery systems. The protein-based nanoencapsulation improves stability, solubility, bioaccessibility and bioavailability of carotenoids thereby exerting enhanced biological activities. Moreover, these complexes act as emulsions in oil-in-water (o/w) systems and as stable coloring agents in aqueous food systems that are produced commercially attractive red, orange, purple and blue carotenoid-based products. As shown in previous studies, in-silico analysis of association between carotenoids and proteins provides a better understanding of the structure of the bioactive compounds and proteins, the factors that favored or disfavored interactions and the stability of complexes that modulate their function. Moreover, the conjugation could enhance beneficial health aspects including potential anti-amyloidogenic, anti-cancer, anti-neurodegenerative, anti-depressive, anti-inflammatory and anti-viral activities. Thus, successful applications of carotenoid-protein conjugates discovered through computational approaches have shown that a deep understanding of the distribution, stability and behavior of carotenoprotein complexes in real matrices is crucial with relevance to the development of functional foods and nutraceuticals.
Acknowledgments
We are grateful to the Natural Science and Engineering Research Council (NSERC) of Canada for financial support.
| References | ▴Top |